Abstract
Liège syrup is a Belgian traditional cooked fruit foodstuff, produced mainly from apples and pears. The process includes several hours of heating at high temperature during which complex chemical reactions occur, such as Maillard condensation between reducing sugars and amino acids. Aiming at understanding the modifications of the fruit juices during heating, different parameters were monitored throughout the process. It was shown that hydoxymethylfurfural was formed during the first step of concentration by heating. At the end of the process, hydroxymethylfurfural had totally disappeared and the deep brown color of the product suggested that this compound was transformed into melanoidins. A parallel increase in antioxidant capacity was also observed. To determine optimal conditions to reach high melanoidin content and high antioxidant capacity, different in vitro model systems were compared. It was shown that different combinations of an amino acid with glucose or fructose led to different levels of hydroxymethyfurfural, of melanoidins and antioxidant capacity. After heating of apple or pear puree, an increase of the antioxidant capacity and the hydroxymethylfurfural and melanoidin contents was observed when the heating time was doubled. An increase of the pH from 5 to 9 in apple marmalade’s also induced an increase in antioxidant capacity and in hydroxymethylfurfural and melanoidins. However it was not the case in pear marmalade where only the increase in antioxidant capacity was observed. These results suggest that some parameters of the processing could be modified to improve the health-promoting effect of this traditional food (antioxidant properties and composition in hydroxymethylfurfural and melanoidins). The main factors affecting the quality of the final product were the cooking times, the temperature, the pH, the addition of reducing sugars or amino acids.
Author Contributions
Academic Editor: Abhishek Sharma, Manager (Q.C.) Konark Herbals and Healthcare
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2015 Claire Kevers, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Liège syrup (in french Sirop de Liège) is a Belgian traditional food produced from different fruits in the northeast of the province of Liège. It is not a jelly, nor jam, nor marmalade. Liège syrup is a bit like apple butter, gummy and super sweet: it is produced by reducing (boiling off the water from) a mixture of fruit juices. After several hours of long, slow cooking of apples and pears with water and sugar, the resulting product is a soft brown paste that is just barely translucent. It takes 400 g of fresh fruit to produce 100 g of Liège syrup. Besides apples and pears, dates or other fruit juices can be used as well.
This foodstuff is a mixture of compounds such as amino acids, carbohydrates, vitamins and minerals. Complex chemical reactions occur as a result of heat treatments. These include Maillard condensation between reducing sugars and amino acids, sugar browning, ascorbic acid browning and destruction of pigments1. It is known that Maillard reaction products are generated during cooking when reducing sugars react with amino acids, peptides or proteins or ascorbic acid2. These products affect important food properties such as color, flavor and stability3. Numerous studies have focused on the formation of intermediate products4 and melanoidins. These last compounds show scavenging activity against hydroxyl radical, superoxide and hydrogen peroxide as well as metal chelation activity5, 6. In vitro model systems were used to study Maillard reaction. It appeared that melanoidins are characteristically different from sugar and amino acid used7. Maillard reaction occurs in three major stages (early, intermediate and final) and it is depending upon factors such as reactant type and reaction conditions, namely concentration, temperature, time, pH and water activity7.
There is controversy concerning whether dietary Maillard reaction products (MRPs) represent potential harmful or beneficial effects. Some products of the Maillard reaction, such as heterocyclic aromatic amines and acrylamide are mutagenic/carcinogenic or neurotoxic. Also, certain melanoidins have negative effects on the structure of DNA and collagen, and could be involved in promoting Alzheimer disease, diabetes and cardiovascular diseases8. However, some studies reported positive effects of some melanoidins: action on intestinal flora, antioxidant activity, antimutagenic activities9, 10 or anti-inflammatory activity of low molecular weight products11. Kitts et al.12 showed that MRP components have bioactive potential, especially in regard to suppressing oxidative stress and inflammation in IFN-γ- and PMA-induced Caco-2 cells.
