Abstract
Although clinical trials in refractory epilepsy are currently carried out, the field of deep brain stimulation (DBS) in epilepsy is still at its initial stage. Little is known about where, when and how to stimulate and what would be the short and long consequences. Animal studies might provide clinicians with new ideas regarding targets for DBS. Here an overview is given regarding old and new targets in rodent models of temporal lobe epilepsy.
The evidence from animal models showed that stimulation of the subiculum – either in responsive or scheduled manner - is anticonvulsant in different seizure and epilepsy models, indicating that the subiculum might be a promising candidate for DBS targets. For the rest, the antiepileptic effects of low frequency stimulation were established mostly in kindling models. The presence of a critical time window in which stimulation was effective following after discharges on kindling acquisition, demonstrates that timing of DBS is an important factor for the anticonvulsant effects of DBS.
Author Contributions
Academic Editor: Gaetano Zaccara, Azienda Santaria di Firenze
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2016 Lili Huang, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction:
Nearly one third patients with epilepsy, despite with treatment of antiepileptic drugs (AEDs), still have incompletely controlled seizures or debilitating medication effects1. Deep brain stimulation (DBS) is a promising treatment for epileptic patients who are not proper candidates for resective surgery. DBS delivers current to the brain via implanted electrode to reduce or control seizures. Compared to the classic resective surgery, DBS is reversible, can be user-customized and has fewer complications.
Stimulation is commonly delivered according to a predefined protocol, that is, scheduled stimulation, independent of the neurophysiologic state of the brain. In contrast, responsive stimulation refers to stimulation that is delivered directly in response to electrographic epileptic activities. Responsive stimulation can target seizure dynamics with higher temporal specificity and save battery power for implanted stimulation devices 2. With development of brain computer interface systems in real time seizure analysis, responsive stimulation has received more attention in clinical trials and animal research. An example of schema of delivery of responsive stimulation in rat model was given in Figure 1.
Figure 1.Schema of delivery of responsive stimulation in a closed-loop BCI (brain computer interface) system. EEG signal was at first obtained via implanted electrodes from rats, amplified, band-pass and notch filtered with a physiological amplifier (made by Electronic Research Group, Radboud University, Nijmegen), fed into Digital Analogue converter (DI-720), and was digitized on a PC. EEG signal was also fed into a seizure detection program 3 to detect seizures and trigger a stimulator to deliver responsive stimulation to rats in order to disrupt or modulate seizures.
One crucial question on DBS treatment for refractory epilepsy is where to stimulate. DBS targets are either the areas directly involved in seizure generation or propagation like the hippocampus, or the areas such as the anterior nucleus of the thalamus (ANT) that might serve as a gate to control an epileptic network. Various degrees of efficacy have been achieved on the established targets including the cerebellum, hippocampus, subthalamic nucleus, caudate nucleus, and ANT in animal research and human trials (see review 4, 5).
Despite the progress of DBS for epilepsy treatment in clinical trials and animal research, not much is known regarding where, when and how to stimulate to obtain better efficacy. The current review will give an overview of application of DBS in the classical targets - hippocampus and amygdala, and then introduce some potential new targets for epilepsy treatment in rodent models, with an emphasis on whether stimulation timing is a factor.
Classical Targets in the Hippocampus/Amygdala
The hippocampus and amygdala are located in temporal lobe and are considered as pivotal epileptogenic areas to investigate seizure generation in the laboratory. Stimulation of the hippocampus and amygdala can induce seizures, as in cases of kindling.
However, DBS in the hippocampus and amygdala with different stimulation protocols can also abort or suppress epileptiform activities. Table 1 summarizes the studies on stimulation of the hippocampus (mostly the CA1 and CA3 area) and amygdala in different rat models.
