Abstract
Parasitic infection by the Fasciola hepatica (F. hepatica) promotes susceptibility towards other infections, such as Mycobacterium bovis. As consequence, could affect diagnostic tests for this disease. Hence, the objective of this study was to assess the impact of F. hepatica coinfection on the most commonly used immunodiagnostic bovine tuberculosis (bTB) tests in field conditions in an enzootic area for both diseases. Thus, from a dairy herd located in Hidalgo State, México, displaying a 59.2% and 28% prevalence of fascioliasis and bTB, respectively. Sixty-one cows were analyzed based on their response towards bTB immunodiagnostic tests, such as Single Intradermal Comparative Tuberculin Test (SICTT), gamma-interferon test (BOVIGAM) and enzyme-linked immunosorbent assay (ELISA), along with the assessment of the F. hepatica parasite load and serodiagnosis by ELISA. Three study groups were formed according to test results. Group 1: coinfected (n=22). Group 2: non-parasitized cows, and positive for bTB tests (n=13) and Group 3: parasitized cows without tuberculosis (n=26). In addition, a group of cows kept in fascioliasis - and tuberculosis-free zones were included (Group 4, n=10). A non-parametric Kruskal-Wallis test and a Dunn test were applied to analyze the results. In Group 1, significant differences were observed regarding IFN-γ production, but not for antibody levels to M. bovis or reactivity towards bovine PPD in relation Group 2. While, Groups 1 and 3 did not display difference in antibody levels against F. hepatica. Differences were observed regarding tuberculosis and Fasciola diagnostic tests when both coinfected and infected groups were compared to controls. It is concluded that F. hepatica coinfection in tuberculous animals studied, depressed the production of IFN-γ towards bovine PPD under in vitro conditions, but its reactivity to the SICTT not show to be altered.
Author Contributions
Academic Editor: Mohammed Elmetwall, Assistant Prof of Theriogenology.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2018 García-López Xitli, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interests
The authors have declared that no competing interests exist.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Fascioliasis is one of the main parasitic infections affecting bovines worldwide and it has a negative impact on both milk production (20%-80%) and meat yield (8%-50%) 1, 2. Nevertheless, losses may be even higher, considered that these diseases promote malnutrition and render the host susceptible to acquire other infections. In these conditions it is frequent to observe herds coinfected by F. hepatica (F. hepatica) and Mycobacterium bovis (M. bovis) 3. Immune response to F. hepatica is oriented towards a Th2-type helper cell response, which is characterized by the proliferation and activation of CD4 T cells specific to parasitic antigens. These cells secrete IL-4 IL-5, IL-9 and IL-13; cytokines promote eosinophilia, platelet and mast cells increase and an enhanced production of both IgG1 and IgE immunoglobulins 4. Whereas cooperating cell immunity of the Th1 type is of greater importance for M. bovis control, this type of response includes the participation of alveolar macrophages, γδ T-lymphocytes, cooperating CD4+ T-lymphocytes and cytotoxic CD8+ lymphocytes, which cytokines secreted (IL-2, IFN-γ-, IL-12, IL-18 and TNF-α), along with the RANTES, MCP-1, MIP-1α and IL-8 chemokines, play a central role during migration of several cell subpopulations towards the infection site in order to trigger granuloma formation 5. Cattle infected by F. hepatica display a Cellular Mediated Immunity (CMI) regulation that decreases both IFN-γ production and lymphocyte proliferative response 6. This may affect the host’s susceptibility towards some bacterial diseases such as bovine tuberculosis (bTB).
On the other hand, different immunoenzymatic methods have been developed and applied in order to diagnose an active F. hepatica infection. These, are distinguished by their sensitivity and specificity that is based on the use of the parasite’s excretion-secretion antigens 7. On the other hand, bTB diagnosis is usually based on the induction of Delayed-type Hypersensitivity (DTH) and the detection of IFN-γ production in vitro by using bovine PPD in both tests 8, 9 .