Thermal treatments are used in the preservation of fruit products and in the manufacture of processed foods. The negative effects of these treatments include non-enzymatic browning, loss of nutrients and formation of undesirable products such as 5-hydroxymethylfurfural (HMF), an intermediate in the Maillard Reaction13.
HFM is formed not only from the Maillard Reaction, but also from hexoses degradation and caramelization for which the presence of amino groups is not needed. Moreover, it is one of the decomposition products of ascorbic acid. Although HMF is nearly absent in fresh and untreated foods, its concentration tends to rise during heating, so it is a useful marker of heat damage in foodstuffs.
HMF is a widespread heat-induced contaminant whose dietary intake is several orders of magnitude higher than that reported for other food toxicants such as acrylamide or furan14. Its amount in foods is directly related to the heat load applied during processing of carbohydrate-rich products. HMF concentrations in food can vary largely, sometimes exceeding 1g/Kg. Up to now there are no available mitigation strategies specifically addressed to reduce HMF content in food.
Based on data reported in literature, it is not clear whether human exposure to HMF represents a potential health risk14. It has been shown that HMF at high concentrations is cytotoxic, irritating to eyes, upper respiratory tract, skin and mucous membranes. But the major risk to HMF is related to its conversion to SMF (5-sulphooxymethylfurfural)15, 16. Concerning safety of furan derivatives, EFSA concluded that, based on mutagenicity of SMF, there is a sufficient evidence to justify concerns about its genotoxic potential14.
The objective of this study was to evaluate the antioxidant capacities and relationship with MRPs during the process of Liège syrup preparation. The content of HMF and antioxidant capacity of aqueous sugar (fructose and glucose) and different amino acid in in vitro model systems was measured in relationship with heating time and pH. Specific conditions were assessed also on fruit marmalade’s.
Material and Methods
Sample Preparation
Various varieties of apples (Boskoop, Granny, Pinova, Elstar, Cox, Jonagold and Jonagored) were used for the production of Liège syrup by Siroperie Meurens (Aubel, Belgium). Sampling was done at various stages:
Homogenate: after the washing and the grinding of the apples (with skin, seeds…)
Juice: after the first cooking (3 h at 90°-95°C) and filtration (filter press) of the homogenate
Concentrate: after concentration of the juice with a concentrator (120°-140°C for 40 min)
Syrup: after mixing concentrates of various fruits at 65°C and heating at 105°C during 20 min. Sugar can be added during mixing.
The samples were diluted in water and centrifuged at 10000 g for 10 min before the various measurements.
In in vitro model systems, the heating procedure was modified from Ajandouz et al.17. An equimolar (100 mM) mixture of one amino acid and glucose or fructose (1 mL), without pH adjustment was heated in a 10 mL screw-sealed tubes in boiling water (100°C) for 20, 60, 120 or 180 min or autoclaved at 120°C for 20 min. The tubes were then cooled down on ice and stored at 4°C. Various pH were also tested at 100°C for 180 min. The following buffers were used: 0.1 M sodium acetate adjusted to pH 3 or 5 with 0.1 M acetic acid, 0.1 M sodium phosphate adjusted to pH 7 or 9 with 0.1 M hydrochloric acid, 0.1 M sodium phosphate adjusted to pH 11 with 0.1 M sodium hydroxide.
Marmalades (lab preparations) were prepared by mixing and crushing fruits (10 g of apple or pear) with sucrose (6 g) and distilled water (6 mL). The mixture was heated in 50 ml disposable plastic tubes for 60 min in boiling water. In some experiments, the heating was extended to 120 min or the mixture was buffered to pH 5 or 9. In one experiment, sucrose was replaced by fructose (5 g instead of 6 g because the sweetness of fructose is higher than this of sucrose).
Determination of the Total Phenolic Content
Total phenolics were determined according to the Folin-Ciocalteu method described by Mihalache Arion et al.18. In a 96-well microplate, 200 µL of water, sample (appropriately diluted), or standard were mixed with 100 µL of Folin-Ciocalteu reagent (10%). After 3 minutes, 80 µL sodium carbonate solution (7.5% w/v) were added. The plate was incubated at 30°C for 1 h in a microplate reader (Multiskan Ascent, ThermoLabsystems, Finland). After incubation, the absorbance at 750 nm was measured. Gallic acid (GA) was used as standard, and results are expressed in mg gallic acid equivalents (GAE) per mL. All assays were done in duplicate.