Table 1. Animal studies for DBS in the hippocampus and amygdala| Study | Animal models | Target | Stimulation parameters | Outcomes |
|---|---|---|---|---|
| Bragin et al, 20026 | KA induced chronic epilepsy model | PP | 5 Hz; 5s for 15 days | Increased AD threshold and reduced spontaneous sz |
| Wyckhuys et al, 2010a7 | KA induced chronic epilepsy model | H | 130 Hz with Poisson distributed stimulation (PDS) and standard HFS | PDS: 7/13 rats with reduced spontaneous sz frequency HFS: 5/11 rats with reduced sz frequency |
| Ullal et al., 19898 | Amg kindling | H and Amg | 4 Hz, square wave, 125 ms | Increased AD threshold during kindling acquisition and in fully kindled animals |
| Weiss et al, 19959 | Amg kindling | H and Amg | 1 Hz, 15 min | Increased AD threshold |
| Cuellar-Herrera et al, 200610 | Amg kindling | H | 130 Hz, 60 us, 120-660 uA, 60 min; immediate after kindling stimulation | Non-responders and responders with no stage 4 or 5 sz |
| Mohammad-Zadeh et al, 200711 | Rapid kindling | PP | 1 Hz, 50-150 μA, 0.1 ms | Slower progression to fully kindled stage |
| Wyckhuys et al, 200712 | Alternative day rapid kindling | H | 130 Hz, square wave, 60 μs | Increased AD threshold, AD latency and decreased AD duration |
| Wyckhuys et al, 2010b13 | Alternative day rapid kindling | H | 5 and 130 Hz, square wave, 60 μs | LFS: no significant change HFS: increased AD threshold and latency |
| Zhang et al, 200914 | Amg kindling | CA3 | 1 Hz, monophasic square wave, 100 μA, 0.1 ms | Decreased AD duration and retarded generalization of sz. |
| Sun et al, 201015 | Amg kindling | CA3 | 1 Hz, 0.1 ms, 100 μA; 15 min immediate before (Pre) or after (Post) kindling stimulation | Post treatment: reduced severity and susceptibility to evoked sz Pre treatment: similar but weaker inhibition of sz |
| Gaito et al, 198016 | Amg kindling | Amg | 3 Hz, 100-196 μA, 30 s, sine wave | Higher sz threshold and suppression of behavioral signs |
| Shao et al, 198217 | Amg kindling | Amg | 60 Hz, till 54 μA, 1 s, sine wave | Long term inhibition |
| Weiss et al, 199818 | Amg kindling | Amg | 1 Hz, 5-15 μA, 15 min (DC) | Increased AD threshold caused by DC leakage |
| Velisek et al, 200219 | Amg kindling | Amg | 1 Hz, 280 μA, 15 min, square wave, 200 μs | Impaired progression to fulled kindled state, decreased AD duration |
| Lopez-Meraz et al, 200420 | Amg kindling | Amg | 1 Hz, 100-400 μA, 15 min, square wave | Impaired progression to fulled kindled state |
| Goodman et al, 200521 | Amg kindling | Amg | 1 Hz, 50 μA, 30 s, sine wave; immediate before kindling stimulation | Decreased AD duration and behavioral score |
| Carrington et al, 200722 | Amg kindling | Amg | 1 Hz, 100 μA, 30 s, sine wave | Increased AD threshold |
| Wu et al, 200823 | Amg kindling | Amg | 1 Hz, 100 μA, 30 s, 15 min, square wave; at different time points: Immediate: after kindling stimulus Delayed: after cessation of AD | In kindling rats: immediate LFS inhibited sz stage and delayed LFS increased sz stage 1 & 2. In fully kindled rats: immediate LFS decreased gz, sz stage and cumulative duration of gz. Delayed LFS: prolonged cumulative duration of gz and AD duration. |
For these classical stimulation targets, kindling models are mostly used. Gaito and group 16 reported that low frequency stimulation (LFS) (3 Hz) in the amygdala led to strong long term inhibition of epileptic activities in fully kindled rats. Since this original report different groups have found that LFS (1 Hz) of the amygdala can increase AD threshold and decrease AD duration, and slow progression of seizure stage in the kindling models in rats. HFS of the amygdala was hardly investigated, except in a single paper in which stimulation at 60 Hz was delivered to the amygdala, resulting in long term inhibition 17.
In the hippocampus, the CA3 was commonly chosen for stimulation and the perforant pathway has also been stimulated in two studies 6, 11. LFS (1 Hz) was applied in the kindling model and was found to increase AD threshold and decrease AD duration during kindling acquisition, and slower progression to fully kindled stage. High frequency stimulation (HFS) (130 Hz) was investigated in the kindling models 10, 13 and a chronic epilepsy model 7, 12. The results showed that HFS can reduce seizure frequency in the chronic epilepsy model, and can increase the AD threshold, latency, or lower generalized seizure number in the kindling models.
