Abstract
Obesity is a worldwide epidemic that features a multifactorial syndrome characterized by a chronic positive energetic unbalance. Neonatal administration of monosodium L-glutamate (MSG) causes lesion on the arcuate nucleus of hypothalamus that led to development of obesity in the adult life in rodents characterized by a notorious accumulation of catecholamine in the adrenal medulla. The amino acid glycine induces catecholamine secretion of adrenal medulla. Thus, the objective of our work was to evaluate the possible effects of glycine administration in the MSG-obesity model in rats and investigate its impact on adrenal catecholamine medulla homeostasis. Male Wistar rats received MSG solution (4mg/g body weight) subcutaneously in the cervical area for 5 days after delivery, controls received saline solution. Animals were also divided in two groups, in which one received tap water added with glycine (0.1g/Kg) after weaning on 21st day until 90 days of life.Biometrical variables, visceral fat pads weight, total content and basal secretion of adrenal cathecolamine were evaluated. Glycine increased Lee index of all tested groups and had no effect on visceral adiposity. However, glycine treatment completely reestablished catecholamine total content and basal secretion of MSG-obese group. In conclusion, although glycine treatment apparently completely reestablishes catecholamine secretion homeostasis it is not sufficient to significant directly reduce visceral adiposity in MSG obesity model in rats.
Author Contributions
Academic Editor: Sebastian P. Galuska, Justus Liebig University Giessen
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2015 Ana Eliza Andreazzi, et al
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Obesity is a multifactorial syndrome characterized by a chronic positive energetic unbalance. Its incidence is increasing in alarming proportion; currently, approximately 500 million people are obese world-wide representing a risk factor for type 2 diabetes and cardiovascular illnesses 1, 2, 3, 4, 5. Extensive academic effort has been put on medical research to better understand the complexity of this disorder.
The experimental obesity model induced by monosodium L-glutamate (MSG) has been widely used for being representative of the metabolic disturbance observed in human obesity 6. The neonatal administration of MSG causes lesion on the arcuate nucleus of hypothalamus 7, 8, 9. Neonatal administration of MSG led to development of obesity in the adult life in rodents 10, 11. Moreover, the animals develop an abnormal deposition of fat 12, 13, 14. Also, MSG-obese animals present hyperinsulinemia, insulin resistance and a notorious accumulation of catecholamines in the adrenal medulla due to impaired secretion process, among other features 6, 10, 15.
Catecholamine from adrenal medulla has an important role in the regulation of glucose, lipids and protein metabolism. The sympathoadrenal system includes the sympathetic nervous system and the chromaffin cells from the adrenal medulla, which secretes catecholamine (primarily epinephrine) into the bloodstream 16. Chromaffin cells are cholinergically innervated by the splanchnic nerve; acetylcholine released upon stimulation of this nerve activates neuronal cholinergic receptors in chromaffin cells, thereby inducing membrane depolarisation and triggering catecholamine secretion 17.The metabolic effect of the epinephrine results in the increase of serum glucose, lipolysis, oxygen consumption and thermogenesis 18, 19. Therefore, abnormalities in the release mechanisms and/or production of catecholamine can contribute for the development of obesity.
Some articles have shown that the amino acid glycine can induce catecholamine secretion of adrenal medulla 20, 21, although its mechanism of action in chromaffin cells is still not clear. Yadid and collaborators 22 had demonstrated preferential release of epinephrine from the chromaffin cells of the adrenal medulla in response to glycine.
Thus, the objective of our work was to evaluate the possible effects of glycine administration in the MSG-obesity model in rats and investigate its impact on adrenal catecholamine medulla homeostasis.