In Vitro Evaluation of the Antioxidant Capacity
The antioxidant capacity was first determined by scavenging of the 2,2-diphenyl-1-picryhydrazyl (DPPH) radical as described by Sipel et al.19. The stock solution was prepared by stirring 7.5 mg DPPH in 100 mL methanol overnight. In the assay, 100 µL of extract, standard (0–100 µM Trolox), or blank (methanol) and 200 µL DPPH solution were mixed in a well of a 96-well microplate. The absorbance of samples, standards, and blanks at 520 nm was determined after 5 min of incubation in a Multiskan Ascent reader (ThermoLabsystems, Finland) at 30°C.
The antioxidant capacity was also determined by the ORAC assay as described by Kevers et al.20. Briefly, AAPH was used as a peroxyl radical generator, Trolox as a standard, and fluorescein as a fluorescent probe. 25 µl of diluted sample, blank, or Trolox calibration solution (0–100 µM) were mixed with 150 µl of 4 µM fluorescein and incubated for 15 min at 37°C before addition of 25 µl AAPH solution (173 mM). The fluorescence was measured every 2 min for 4 h on a Victor 3 plate reader (Perkin Elmer) at 37°C. Filters were used to select an excitation wavelength of 485 nm and an emission wavelength of 520 nm. All samples were analysed in duplicate at three different dilutions. The final ORAC values were calculated from the net area under the decay curves.
The results obtained with both assays are expressed in µmol Trolox equivalents (TE) per mL.
Determination of Hydroxymethylfurfural Content (Modified from Martysiak-Zurowska & Borowicz) 21
In a 96-well microplate, 50 µL of water, sample (appropriately diluted), or standard (HMF) were mixed with 125 µL of p-toluidine solution (10% in isopropanol). 25 µL of barbituric acid (0.5%) were added. The absorbance at 550 nm was measured with a microplate reader (Multiskan Ascent, ThermoLabsystems, Finland). Results are expressed in µg HMF per mL.
Even if this method is not specific (it can also detect the presence of aldehydes other than HMF), it allows evaluating the modifications of HMF in in vitro reactions where there is no interference with other compounds.
Evaluation of Melanoidins
Melanoidin formation was evaluated by measurement of OD at 405 nm of 150 µL samples (triplicates) in a 96-well microplate according to Echavarria et al.22. Results are expressed in OD of the sample without dilution.
Determination of Protein Content by BC Assay
The assay was done with the Thermo scientific Pierce BCA protein assay kit according to the manufacturing instructions. Albumin was used as standard.
Statistical Analysis
All results presented are means (±SEM) of three independent experiments except for table 1 for which five experiments were done.
Table 1. Evolution of antioxidant capacity (DPPH, ORAC, µmol TE/mL), total phenolic compounds (mg GAE/mL), proteins mg/mL) and HMF (mg/mL) during the different steps of LiegeLiège syrup preparation.| Homogenate | Juice | Concentrate | Syrup | |
| DPPH | 34.1 ± 2.6a | 38.9 ± 2.3a | 29.6 ± 2.4a,b | 22.9 ± 1.9b |
| ORAC | 571.4 ± 53.2a | 845.2 ± 36.8b | 857.1 ± 43.9b | 594.4 ± 29.2a |
| Total phenolics | 45.6 ± 2.4a | 55.1 ± 2.2b | 75.6 ± 4.9c | 45.6 ± 3.1a |
| Proteins | 0.448 ± 0.028a | 0.415 ± 0.014a | 0.078 ± 0.003b | 0.012 ± 0.001c |
| HMF | 0a | 0a | 0.153 ± 0.011b | 0.009 ± 0.001c |
The data were compared by ANOVA to evaluate the significant differences between samples using Tukey HSD’s post test P<0.05.