While most studies used the kindling models, much less work was done in the chronic epilepsy models. Exceptions were the two studies with hippocampal stimulation in chronic epilepsy models 6, 7. Especially, Wyckhuys and group 7 showed that HFS of the CA3 can suppress seizures in the kainate induced SE model: Poison distributed stimulation reduced seizure frequency in nearly 50% of rats (7/15) compared to HFS in 33% rats (5/15). The same group also compared LFS (5 Hz) and HFS (130 Hz) in a kindling model in rats 13. They found that HFS increased AD threshold and decreased AD latency, whereas LFS did not show significant changes. This is one of the few studies that compared effects of LFS and HFS in the hippocampus on kindling acquisition.
Most these studies were conducted in the kindling models with scheduled stimulation. Considering the advantages of time contingent stimulation, responsive stimulation deserves to be investigated in the hippocampus in different models. It is interesting to investigate which stimulation – HFS or LFS - is more effective to obtain immediate seizure suppression or to induce long term depression with specific stimulation protocols. More studies are also needed to further compare effects of stimulation in these classical targets with the new targets.
New DBS Targets
It is critical yet difficult to choose a proper target DBS for epilepsy treatment. Apart from the classical DBS targets mentioned above, some potential new targets have also been investigated (see Table 2).
Table 2. Animal studies for deep brain stimulation (DBS) in the new targets| Study | Animal models | Target | Stimulation parameters | Outcomes |
| Huang & van Luijtelaar, 201224 201325 201426 Zhong et al, 201227 | KA induced seizure model KA induced seizure model KA induced epilepsy model Kindling model Pilocarpine induced epilepsy model | Sub Sub Sub Sub | 125 Hz, 0.1 ms, 100-300 μA 125 Hz, 0.1 ms, 100-300 μA (RS & SS) 125 Hz, 0.1 ms, 100-300 μA (RS & SS) 1 Hz, 0.1 ms | Focal sz suppressed on Day 1 RS and SS suppressed focal sz on Day 1 and 15 but generalized sz only Day 15 in non-SE rats Both RS and SS suppressed focal spontaensous sz Reduce sz stage and shorten AD during kindling acquisition Prevent pilocarpine induced spontaneous generalized sz |
| Xu et al, 201028 Zhong et al, 201227 | Kindling model Kindling model | EC EC | 1 Hz 1 Hz, 0.1 ms at 4s, 10s or ADD delay | Reduce sz stage Suppress generalized sz slow progression of stage at 4s delay but not at 10 s or ADD delay |
| Kile et al, 201029 | Genetic mouse model | VHC | 14 Hz | Suppress sz frequency |
| Rashid et al, 201230 | SE induced epilepsy model | VHC | 1 Hz | Reduce spontaneous sz and interictal spikes |
| Chiang et al, 201331 | 4-aminopyridine (AP) Induced seizure model | VHC H | 100 Hz at100, 300, 500μA | Both VHC and H stimulation produce global suppression |
| Siah et al, 201432 | 4-AP Induced seizure model | VHC | Closed-loop theta burst stimulation | Suppress sz |
| Ozen et al, 200933 | Kindling model | CC | 1 Hz | Suppress sz stage and shorten AD duration |
1.Subiculum
One of them is the subiculum of the hippocampus. The subiculum is relatively less studied as a DBS target, compared to the CA1 and CA3 area, but is receiving increasing attention, driven by its role in spatial encoding 34, 35 and epilepsy 36.
The subiculum, situated between the CA1 area and entorhinal cortex (EC), is considered as the major output of the hippocampus proper (Figure 2). In the classic tri-synaptic pathway, the subiculum receives primary inputs from the CA1 and projects to the EC 37. The subiculum also projects to the pre- and para-subiculum 38, which projects in turn to the superficial layers of the EC 39, 40, 41. Besides the major EC and hippocampal connections, a variety of small circuits has been reported. An in vitro study 42 showed functional pathways in which synchronous activities could propagate backward to the CA1. Besides constitution of the entorhinal-hippocampal circuits, the subiculum also projects to a range of cortical and subcortical structures such as the perirhinal cortex 43, 44, amygdala 45 and thalamus 46. In vitro studies on human hippocampal tissue showed spontaneous rhythmic activities in the subiculum 36, 47, reminiscent of interictal spikes observed in epilepsy patients. Therefore, the cellular and network properties of the subiculum suggest that it is susceptible to synchronous activities and could participate in seizure generation and propagation within and outside the hippocampal area.