Material and Methods
Animals and Treatment
All animal protocols were performed according to Brazilian College of Animal Experimentation (COBEA) 23 and Brazilian Federal Law and the procedure protocols were approved by the Ethical Committee for Animal Handling (UFJF – Juiz de Fora, Minas Gerais State, Brazil) (Permit Number: 002/2012). Male Wistar rats were used in this study (n=60 for all experimental procedures). The animals were obtained from the Center of Reproductive Biology (CRB) of the Federal University of Juiz de Fora. During the first 5 days after delivery, animals were injected subcutaneously in the cervical area with MSG solution (4mg/g body weight) 7. Control animals received equimolar saline solution. The weaning of the animals was performed at the 21st day. The animals were divided in four groups: Control and MSG which received tap water; and, Control-Glycine and MSG-Glycine which were treated with tap water added with glycine (0.1g/Kg) according to Alarcon-Aguilar et al 24. After weaning, all animals groups were weighted weekly. Animals received water and commercial chow (Nuvital, Curitiba, Brazil) ad libitum and placed in an environmentally controlled room (23 ± 3 °C and 12h light / darkness photocycle (07:00-19:00 h)) during the whole protocol period.
Biometrical Analysis
To evaluate obesity onset, all 90 day old rats were euthanized by exsanguination through cardiac puncture under sodium pentobarbital (45mg/100g body weight i.p.) anesthesia. Epidydimal and retroperitoneal fat pads were removed and weighted to estimate obesity induced by MSG treatment 10. The Lee index was calculated from the ratio [body weight (bw)1/3 (g)/nasoanal length (NAL) (cm)]×1000 and used as a predictor of obesity in MSG-rodents 25.
Adrenal Glands
Adrenal glands were removed and weighted. During handling, glands were maintained in standard Krebs–Hepes solution composed of (in mM): Cl- 154.2; Na+ 144.0; Ca2+ 2.5; Mg2+ 1.18; SO42- 1.2; K+ 3.5; glucose 11.1; Hepes (acid N-(2-hydroximethylpiperazine)-N-(2-ethanosulfonic)) 25.0; bovine serum albumin (BSA) 0.5%, on an ice bath. Right glands of all experimental groups were used to evaluate the total catecholamine content – epinephrine and norepinephrine – quantified by using the trihydroxyindole fluorescence method 26. Results were obtained by plotting the values on a linear regression of the standard epinephrine curve. On the other hand, left adrenal glands were used in secretion experiments. Dissection of adrenal medulla was undertaken with stereoscopic lens and ophthalmologic surgical instruments. Isolated medullae were, moreover, impaled on steel sticks for better manipulation, and left in a rest of 40 minutes in standard Krebs-Hepes solution. Costar 96-well cell culture plates were used. Each well contained 200 µL of standard Krebs-HEPES or modified Krebs-HEPES solution containing5 µM of glycine 27. Catecholamine secretion experiments were realized according to previous described method 6.
Statistical Analysis:
All results are presented as mean ± SEM. p < 0.05 was considered statistically significant. One-way ANOVA with Bonferroni post-test was performed using GraphPad Prism version 5 for Windows (GraphPad Software, San Diego California USA).
Results
The biometrical analysis of the studied groups is shown in the Table 1. Control animals has a 3.5% increased on NAL after glycine treatment (p<0.05). However, the NAL did not differ between the MSG groups. Furthermore, glycine treatment enhanced the Lee Index in both groups (p<0.001). The MSG animals have shown diminished weight of the adrenal glands compared to Control animals, but no differences were seen after the glycine treatment when we compared to their respective pairs.
Table 1. Effects of MSG treatment and glycine intake on adult rats| Variable | Control | Control-Glycine | MSG | MSG-Glycine |
| Body weight (g) | 277.3±8.3 | 308.8±4.4a, c, d | 213.4±6.2a, b | 221.4±8.1a, b |
| NAL (cm) | 22.8 ± 0.2 | 23.6± 0.2 | 19.3± 0.2a, b | 19.4 ± 0.3a, b |
| Lee Index | 281.2±1.07 | 286.7± 2.2 | 300.7±1.6a, b, d | 312.2 ± 3.7a, b, c |
| Adrenal (mg/100g bw) | 8.57 ± 0.12 | 8.20 ± 0.18 | 6.026 ± 0.29a, b | 5.79 ± 0.14a, b |
The results of the body weight after the MSG and glycine treatment are presented in the Figure 1. Neonatal treatment with MSG reduced body weight AUC gain by 19.5% compared to control ones (p<0.05). There were no significant differences on the body weight gain with glycine treatment when we compared each group to their pairs. The amino acid glycine did not alter food and water ingestion (data not shown).