Results and Discussion
Antioxidant Capacity and Maillard Reaction During Syrup Preparation
During the process, the fruits were first washed and grinded to obtain a homogenate. Then this homogenate was cooked at 90-95°C for 3 h and then filtered. The juice so obtained was concentrated at around 130°C for 40 min. and finally, the Liège syrup was obtained after cooling and further heating to 105°C. Thus this process includes at least 4 hours of heating at temperatures between 90°C and 140°C.
The antioxidant capacity (ORAC) and the total phenolic content were higher in juice than in homogenate (table 1). The process of cooking was probably responsible of this increase23 because cooking induces thermal inactivation of oxidative enzymes and /or the destruction of cell walls and subcellular compartments that causes the release of antioxidant compounds as phenolics24. The heating of the juice induced another increase of total phenolics but no modification (ORAC) or a decrease of the antioxidant capacity (DPPH). The decrease was confirmed in the syrup for these three parameters. The final values of antioxidant capacity (ORAC) and total phenolics were similar to the values of the homogenate. Finally, all the process of syrup preparation did not decrease the antioxidant capacity nor the phenolic content. Correlations were already observed between total phenolics and antioxidant capacity suggesting that phenolic compounds were partially responsible of the antioxidant capacity18, 20.
The proteins present in the homogenate drastically decreased during the concentrate preparation. Proteins are easily denatured at high temperature and partially hydrolysed in acidic conditions. The released amino acid can then be used in the Maillard reaction. Indeed, in parallel, HMF appeared in the concentrate while in syrup, the final product, the content of this compound was very low. HMF is a furanic compound which forms as an intermediate in the Maillard reaction13. The amount of HMF detectable in foods is directly related to the heat load applied during processing of carbohydrate-rich products14. In Liège syrup, the protein content was very low and the HMF had almost totally disappeared. We can suppose that the Maillard reaction has continued during syrup mixing and heating and that melanoidins were formed from the HMF. These compounds as other MRPs are known to have an antioxidant capacity4, 11 and are probably responsible of the deep brown color of the Liège syrup and of its taste and smell.
Maillard Reaction Between an Amino Acid and a Reducing Sugar
Aiming at understanding how antioxidant capacity was modified and how the content in HMF and melanoidins varied during the process, in vitro model systems were studied. Each consisted in mixing one amino acid with either glucose or sucrose in equimolar concentration (100 mM). The different mixtures were heated at 100°C during 180 min.
Antioxidant Capacity of MRPs
For many amino acids, heating in the presence of fructose led to higher DPPH radical scavenging activity than heating it in the presence of glucose (table 2), as already observed by Echavarria et al.25 and Liu et al.26. Exceptions were observed for leucine, lysine and glutamic acid showing higher antioxidant capacity in the presence of glucose. In the case of some amino acids such as glycine, no antioxidant capacity was measured with glucose after the 180 min heating treatment (table 2). ORAC antioxidant capacity was also higher after heating in the presence of fructose than in the presence of glucose except for the leucine, glutamic acid and tyrosine. Higher biological activities of MRPs derived from fructose-amino acid model mixtures were also reported by Hwang et al.7