Figure 2.The main intrahippocampal circuit. Inputs of the EC project to the DG via the perforant pathway, then to the CA3 through mossy fibers, which in turn projects to the CA1 area via the Schaffer collateral pathway. CA1 neurons further project to the subiculum and then project back to the EC and other areas such as the perirhinal cortex, amygdala and thalamus. DG: dental gyrus; EC: entorhinal cortex; Sub: subiculum; MF: mossy fibers; PP: perforant pathway; SC: Schaffer collateral. Adapted from Moser (2011) 48.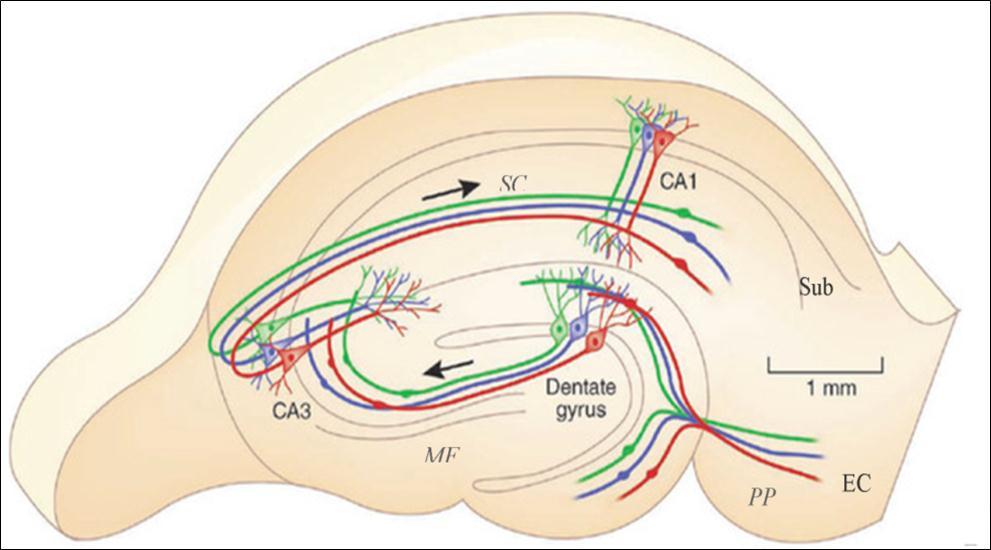
DBS of the Subiculum
So far, a few studies have applied DBS to the subiculum for seizure control in animal models (Table 2).
Zhong and colleagues 27 applied LFS to the subiculum in a series of experiments in the amygdala kindling and pilocarpine induced epilepsy models in rats. They found that LFS of the subiculum (1 Hz) immediately before and after kindling stimulation or after the cessation of the afterdischarge (ADD, afterdischarge duration) reduced seizure stages and shortened the duration of the AD during kindling acquisition. LFS of the subiculum with immediate and double ADD delay reduced the incidence of generalized seizures and average seizure stage in fully kindled animals. In the pilocarpine induced chronic epilepsy model, LFS was also applied daily for 15 min for 4 weeks since 8 days after status epilepticus (SE) and was found to prevent spontaneous generalized seizures. Importantly, immediate, ADD-delayed and double ADD-delayed LFS of the subiculum can inhibit progression in amygdaloid-kindling rats, suggesting that the subiculum is more suitable for a responsive stimulation pattern, as there is inevitably a time delay between seizure detection and stimulation delivery.
Meanwhile, responsive HFS was applied to the subiculum in different seizure and epilepsy models at our lab 24, 25, 26. In the first study 24, rats received responsive HFS (130Hz) at the subiculum in an acute seizure model induced by repeated injections of kainic acid (KA) in the CA3 area in rats. The results showed that responsive stimulation suppressed seizures (less focal seizure number and longer seizure interval). Meanwhile, a real-time seizure detection program with high sensitivity and specificity was developed 3 and was later applied to a similar seizure model to deliver responsive stimulation. In that model 25, either scheduled or responsive stimulation was applied to the rats during the first 24 hours after KA administration. The results showed that both types of stimulation were effective only on the rats that did not reach SE. Such anticonvulsant effects of stimulation were different for focal and generalized seizures: immediate and lasting effects on focal seizures but only delayed effects on generalized seizures. In a third study 26, both responsive and scheduled types of stimulation for two days were compared in the KA induced chronic model of TLE. Both types of stimulation suppressed the spontaneous focal seizures several weeks after the induction of SE.