Studies regarding influence of coexisting infections on the host’s immune response as well as its effect on the results from diagnostic tests and the epidemiologic situation of such diseases have been scarce. However, it has been observed that parasite elimination in coinfected humans enhance cellular response towards M. tuberculosis antigens 10. Whereas that the effectiveness of the BCG vaccine is decreased against infections mediated by M. tuberculosis in mice, in those cases where a coinfection with Schistosoma mansoni occurs 11, 12.
The assessment of the impact caused by F. hepatica and other common helminths on the results obtained from the tuberculin test and the IFN-γ assay is important in order to control bTB. In relation to, an altered CMI has been reported in cattle coinfected by F. hepatica and M. bovis. This effect was observed by performing the tuberculin diagnostic test and evaluating the expression levels of some cytokines 13, 14, 15. Moreover, it has been demonstrated a negative association between F. hepatica parasitic infections and bTB diagnosis as the percentage of positive animals by the tuberculin test is lower on parasitized bovines 16, 17.
The aim of this work was to evaluate in field conditions the effect of F. hepatica coinfection on the immunodiagnostic tests commonly used to detect bovine tuberculosis in an enzootic area for both diseases, located in Hidalgo State, México.
Materials and Methods
Ethics Statement
The study design and sampling methodology was reviewed and approved by Bioethics Committee for Care and Use Reasonable Animals for Experimentation in Research Projects of National Disciplinary Research Center in Animal Microbiology (CENID-Animal Microbiology) belonging to the National Institute for Forestry, Agriculture and Livestock Research (INIFAP). Who granted permission to realize the fieldwork. Furthermore, the study was conducted in accordance with the Animal Welfare Legislation of Mexico. Collection of blood samples and administering of the Single Intradermal Comparative Tuberculin Test (SICTT) were performed by qualified veterinarians following official procedures from the Norma Oficial Mexicana (NOM-041-ZOO1995) of the National Campaign against tuberculosis in Animals.
Animals And Experiment Design
The study was conducted in a dairy herd under a semi-intensive system located in a rural region of the State of Hidalgo, México, in the Municipality of Acatlan, situated 20° 09' north latitude and 98° 26' east longitude. The dairy herd comprising 122 cows of the Holstein-Friesian breed in production stage and older than 12 months. According to your records, displaying a 59.2% and 28% prevalence of parasite infections by F. hepatica and tuberculosis, respectively. In a first work session, the SICTT was applied to all the animals of the herd, that same day blood samples were taken from each of the animals to carry out the different immunodiagnostic tests, along with the first stool samples for perform the coproparasitoscopic analysis. On the day of the reading of the SICTT, the second stool samples were collected and in the following week, the third samples to complete the series of three samples. According to the results obtained in the different diagnostic tests for both diseases, 61 animals were selected based on the inclusion criteria that considered positivity towards the diagnostic tests for one or both diseases. Thereby, three groups were established. Group 1, coinfected, positive towards both F. hepatica (coproparasitoscopic examination) and bTB diagnostic tests (SICTT, IFN-γ and ELISA) (n=22). Group 2: non-parasitized cows positive to bTB tests (n=13). Group 3: parasitized cows negative to bTB tests (n=26). Mostly it was included a ten cows group belonging to a F. hepatica- y bTB-free zone was considered (Group 4, n=10) for comparison purposes.
Coproparasitoscopic Test, Sedimentation Technique
The identification of cows infected with F. hepatica was made by coproparasitoscopic analysis, using three samples per animal on alternating days, as stated above, employing the sedimentation technique 18.
Preparation of Metabolic (excretion/secretion) F. Hepatica (FhSE) Antigen
The metabolic antigen was obtained according to the procedures described by Espino., et al (1988) 19 and De Almeida., et al (2007) 20. Adult flukes of F. hepatica were collected from bovine livers and they were placed on sterile phosphate buffer saline (PBS) pH 7.4 at room temperature. Parasites were washed five times with Hank’s solution supplemented with antibiotics (100 U/mL penicillin G and 100 μg/mL streptomycin) (In Vitro S.A. México). After the last wash, parasites were placed on RPMI 1640 culture media (Gibco, USA) supplemented with the above antibiotic/antifungal mixture and they were incubated at 37ºC under constant stirring (Labnet, 311DS, Woodbridge, NJ USA) for 12 h. Subsequently, the culture supernatant containing the secretion-excretion antigens was centrifuged at 10,000 rpm at 4ºC during 30 min. The pellet was suspended in 10 mL of sterile PBS containing 200 µg/mL phenylmethylsulphonyl fluoride (Sigma Aldrich, St. Louis, MO, USA) and it was filtered through a 0.20 μm membrane (Millipore Billerica, MA. USA). Finally, protein was quantified by the Bradford method 21 and the sample was stored at -80ºC.