Table 2. Antioxidant capacity (µmol.mL-1) of the reaction between an amino acid and glucose or fructose after 180 min at 100°C.| DPPH | ORAC | |||
| Glucose | Fructose | Glucose | Fructose | |
| Alanine | 10.7 + 1.0 | 89.1 ± 1.5* | 231.7 ± 34.6 | 432.9 ± 5.3* |
| Glycine | 0 | 24.0 ± 1.4* | 113.7 ± 5.5 | 268.6 ± 5.6* |
| Valine | 27.0 ± 4.2 | 159.0 ± 7.1* | 230.7 ± 6.9 | 291.0 ± 14.0* |
| Leucine | 459.2 ± 5.9 | 311.7 ± 18.8* | 4759.2 ± 128.6 | 4357.1 ± 59.6* |
| Isoleucine | 207.1 ± 2.9 | 277.7 ± 12.3* | 15799.4 ± 269.4 | 15650.6 ± 125.0 |
| Proline | 0 | 41.2 ± 0.6* | 127.2 ± 2.4 | 268.5 ± 5.4* |
| Serine | 0 | 27.7 ± 1.4* | 923.4 ± 87.0 | 831.7 ± 19.9 |
| Threonine | 117.0 ± 21.2 | 132.0 ± 10.2 | 1331.1 ± 380.1 | 1811.0 ± 154.4 |
| Aspartic acid | 105.5 ± 1.9 | 122.9 ± 1.5* | 2030.9 ± 323.2 | 3678.1 ± 87.9* |
| Glutamic acid | 64.3 ± 0.8 | 21.2 ± 1.8* | 597.6 ± 6.4 | 221.9 ± 7.3* |
| Lysine | 47.4 ± 0.5 | 33.9 ± 0.2* | 264.9 ± 2.3 | 371.4 ± 11.9* |
| Arginine | 910.0 ± 49.1 | 1110.0 ± 26.5* | 6354.7 ± 256.2 | 6621.5 ± 526.5 |
| Histidine | 0 | 28.8 ± 2.8* | 18792.4 ± 2320.7 | 21393.2 ± 794.7 |
| Phenylalanine | 2815.0 ± 229.0 | 3302.5 ± 21.7* | 14281.3 ± 369.7 | 18435.0 ± 541.6* |
| Tryptophane | 1701.3 ± 5.3 | 2376.7 ± 91.4* | 425281 ± 14950 | 377195 ± 8050 |
| Tyrosine | 6293.3 ± 219.4 | 6893.3 ± 204.3 | 546364 ± 10624 | 521219 ± 2199* |
Effects of Heating Time and Temperature
The HMF content was monitored during heating of one amino acid in the presence of glucose or fructose. Different amino acids showed different behaviours. Phenylalanine yielded similar high HMF amounts in the presence of glucose or fructose (figure 1A). However, HMF was not detectable after 180 min of heating for glycine, isoleucine and lysine whatever the sugar (data not shown). Melanoidin level was also monitored (absorbance at 405 nm) and it was shown to increase with heating time. It was very low with glycine and isoleucine (OD< 0.05), the highest with phenylalanine (figure 1B). For glycine and lysine, absorbance at 405 nm of the glucose/amino acid mixture were lower than those observed for the fructose/amino acid mixture (data not shown), as previously noted by Echavarria et al.25. However no difference could be shown for lysine and phenylalanine. For Echavarria et al.22, colour can be considered as indicative of the overall antioxidant properties of melanoidins. The correlation between colour and antioxidant properties can be assigned to melanoidins as these compounds are the prevalent MRPs formed during heating.
Figure 1.Evolution of the hydroxymethylfurfural content (A,C) and optical density at 405 nm (indicative of melanoidin content, B,D) of model systems consisting in heating sugars (glucose or fructose, 100 mM) at 100°C during various times with phenylalanine (100 mM) in non buffered conditions (A, B) or with phenylalanine during 180 min at various pH (C,D). Values with different superscript letters are significantly different at p<0.05 using Tukey HSD’s post test (n=3).
The antioxidant capacity (DPPH assay) of the reaction mixture increased with the heating time (as illustrated for glycine, isoleucine, lysine and phenylalanine in figure 2. Similar observations were reported by Liu et al.26 with TEAC assay and by Kim and Lee4 with DPPH, TEAC and FRAP assays. This was true for all the amino acids but the antioxidant capacity varied to a large extent between them. It was maximum with phenylalanine, tryptophan and tyrosine (table 2). Heating at 120°C for 20 min often gave similar results as heating at 100°C for 180 min figure 2. The variation of the antioxidant capacity measured by ORAC assay gave similar trends (data not shown).