One main finding is that HFS, irrespective of responsive and scheduled stimulation can suppress seizures, suggesting that the subiculum can be a potential target for DBS. It is the first time that the anticonvulsant effects of responsive stimulation of the subiculum were reported. The first study demonstrated a lower focal seizure number and longer seizure interval; the second confirmed these positive effects of responsive stimulation in the non-SE rats, while positive effects were also observed on spontaneous focal seizures in the chronic epilepsy model.
Furthermore, the effects of stimulation were highly dependent on the severity of the seizures anticonvulsant effects for the non-SE rats, whereas no effects or even pro antiepileptic effects for the SE rats. This is the first study which demonstrated in rats that DBS, applied immediately after SE could worsen seizures if subjects reach a severe seizure state such as SE. This finding has a clear clinical implication: patients with SE should not be given DBS within 24 h after SE.
In summary, outcomes from preclinical studies from two groups, suggest that the subiculum might be a proper target for responsive stimulation. It still remains unclear whether there are lasting effects of stimulation after stimulation stops in these models. It is also worthy to investigate whether chronic stimulation of the subiculum remains anticonvulsant, which is obviously of the larger clinical importance.
2. Entorhinal Cortex:
The Entorhinal cortex (EC) is another structure receiving attention as a potential new DBS target. The EC is an important parahippocampal structure (Figure 2), sending projections via its shallow layers (Layer II and III) to the dental gyrus (DG), CA3, CA1, subiculum and projecting back to its deep layers (Layer IV and V). The EC is considered to serve as a gate connecting the hippocampal formation and extra-hippocampal areas.
Gnatkovsky and colleagues49 performed intracellular and extracellullar recordings of principal neurons in the EC in the isolated brain by perfusion of bicuculine in guinea pig. They found that during ictal transition sustained inhibition without firing in the EC correlates with the onset of seizures, indicating an inhibitory network in the EC during the transition of seizures. Xu and group 28 demonstrated that LFS of the EC can indeed reduce the progression rate of seizure stages on kindling acquisition and suppress generalized seizures in fully kindled rats. These anticonvulsant effects were present when responsive LFS was applied immediate or with a 4s delay after kindling stimuli, but were not effective with a 10s delay or with a delay as long as the ADD. This suggests that there is a time window for LFS of the EC, in agreement with the outcomes of Zhong et al’s study 27. In the latter study, ADD-delayed LFS was delivered to different areas such as the amygdala, EC and subiculum on kindling acquisition. The results showed that LFS of the EC with ADD delay could not slow the progress of seizure stages, indicating that the EC has a shorter time window for LFS compared to the subiculum.
Does it mean that the EC is less suitable for responsive stimulation compared to the subiculum? It is too early to draw firm conclusions, considering the small number of rats and the fact that responsive stimulation was only studied in the kindling model. More experiments and different models need to be conducted to investigate whether HFS of the EC is anticonvulsant and if so, which type of stimulation acts better
3. Hippocampal Commissure and Corpus Callosum:
These above mentioned areas subiculum and EC are gray matter targets involved in seizure generation or propagation. Another category of targets could be white matter tracts that serve as functional pathway for seizure propagation such as the ventral hippocampal commissure (VHC) and corpus callosum (CC).
The VHC connects heavily to the hippocampus and is thought to participate in seizure propagation. Kile and group 29 demonstrated that LFS (14 Hz) of the VHC suppressed seizure frequency in the Q54 transgenic mice that can display spontaneous seizures due to mutation of sodium channel. In a more recent study, Rashid and colleagues 30 showed that continuous LFS (1 Hz) of the VHC for two weeks resulted in reduction of spontaneous seizure frequency and interictal spikes on seven rats in the SE induced model of TLE. HFS (100 Hz) was applied to the ventral HC and focus site at different current (100, 300, 500 μA) in five rats that received the injections of potassium channel blocker 4-aminopyridine (4-AP) in the CA3 area to induce seizures 31. Both HFS in the focus site and VHC showed amplitude dependent suppression on seizures and HFS at the focus site had a higher suppression rate, suggesting that both stimulations suppressed acutely induced seizures and focal stimulation was more effective than more remote sites of stimulation.
Later, Siah and colleagues 32 compared the theta burst stimulation (5 Hz burst train with bursts at 100 Hz) and continuous HFS to the VHC in a closed-loop system in the same 4-AP rat model. They found that rats with theta burst stimulation experienced longer seizure suppression compared to continuous HFS.