Culture Filtrate Protein Extract of M. Bovis
M. bovis strain AN5 was used to produce the Culture Filtrate Protein Extract (CFPE); for which, bacteria was cultured in Dorset-Henley liquid medium at 37ºC for 6 weeks. After this, the culture was filtered in order to obtain a cell-free filtrate 22. The protein present on the residual liquids were precipitated with ammonium sulfate (Sigma Aldrich, St. Louis, MO, USA) at 80% saturation while kept under constant stirring at 4ºC for 24 h. In order to recover the precipitate, the mixture was centrifuged at 15,000 g at 4ºC for 90 min. It was further submitted to dialysis by using a membrane with a 6-8 kDa cutoff value (Membrane Filtration Products, Inc. Tx, USA) 23. Protein concentration was assessed by using the Bradford method 23 and it was finally stored at -70ºC.
ELISA for F. hepatica and M. bovis
Enzyme-linked immunosorbent assays (ELISA) for determination of IgG-class antibodies to M. bovis in serum were made according to the methodology described by Díaz-Otero., et al (2003) 23. While the ELISA to F. hepatica was established and Receiver Operating Characteristics curve (ROC) was used to determine the cut off.
Suitable plates (Nunc MaxiSorp® Roskilde, Denmark) were coated with the respective antigens diluted in carbonate-bicarbonate buffer pH 9.6, using for the purpose 1 µg/well of the CFPE of M. bovis and 2.5 µg/well of the FhSE antigen in the corresponding plates. After performing plates respective coating, the same protocol was followed. Plates were washed five times with 0.1% PBS-Tween 20 (PBS-T) in order to eliminate an antigen excess. Then, were blocked with 3% non-fat milk in PBS-T at 37ºC for 1 h and other washing step with PBS-T was made. Next incubated with 100 µL of the respective sera PBS-diluted (1:100) for 1 h at 37°C. Plates were washed and 100 µL of a G protein-peroxidase conjugate (Sigma Aldrich, St. Louis, MO, USA) from 1: 10,000 dilution was added at 37ºC for 1 h. Another stage of washes was performed and subsequently 100 µl chromogenic substrate solution was added for detection of enzyme reaction; containing 0.04% O-phenylenediamine (Sigma P-3804) and 0.04% of hydrogen peroxide in citrate buffer pH 4.5. Reaction was stopped with 50 μl/well of 2 M sulfuric acid. Optical densities were obtained at 492 nm (OD492nm) in a microplate spectrophotometer (Spectrophotometer Benchmark Plus, BIO-RAD, Japan). The optical density cutoff value for both ELISA tests was that of healthy cows plus two standard deviations. These OD492 cutoffs ELISA values were 0.30 and 0.350 for F. hepatica and M. bovis, respectively.
Single Intradermal Comparative Tuberculin Test
SICTT was realized according to the NOM-031-1995 Official Mexican Standard 24, by intradermal injecting cattle with 0.1 mL (1mg/mL) bovine PPD and 0.1 mL (0.5 mg/mL) avian PPD (PRONABIVE, México City, Mexico). At 72 h post-inoculation, the skin thicknesses was measured. A result was considered positive if the increased skin thickness at site of injection was at least 4 mm greater when compared to avian PPD at the injection site.