Figure 1.Effect of glycine intake on epidydimal (A) and retroperitoneal (B) fat pad accumulation in MSG-obese rats. ANOVA were performed with Bonferroni post-test (n=15). Letters over bars represent significant differences with p<0.05 between groups: a- Control; b-Control-Glycine; c-MSG and d-MSG-Glycine.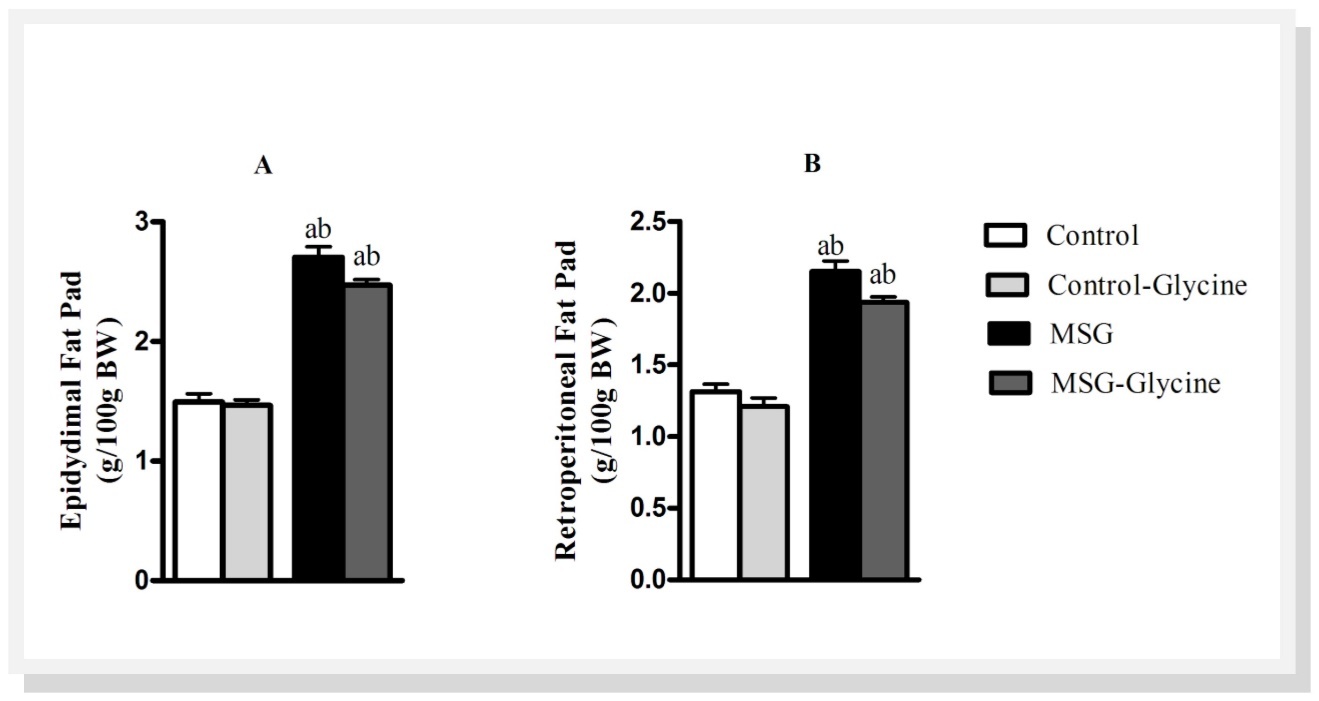
As shown in Figure 2A and Figure 2B, MSG treatment induced substantial enhancement of epidydimal and retroperitoneal fat pads, 65.5% and 48.12% respectively, when compared to control animals (p<0.001). The glycine treatment reduced the fat pad weights in both groups, excepting the epidydimal fat pad in the control animals. The control-glycine group presented 12% lighter retroperitoneal fat pad compared to control group after the glycine treatment (p<0.05). Furthermore, the glycine treatment reduced 9% epidydimal and retroperitoneal fat pads in MSG animals (p<0.01).