Meanwhile, the corpus callosum (CC) was also considered as a target of DBS. Ozen et al. 33 reported that LFS of the CC 1 min after cessation of AD suppressed seizure stage and shortened AD duration on kindling acquisition in rats. They also found that LFS concurrent with seizures (1s after seizure) led to less severe seizures and shorter AD duration, indicating the potential effects of responsive stimulation of the CC. These outcomes suggest that the CC might be a potential target for responsive stimulation. It would be interesting to further investigate whether responsive stimulation of the CC has anticonvulsant effects.
Conclusions
Subicular stimulation was applied in different rat models such as amygdala kindling and in chronic epilepsy models with different types of stimulation – HFS and LFS, responsive and scheduled stimulation. Despite the small number of rats that were used, the evidence of these studies consistently suggests that subicular stimulation – either responsive or scheduled stimulation is anticonvulsant in different seizure and epilepsy models. Yoked control studies are necessary in order to establish whether the timing of stimulation, during the seizure or immediately after the stimulation, is an important factor affecting the efficacy of DBS. More studies are also needed to investigate longer lasting effects of subicular stimulation as well as effects of chronic stimulation in the subiculum.
As for targets such as EC, VHC and CC, the establishment of putative beneficial effects of stimulation are still at embryo stage. Evidence of LFS on the kindling models suggests that EC and amygdala have shorter time windows compared to the subiculum, whereas the CC seems sensitive for stimulation timing. So far only small sample size and limited animal models were reported. It deserves to further explore stimulation of these structures in controlled studies with larger number in different animal models, with different manner responsive and scheduled stimulation. The presence of time windows emphasizes that timing does matter and that responsive stimulation might have a bright future.
References
- 1.Kwan P, Brodie M J. (2000) Early identification of refractory epilepsy. , N Engl J Med 342, 314-9.
- 2.Sunderam S, Gluckman B, Reato D, Bikson M. (2010) Toward rational design of electrical stimulation strategies for epilepsy control. , Epilepsy Behav 17, 6-22.
- 3.Huang L, G van Luijtelaar.(2011)Evaluation of real-time program for seizure and spike detection in rats, Biophysical standards and information technologies. in medicine:Proceedings of the Jubilee Conference dedicated to the 10th anniversary of theOdessaNationalMedicalUniversityandInternationalKazakh-TurkishUniversity 138-146.
- 4.Ellis T L, Stevens A. (2008) Deep brain stimulation for medically refractory epilepsy. Neurosurg Focus.25,E11.
- 5.Saillet S, Langlois M, Feddersen B, Minotti L, Vercueil L et al. (2009) Manipulating the epileptic brain using stimulation: a review of experimental and clinical studies. , Epileptic Disord 11, 100-12.
- 6.Bragin A, Wilson C L, Engel J. (2002) Rate of interictal events and spontaneous seizures in epileptic rats after electrical stimulation of hippocampus and its afferents. , Epilepsia 43, 81-5.
- 7.Wyckhuys T, Boon P, Raedt R, B Van Nieuwenhuyse, Vonck K et al. (2010) Suppression of hippocampal epileptic seizures in the kainate rat by Poisson distributed stimulation. , Epilepsia 51, 2297-304.
- 8.Ullal G R, Ninchoji T, Uemura K. (1989) Low frequency stimulation induces an increase in after-discharge thresholds in hippocampal and amygdaloid kindling. Epilepsy Res. 3, 232-5.
- 9.Weiss S R, Li X L, Rosen J B, Li H, Heynen T et al. (1995) Quenching: inhibition of development and expression of amygdala kindled seizures with low frequency stimulation. , Neuroreport 6, 2171-6.
- 10.Cuellar-Herrera M, Neri-Bazan L, Rocha L L. (2006) Behavioral effects of high frequency electrical stimulation of the hippocampus on electrical kindling in rats. , Epilepsy Res 72, 10-7.
- 11.Mohammad-Zadeh M, Mirnajafi-Zadeh J, Fathollahi Y, Javan M, Ghorbani P et al. (2007) Effect of low frequency stimulation of perforant path on kindling rate and synaptic transmission in the dentate gyrus during kindling acquisition in rats. , Epilepsy Res 75, 154-61.