Figure 2.Evolution of the antioxidant capacity (DPPH assay) of model systems consisting in heating one amino acids (glycine, isoleucine, lysine or phenylalanine, 100 mM) with sugars (glucose or fructose,100 mM) during various times (0 to 180 min) at 100°C or 20 min at 120°C (autoclave). Values with different superscript letters are significantly different at p<0.05 using Tukey HSD’s post test (n=3).
3.2.3 Effect of pH
Increasing the pH induced an increase in the HMF content and of the absorbance at 405 nm (figure 1C-D for phenylalanine). On another hand, an increase of the pH of the mixture increased the antioxidant capacity (DPPH scavenging activity) of the MRPs figure 3. The values obtained at pH 3 and 5 were very low while they were higher at pH 9 and 11. The same trend was observed when the antioxidant capacity was measured by the ORAC assay (data not shown). With some amino acids, the values were similar in the presence of glucose or fructose while for others such as lysine, in the presence of glucose the antioxidant capacities were higher. It was already reported that heating at higher pH led to an increase in the initial rate of degradation of both reducing sugars and amino acids17.
Figure 3.Evolution of the antioxidant capacity (DPPH assay) of model systems consisting in heating amino acids (glycine, isoleucine, lysine or phenylalanine, 100 mM) with sugars (glucose or fructose 100 mM) during 180 min at 100°C at various pH (3 to 11). Values with different superscript letters are significantly different at p<0.05 using Tukey HSD’s post test (n=3).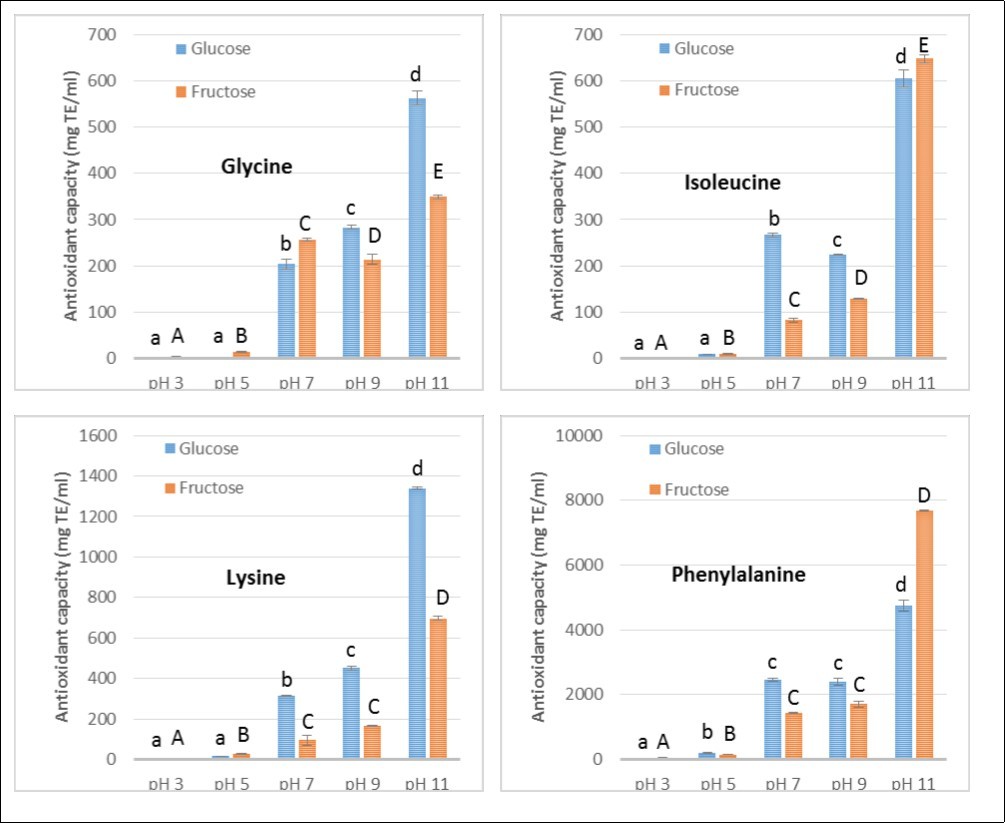
Many studies have reported beneficial effects associated with MRPs, including antioxidative properties5, 6. Other studies about the antioxidant properties of MRPs suggest that melanoidins are the main contributors to the antioxidant capacity10, 27. This can explain the similarity of the observed profiles between antioxidant capacity and absorbance at 405 nm in all the treatments. However, recent studies showed that melanoidins can show a pro-oxidant activity as well10, 27, 28. This last property can be related with the formation of radicals by the Fenton mechanism in the presence of iron or copper cations29.