IFN-γ test for M. bovis
Heparin-treated blood samples collected from each animal group were dispensed in sterile conditions in three consecutive wells on 48-well culture plates (Nunclon, Roskilde, Denmark) at a 750 µL blood/well ratio. 50 µL of bovine and avian PPD (both at 0.3 mg/mL) were added to the respective wells whereas one was kept without antigen to be used as control. Plates were incubated at 37ºC for 24 h under a 5% CO2 saturated atmosphere. After incubation, plasma was obtained from each culture in order to assess IFN-γ production induced by antigenic stimulus by using a commercial sandwich ELISA kit (Bovigam®, Prionics AG, Schlieren- Zurich, Switzerland) 25. A sample was considered positive when the OD value difference between either a sample stimulated with bovine PPD or that stimulated with avian PPD and the value without antigen is equal or higher than 0.1. A sample was considered negative when this difference is less than 0.1, according to Rothel., et al. (1990) 26.
Statistical Analysis
The results obtained for all groups in these tests were analyzed by using the Kruskal-Wallis test by considering their independence and a Dunn test was subsequently applied. A p<0.05 probability was considered significant. The Pearson test was used in order to analyze correlation between the number of eggs and antibodies on the parasitized group.
Results
Group: Coinfected by F. hepatica and M. bovis
Parasite load was heterogeneous in this group, displaying a mean value of 16 eggs/g (range: 1-113 eggs/g). Most of all cows were positive towards FhSE ELISA tests displaying an OD492 mean value of 0.686. Regarding the bTB tests, animals showed a moderate to high reactivity to the SICTT and the mean increased DTH response to bovine PPD was 33 mm. The mean IFN-γ production in cultures stimulated with bovine PPD displayed a 0.493 OD450 value. All animals were positive for M. bovis, as identified by ELISA, and their mean OD492 value was 0.521 (Table 1).
Table 1. Cows Coinfected by M. bovis and F. hepatica Positive for Diagnostic Tests Detecting both Diseases (Group 1, N=22).| Cow number | Amount of F.hepatica eggs/g | SICTT (mm)a | IFN-γ (OD450nm)b | ELISA (OD492nm)c | ||
| PPD-A | PPD-B | PPD-B | FhSE | CFPE | ||
| 2 | 11 | 7 | 14 | 0,209 | 0,963 | 0,449 |
| 3 | 3 | 12 | 26 | 0,375 | 0,856 | 0,565 |
| 10 | 2 | 9 | 16 | 0,486 | 0,587 | 0,467 |
| 12 | 6 | 11 | 22 | 0,346 | 0,62 | 0,467 |
| 20 | 3 | 7 | 24 | 0,327 | 0,629 | 0,686 |
| 22 | 55 | 5 | 27 | 0,345 | 0,917 | 0,486 |
| 22 | 1 | 3 | 49 | 0,387 | 0,565 | 0,846 |
| 78 | 3 | 13 | 21 | 0,123 | 0,46 | 0,358 |
| 81 | 2 | 13 | 78 | 0,2 | 0,863 | 0,385 |
| 95 | 4 | 4 | 18 | 1,48 | 0,507 | 0,439 |
| 95 | 3 | 15 | 59 | 0,379 | 0,839 | 0,663 |
| 108 | 1 | 8 | 41 | 0,259 | 0,903 | 0,382 |
| 224 | 34 | 8 | 17 | 0,108 | 0,866 | 0,555 |
| D | 4 | 12 | 62 | 0,179 | 0,46 | 0,516 |
| E | 5 | 9 | 49 | 0,105 | 0,46 | 0,68 |
| 3 | 1 | 7 | 57 | 0,68 | 0,78 | 0,706 |
| 8 | 11 | 5 | 52 | 0,398 | 0,645 | 0,58 |
| 11 | 80 | 9 | 34 | 0,115 | 0,943 | 0,415 |
| 24 | 3 | 18 | 28 | 1,269 | 0,685 | 0,566 |
| 25 | 2 | 5 | 14 | 0,192 | 0,423 | 0,383 |
| 32 | 9 | 8 | 19 | 0,512 | 0,726 | 0,429 |
| 8 | 113 | 7 | 13 | 2,39 | 0,399 | 0,45 |
| MEAN | 16 | 8,8 | 33 | 0,493 | 0,686 | 0,521 |
Group: Infected by M. bovis, non-parasitized
The mean IFN-γ production level in this group was 0.852 OD450 after antigenic stimulus with bovine PPD. This was approximately a two-fold value when compared to that obtained for the coinfected Group 1 (p< 0.001), Figure 1. However, no difference regarding antibody levels towards M. bovis CFPE was detected between these groups (p=0.34). A similar effect regarding the DTH response was observed after bovine PPD application (p = 0.32). Furthermore, antibody levels against F. hepatica were low in the animals of this group. This observation along with the coproparasitoscopic analysis confirmed the absence of a parasitic infection (Table 2). The antibodies mean value for FhSE, as assessed by ELISA, was 0.203 OD492, excepting one animal on the group that displayed levels above the cutoff.