- 12.Wyckhuys T, T De Smedt, Claeys P, Raedt R, Waterschoot L et al. (2007) High frequency deep brain stimulation in the hippocampus modifies seizure characteristics in kindled rats. , Epilepsia 48, 1543-50.
- 13.Wyckhuys T, Raedt R, Vonck K, Wadman W, Boon P. (2010) Comparison of hippocampal Deep Brain Stimulation with high (130Hz) and low frequency (5Hz) on afterdischarges in kindled rats. , Epilepsy Res 88, 239-46.
- 14.S H Zhang, H L Sun, Fang Q, Zhong K, D C Wu et al. (2009) Low-frequency stimulation of the hippocampal CA3 subfield is anti-epileptogenic and anti-ictogenic in rat amygdaloid kindling model of epilepsy. , Neurosci Lett 455, 51-5.
- 15.H L Sun, S H Zhang, Zhong K, Z H Xu, Zhu W et al. (2010) Mode-dependent effect of low-frequency stimulation targeting the hippocampal CA3 subfield on amygdala-kindled seizures in rats. , Epilepsy Res 90, 83-90.
- 16.Gaito J, J N Nobrega, S T Gaito. (1980) Interference effect of 3 Hz brain stimulation on kindling behavior induced by 60 Hz stimulation. , Epilepsia 21, 73-84.
- 17.Shao J, E S Valenstein. (1982) Long-term inhibition of kindled seizures by brain stimulation. , Exp Neurol 76, 376-92.
- 18.S R Weiss, Eidsath A, X L Li, Heynen T, R M Post. (1998) Quenching revisited: low level direct current inhibits amygdala-kindled seizures. , Exp Neurol 154, 185-92.
- 19.Velisek L, Veliskova J, Stanton P K. (2002) Low-frequency stimulation of the kindling focus delays basolateral amygdala kindling in immature rats. , Neurosci Lett 326, 61-3.
- 20.Lopez-Meraz M L, Neri-Bazan L, Rocha L. (2004) Low frequency stimulation modifies receptor binding in rat brain. , Epilepsy Res 59, 95-105.
- 21.Goodman J H, Berger R E, Tcheng T K. (2005) Preemptive low-frequency stimulation decreases the incidence of amygdala-kindled seizures. , Epilepsia 46, 1-7.
- 22.Carrington C A, Gilby K L, McIntyre D C. (2007) Effect of focal low-frequency stimulation on amygdala-kindled afterdischarge thresholds and seizure profiles in fast- and slow-kindling rat strains. , Epilepsia 48, 1604-13.
- 23.D C Wu, Z H Xu, Wang S, Fang Q, D Q Hu et al. (2008) Time-dependent effect of low-frequency stimulation on amygdaloid-kindling seizures in rats. , Neurobiol Dis 31, 4-9.
- 24.Huang L, G van Luijtelaar. (2012) The effects of acute responsive high frequency stimulation of the subiculum on the intra-hippocampal kainic acid seizure model in rats. , Brain and Behavior 2, 532-40.
- 25.Huang L, G van Luijtelaar. (2013) The effects of responsive and scheduled subicular high frequency stimulation in the intra-hippocampal kainic acid seizure model. Epilepsy Res. 106, 326-337.
- 26.Huang L, G van Luijtelaar. (2014) Effects of responsive and scheduled stimulation in the subiculum: on seizures and excitability in the kainite induced epilepsy model of TLE. , Int J Neurorehabilitation 1, 127-10.
- 27.Zhong K, D C Wu, M, Z H Xu, Wang Y et al. (2012) Wide therapeutic time-window of low-frequency stimulation at the subiculum for temporal lobe epilepsy treatment in rats. , Neurobiol Dis 48, 20-6.
- 28.Z H Xu, D C Wu, Fang Q, Zhong K, Wang S et al. (2010) Therapeutic time window of low-frequency stimulation at entorhinal cortex for amygdaloid-kindling seizures in rats. , Epilepsia 51, 1961-1864.
- 29.Kile K B, Tian N, Durand D M. (2010) Low frequency stimulation decreases seizure activity in a mutation model of epilepsy. , Epilepsia 51, 1745-53.
- 30.Rashid S, Pho G, Czigler M, Werz M A, Durand D M. (2012) Low frequency stimulation of ventral hippocampal commissures reduces seizures in a rat model of chronic temporal lobe epilepsy. , Epilepsia 53, 147-56.