Antioxidant Capacity and HMF Content Evolution during Fruit Cooking
Development of antioxidant activity due to MRPs can be influenced by the characteristics of the food matrix. Therefore we also analysed the antioxidant capacity (DPPH and ORAC), the HMF content and absorbance at 405 nm during apple or pear marmalade cooking. According to the previous results, the cooking conditions used were: 100°C during 60 and 120 min in non buffered condition, or during 60 min at pH 5 or pH 9, or during 60 min with fructose added instead of sucrose).
Cooking increased both the DPPH antioxidant capacity (figure 4A) and ORAC values (data not shown) of apple and pear marmalade’s. Doubling the heating time from 60 to 120 min in non buffered conditions led to an increase of HMF content (figure 4B). Absorbance at 405 nm (indicative of melanoidin content) increased also during the first hour (figure 1C), but was stable during the following hour for pear marmalade. The slight decrease observed with apples indicated that there was probably an aggregation of particles of melanoidin with time at high temperature.This behavior is quite different from what was observed in amino acids / sugar in vitro model systems. However melanoidin composition in real foods is probably more complex than in model systems due to the more diverse pool of reactants.
Figure 4.Antioxidant capacity (DPPH assay, A), hydroxymethylfurfural content (B) and melanoidin evaluation (C) of marmalade’s of apples and pears boiled during 60 or 120 min in non buffered conditions, during 60 min at pH 5 or 9 with sucrose or during 60 min with fructose instead of sucrose. Values with different superscript letters are significantly different at p<0.05 using Tukey HSD’s post test (n=3).
When the mixture was buffered to pH 5, the antioxidant capacity after 60 min of heating was similar to that observed in non buffered conditions (figure 4A). But at pH 9, an increase of the antioxidant capacity was observed for apples and pears. Such an increase was already observed in this study in model aminoacid – sugar mixtures. On another hand, shifting the pH from 5 to 9 led to an increase of the HMF content in apple but not in pear marmalades. The melanoidins (OD at 405 nm) increased with the increase of pH from 5 to 9 in apple marmalade’s, but was stable in pear marmalade’s. Although melanoidins are chemically diverse, many studies reported that they are negatively charged in both real foods and in in vitro model systems at neutral pH 9. Under these conditions, the type of amino acid present during the reaction determined the anionic properties of the melanoidins30. The antioxidant properties of melanoidins have been partly ascribed to the metal chelating capacity of these compounds. This can explain the difference observed between apple and pear marmalade’s.
The replacement of sucrose by fructose induced an increase of the antioxidant capacity in apple marmalade. Reducing sugars in the fruit puree, mainly glucose and fructose, participate directly in the non enzymatic browning reactions while some disaccharides, such as sucrose are less reactive because they must be first hydrolyzed during thermal treatment, leading to the formation of glucose and fructose31. This fact can explain the rapidity of Maillard reaction in the presence of fructose.
Practical Implications
The results of this study may have some useful practical implications at both the food technology and nutritional levels. Melanoidins are produced during the processing and storage of foods. The antioxidant properties of melanoidins can inhibit the oxidation of unsaturated lipids and functional food ingredients, such as vitamins, polyphenols and flavonoids. Moreover, their antimicrobial activity can inhibit the growth of microorganisms32 and prevent the spoilage and deterioration of foods. Furthermore, at the end of the Maillard reaction, the volatile aromatic compounds contribute directly to the attractiveness of the products. Consequently, the Maillard reaction has both desirable and undesirable effects on products33. Hence, researchers should optimize the formation of these components.