Table 2. Tuberculous Cows Non-Parasitized by F. hepatica and Positive for the SICTT, IFN-γ and ELISA Diagnostic Tests (Group 2, N=13)| Cow number | |||||
| SICTT (mm)a | IFN-γ (OD450nm)b | ELISA (OD492nm)c | |||
| PPD-A | PPD-B | FhSE | CFPE | ||
| 3 | 7 | 37 | 0,355 | 0,14 | 0,54 |
| 6 | 13 | 63 | 0,296 | 0,181 | 0,513 |
| 25 | 9 | 31 | 0,342 | 0,256 | 0,754 |
| 16 | 5 | 12 | 0,428 | 0,18 | 0,311 |
| 18 | 6 | 14 | 1,422 | 0,12 | 0,686 |
| 26 | 7 | 48 | 0,673 | 0,21 | 0,339 |
| 28 | 9 | 61 | 1,224 | 0,242 | 0,407 |
| 38 | 8 | 24 | 0,407 | 0,391 | 0,517 |
| 75 | 6 | 18 | 0,2 | 0,16 | 0,516 |
| 131 | 8 | 26 | 0,308 | 0,107 | 0,439 |
| E | 9 | 53 | 0,926 | 0,13 | 0,68 |
| 17 | 8 | 32 | 2,546 | 0,245 | 1,062 |
| 21 | 8 | 48 | 1,95 | 0,278 | 0,359 |
| MEAN | 7,92 | 35,9 | 0,852 | 0,203 | 0,547 |
Figure 1.Data are presented as mean ± standard deviation (DS). Kruskal Wallis test and post hoc Dunn´s multiple comparison test were used for the comparison of results obtained in the different diagnostic tests used for both diseases with *Significant differences (p˂ 0.001); **(p<0.05). Mean antibody levels towards F. hepatica secretion/excretion antigens (FhSE) and to the protein extract from a M. bovis culture filtrate (PECF) as assessed by indirect ELISA (OD492 nm) and mean IFN-γ production as quantified by sandwich ELISA in supernatants collected after antigen stimulation of whole blood cultures with bovine PPSD (OD450 nm) in the different study groups. Group 1: tuberculous cows coinfected by F. hepatica; Group 2: tuberculous cows; Group 3: parasitized cows; and Group 4: non-tuberculous, non-parasitized cows.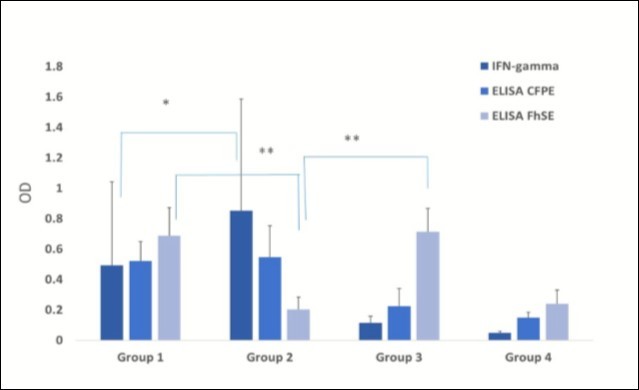
Group: Parasitized and Uninfected by M. bovis
A moderate correlation (r=0.50) was observed between parasite load and FhSE antibody levels among the animals in this group. The mean number of F. hepatica eggs was 14 eggs/g (range 1-91 eggs/g), whereas the mean antibody levels towards FhSE was OD492 0.712 (Table 3). This value was similar to that observed within the coinfected group. Regarding the results obtained for the tuberculosis tests, they were all negative for SICTT and IFN-γ, whereas the M. bovis analysis by ELISA displayed values above the cutoff for three animals.