- 31.Chiang C C, Lin C C, Ju M S, Durand D M. (2013) High frequency stimulation can suppress globally seizures induced by 4-AP in the rat hippocampus: an acute in vivo study. , Brain Stimulation 6, 180-189.
- 32.Siah B H, Chiang C C, Lin C C, Ju M S. (2014) Suppression of acute seizures by theta burst electrical stimulation of the hippocampal commissure using a closed-loop system. , Brain Research 1593, 117-125.
- 33.L J Ozen, G C Teskey. (2009) One hertz stimulation to the corpus callosum quenches seizure development and attenuates motor map expansion. , Neuroscience 160, 567-75.
- 34.Sharp P E, Green C. (1994) Spatial correlates of firing patterns of single cells in the subiculum of the freely moving rat. , J Neurosci 14, 2339-56.
- 35.Taube J S. (1995) Place cells recorded in the parasubiculum of freely moving rats. , Hippocampus 5, 569-83.
- 36.Cohen I, Navarro V, Clemenceau S, Baulac M, Miles R. (2002) On the origin of interictal activity in human temporal lobe epilepsy in vitro. , Science 298, 1418-21.
- 37.Witter M P, Ostendorf R H, Groenewegen H J. (1990) Heterogeneity in the Dorsal Subiculum of the Rat. Distinct Neuronal Zones Project to Different Cortical and Subcortical Targets. , Eur J Neurosci 2, 718-725.
- 38.Funahashi M, Harris E, Stewart M. (1999) Re-entrant activity in a presubiculum-subiculum circuit generates epileptiform activity in vitro. , Brain Res 849, 139-46.
- 39.O’Mara S M, Commins S, Anderson M, Gigg J. (2001) The subiculum: a review of form, physiology and function. , Prog Neurobiol 64, 129-55.
- 40.O’Mara S. (2005) The subiculum: what it does, what it might do, and what neuroanatomy has yet to tell us. , J Anat 207, 271-82.
- 41.O’Mara S. (2006) Controlling hippocampal output: the central role of subiculum in hippocampal information processing. , Behav Brain Res 174, 304-12.
- 42.Harris E, Stewart M. (2001) Propagation of synchronous epileptiform events from subiculum backward into area CA1 of rat brain slices. Brain Res. 895, 41-9.
- 43.Deacon T W, Eichenbaum H, Rosenberg P, Eckmann K W. (1983) Afferent connections of the perirhinal cortex in the rat. , J Comp Neurol 220, 168-90.
- 44.Swanson L W, Wyss J M, Cowan W M. (1978) An autoradiographic study of the organization of intrahippocampal association pathways in the rat. , J Comp Neurol 181, 681-715.
- 45.Canteras N S, Swanson L W. (1992) Projections of the ventral subiculum to the amygdala, septum, and hypothalamus: a PHAL anterograde tract-tracing study in the rat. , J Comp Neurol 324, 180-94.
- 46.Stafstrom C E. (2005) The role of the subiculum in epilepsy and epileptogenesis. , Epilepsy Curr 5, 121-9.
Cited by (5)
- 1.Zhang Fang, Yang Yufang, Zheng Yongte, Zhu Junming, Wang Ping, et al, 2021, Combination of Matching Responsive Stimulations of Hippocampus and Subiculum for Effective Seizure Suppression in Temporal Lobe Epilepsy, Frontiers in Neurology, 12(), 10.3389/fneur.2021.638795
- 2.Mohammad-Ali-Nezhad Sajad, Rezvani-Ardakani Samira, Ghasemi Reza, 2023, Closed-Loop Deep Brain Stimulation Using a Type of Fixed-Time Sliding Mode Controller for Avoiding Epileptiform Discharge in a Human Cortical Model, Journal of Computational Biophysics and Chemistry, 22(06), 751, 10.1142/S2737416523500382
- 3.Akyuz Enes, Ozenen Cansu, Pinyazhko Oleh R., Poshyvak Olesya B., Godlevsky Leonid S., 2021, Cerebellar contribution to absence epilepsy, Neuroscience Letters, 761(), 136110, 10.1016/j.neulet.2021.136110
- 5.Wang Zhihui, Wang Qingyun, 2017, Eliminating Absence Seizures through the Deep Brain Stimulation to Thalamus Reticular Nucleus, Frontiers in Computational Neuroscience, 11(), 10.3389/fncom.2017.00022