Considering that melanoidins may preserve the quality and safety of foods10, some parameters of the processing could be modified:
the cooking times and temperature,
the pH of the preparation,
the relative proportion of fruits or the addition of amino acids and reducing sugars responsible of the formation of the MRPs (reducing sugars are more rapidly transformed in MRPs than sucrose),
the addition of plant extract containing polyphenolics. The composition in polyphenols themselves responsible of antioxidant capacity can be an important factor that affects MRP formation4, 34.
Concerning the cooking time and temperature, we have also to take in account that the degradation of ascorbic acid in food is one of the major sources of furan compounds35, 36. In our in vitro model, we have evidenced that an equimolar mixture of ascorbic acid with amino - acids is associated with a dramatic increase of HMF (data not shown).
An increase of health properties of cooked fruits is associated with a higher antioxidant capacity, a decrease in HMF and an increase of melanoidins. Some phenolic compounds and plant extracts containing phenolic compounds could be used to prevent the formation of some MRPs before thermal process applications or during storage37.
Conclusion
The process of Liège syrup production includes several hours of heating at high temperature during which complex chemical reactions occur such as Maillard condensation between amino acids and reducing sugars present in the mixture. At the end of the process, the HMF formed have totally disappeared and the deep brown color of the product is probably due to the formation of melanoidins. These compounds could also contribute to the antioxidant capacity. In apple and pear marmalade’s, an increase of the antioxidant capacity, and of HMF and melanoidins contents was observed with time at high temperature. An increased of the pH also induced an increase in antioxidant capacity in the two marmalade’s while the HMF and melanoidins contents were increased by a shift of the pH from 5 to 9 in apple but not in pear marmalade’s. However, it is known that Maillard reaction has both desirable and undesirable effects on food products. Some parameters of the processing can be modified to improve the antioxidant properties and composition in HMF and melanoidins of cooked fruits such as cooking time, temperature, pH, addition of reducing sugars or amino acids,…
Acknowledgment:
We thank ‘Siroperie Meurens’ for their cooperation and M. Chenut (student at the University of Lille 1) for his technical assistance. FB gratefully acknowledges the “Ministère des technologies nouvelles” (First subvention granted to « Haute Ecole de la Province de Liège »). The skillful assistance of the APE personnel (provided to CEDEVIT by the regional government of Wallonia) was greatly appreciated.
References
- 5.Maillard M N, Billaud C, Chow Y N, Ordonaud C, Nicolas J. (2007) LWT - Food Sci. , Technol 40(8), 1434-1444.
- 9.Valls-Bellés V, Torres M C, Muñiz P, Boix L, González-Sanjose M L.. , J Sci Food Agric 84(13), 1701-1707.
- 13.J M Ames. (1992) . , InB.J.F.Hudson (Ed.), Biochemistry of Food Proteins, London:Elsevier 99-153.
- 17.E H Ajandouz, L S Tchiakpe, Ore Dalle, Benajiba F, A et al. (2001) . , J. Food Sci 66(7), 926-931.
- 18.Arion Mihalache, Tabart C, Kevers J, Niculaua C, Filimon M et al. (2014) Food Chem. 146, 485-491.
- 19.Sipel A, Kevers C, Pincemail J, P G Grygiel, J O Defraigne. (2013) Food Anal. , Meth 6(5), 1485-1491.
- 20.Kevers C, Pincemail J, Tabart J, J O Defraigne, Dommes J. (2011) . , J. Agric. Food Chem 59(11), 6165-6171.
- 23.Aguedo M, Kohnen S, Rabetafika N, Bossche Vanden, Sterckx S et al. (2012) . , J. Food Comp. Anal 27(1), 61-69.
- 29.Hoff S, M N Lund, M A Petersen, B M Jespersen, M L Andersen. (2012) . , J. Agric. Food Chem 60(22), 5652-5659.
- 33.Martins S I F S, Jongen W M F, Boekel M A J S Van. (2000) Trends Food Sci. , Technol 11(9), 364-373.