Figure 2.Effect of glycine intake on total catecholamine content (A) and catecholamine basal (B) secretion in MSG-obese rats. ANOVA was performed with Bonfferoni post-test (n=15). Letters over bars represent significant differences with p<0.05 between groups: a- Control; b-Control-Glycine; c-MSG and d-MSG-Glycine.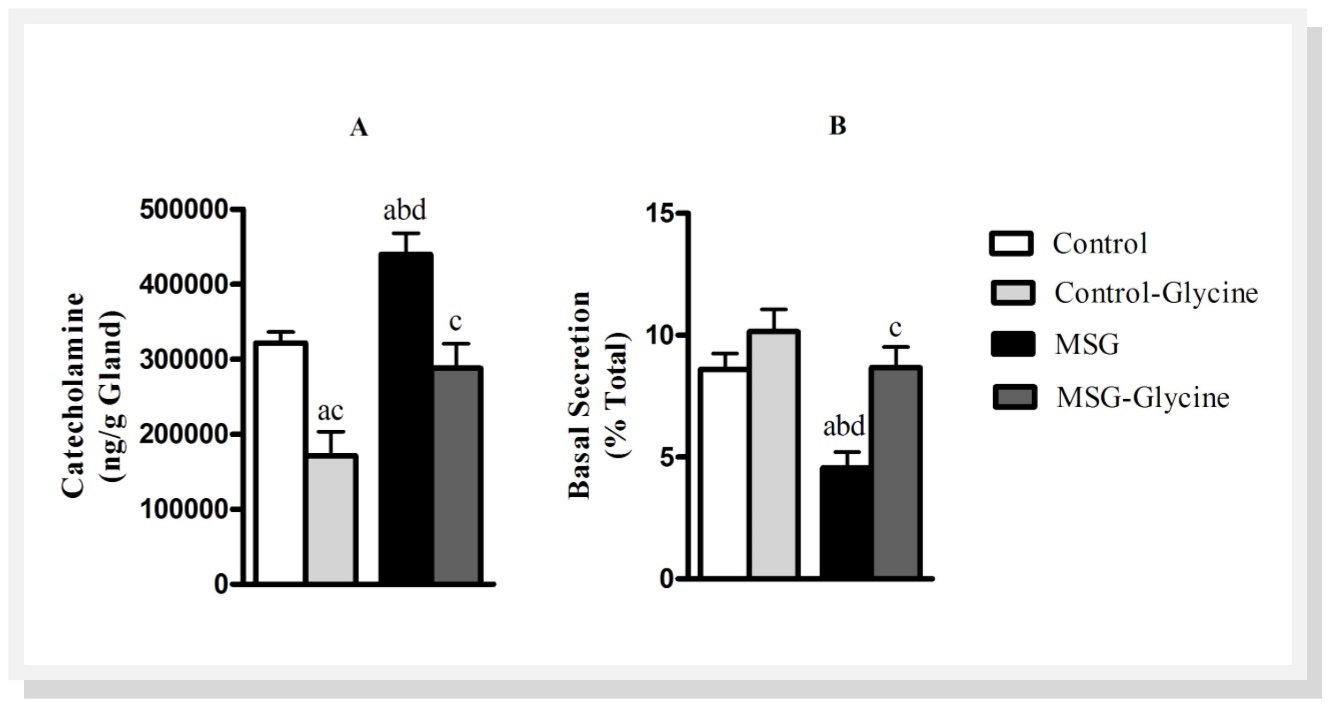
Figure 2 shows total catecholamine stores in adrenal glands of rats and basal catecholamine secretion. Neonatal treatment with MSG caused a 41.2% enhancement in total catecholamine content of the adrenal gland when compared with control group (p<0.01). On the other hand, glycine supplementation restored total catecholamine content in adrenal medulla of MSG obese rats compared to control levels. Surprisingly, we found 46.9% reduction in total catecholamine content in the adrenal of control-glycine rats (p<0.01). As shown in Figure 2B, MSG group showed marked reduction on basal catecholamine secretion compared with control group. Basal catecholamine secretion from the adrenal medulla was reduced by 40.1% with neonatal MSG treatment (p<0.01). However, animals which received glycine after weaning presented an increase on basal catecholamine secretion, but only in MSG-Glycine group there was a significantly enhancement (83.3%, p<0.001). Glycine has no effect on ‘in vitro’ catecholamine secretion (data not shown)
Discussion
Previous studies have addressed obesity as an epidemic worldwide phenomenon and showed that factors related with body fat accretion are narrowly associated to chronic diseases, e.g, type 2 diabetes, many cancers and cardiovascular diseases 28, 29. Important recent reports in the literature have been shown the negative association between glycine and body fat accretion, insulin resistance and impaired lipid metabolism 30, 31. In present study we show that the glycine treatment after weaning induced morphophysiological adjustments in MSG obesity model in rats.
Biometrical analysis has shown that NAL was higher in control animals after glycine treatment. Alike, highly significant increase was found in the Index in the animals under glycine treatment. Glycine has previously been shown to stimulate the growth hormone (GH) production 32, 33, corroborating the results for NAL and Lee Index, due the anabolic effect of GH. Opposed to glycine, neonatal MSG treatment caused shorter NAL and lower Lee Index compared to control animals. This is consistent with other studies showing that MSG treatment induced a growth, endocrine and behavioral deficit 6.
MSG animals presented greater accumulation and impaired secretion of adrenal catecholamine. This data corroborates to previous works 6, 34. Glycine administration on the other hand, was able to complete reestablish adrenal catecholamine content and basal secretion in MSG obese rats. In our protocol, glycine was not able to stimulate catecholamine secretion in vitro. Like already mentioned, glycine is capable to stimulate catecholamine release from adrenal medulla 20, 21, 22. However the mechanism of action remains unclear, for the first time we show a direct impact of glycine in restoring adrenal medulla homeostasis.
A slight and non-significant reduction in adiposity occurred in both control and MSG groups that received glycine; however it was more pronounce in the obese group than in control rats. Similar results was observed by Hafidi and collaborators 35 using an animal model of intra-abdominal fat accumulation induced by the addition of sucrose to the drinking water, showed that glycine supplementation, at adult life for 4 weeks, reverted the elevation of plasma triglycerides and reduced intra-abdominal fat accumulation; however, in control animals glycine intake did not affect these variables. Authors suggested an increase of mitochondrial fatty acid oxidation on sucrose rats treated with glycine.
Conclusion
With this current work we were able to demonstrate that, although glycine treatment apparently completely reestablish catecholamine secretion homeostasis it is not sufficient to significant reduce the visceral adiposity in MSG obesity model in rats.
Acknowledgment
This research was supported by the Brazilian Science Foundation CNPq, CAPES, FAPEMIG and UFJF.
References
- 6.Andreazzi A E, Grassiolli S, Marangon P B, Martins A G, Oliveira J C. (2011) . Exp. Diabetes Res.10.1155/2011/947917 .
- 11.Dolnikoff M, Martin-Hidalgo A, U F Machado, Lima F B, Herrera E. (2001) . , Int. J. Obes. Relat. Metab. Dis 25, 426-433.
- 12.Scomparin D X, Grassiolli S, Marçal A C, Gravena C, Andreazzi A E. (2006) . , Life Sci 79, 2151-2156.
- 14.Andreazzi A E, Scomparin D X, Mesquita F P, Balbo S L, Gravena C. (2009) . , J. Endocrinol 201(3), 351-359.
- 21.Yadid G, Goldstein D S, Pacak K, Kopin I J, Golomb E. (1995) . , Eur. J. Pharmacol 288(3), 399-401.
- 23.VBV Lapchik, VGM Mattaraia, Ko M G. (2009) . Cuidados e Manejo de Animais de Laboratório.Ed.Atheneu,Rio de Janeiro .
- 24.Alarcon-Aguilar F J, Almanza-Perez J, Blancas G, Angeles S, Garcia-Macedo R. (2008) . , Eur. J. Pharmacol.599(1-3) 152-158.
- 30.Lustgarten M S, Price L L, Phillips E M, Fielding R A. (2013) . PlosOne. doi: 10.1371/journal.pone.0084034
- 31.Tastesen H S, Keenan A H, Madsen L, Kristiansen K, Liaset B. (2014) . , Amino Acids 46(7), 1659-1671.
- 33.Florea I, Popa M, Simionescu L, Ciovirnache M, Ionescu V. (1976) . , Endocrinologie 14(4), 291-295.