Table 3. Cows Parasitized by F. hepatica Negative for the SICTT, IFN-γ and ELISA Tuberculosis Tests (Group 3, N=26)| Cow number | Amount ofF. hepatica eggs/g | SICTT (mm)a | IFN-γ (OD450nm)b | ELISA (OD492nm)c | ||
| PPD-A | PPD-B | PPD- B | FhSE | CFPE | ||
| 5 | 1 | 0 | 0 | 0,18 | 0,53 | 0,125 |
| 13B | 2 | 2 | 3 | 0,12 | 0,48 | 0,289 |
| 15 | 4 | 3 | 2 | 0,08 | 0,567 | 0,241 |
| 19 | 2 | 0 | 0 | 0,065 | 0,64 | 0,159 |
| 23 | 9 | 2 | 0 | 0,065 | 0,574 | 0,248 |
| 24 | 10 | 0 | 0 | 0,081 | 0,733 | 0,121 |
| 40 | 7 | 0 | 0 | 0,091 | 0,855 | 0.450* |
| 46 | 2 | 2 | 0 | 0,083 | 0,578 | 0.566* |
| 54 | 15 | 3 | 0 | 0,13 | 0,799 | 0.471* |
| 66 | 2 | 0 | 0 | 0,093 | 0,884 | 0,158 |
| 83 | 91 | 2 | 2 | 0,17 | 0,947 | 0,248 |
| 84 | 2 | 4 | 3 | 0,19 | 0,581 | 0,236 |
| 97 | 3 | 4 | 2 | 0,21 | 0,758 | 0,178 |
| B | 16 | 0 | 0 | 0,095 | 0,531 | 0,156 |
| F | 14 | 2 | 0 | 0,084 | 0,684 | 0,256 |
| 2 | 4 | 0 | 0 | 0,17 | 0,849 | 0,018 |
| 27 | 2 | 0 | 3 | 0,091 | 0,764 | 0,175 |
| 28 | 1 | 0 | 0 | 0,083 | 1,101 | 0,286 |
| 1 | 7 | 3 | 0 | 0,173 | 0,873 | 0,189 |
| 7 | 5 | 0 | 0 | 0,084 | 0,561 | 0,164 |
| 9 | 40 | 0 | 0 | 0,097 | 0,668 | 0,167 |
| 11 | 43 | 2 | 2 | 0,1 | 0,776 | 0,181 |
| 1515 | 2 | 0 | 0 | 0,086 | 0,827 | 0,173 |
| 18 | 4 | 0 | 0 | 0,08 | 0,781 | 0,248 |
| 46 | 57 | 3 | 2 | 0,1 | 0,553 | 0,138 |
| 79 | 25 | 4 | 2 | 0,183 | 0,627 | 0,191 |
| MEAN | 14,23 | 1,38 | 0,81 | 0,115 | 0,712 | 0,224 |
Group: Non-parasitized, Uninfected by M. bovis
Animal within this group were selected from a free-disease zone. This was verified after negative results were obtained for several tuberculosis and F. hepatica diagnostic tests (Table 4). However, two animals within the group showed antibody levels above the cutoff value established for FhSE by ELISA. Significant differences were observed when the results obtained from different diagnostic tests were compared to other study groups (p<0.001).
Table 4. Cows Negative Towards both F. Hepatica and Bovine Tuberculosis Kept in a Zone Free from Such Diseases (Group 4, N=10).| Cow number | SICTT (mm)a | IFN-γ (OD450nm)b | ELISA (OD492nm)c | ||
| PPD-A | PPD-B | PPD-B | FhSE | CFPE | |
| 173 | 2 | 0 | 0,05 | 0,201 | 0,128 |
| 176 | 0 | 0 | 0,038 | 0,157 | 0,129 |
| 213 | 0 | 0 | 0,04 | 0,378 | 0,199 |
| 214 | 0 | 0 | 0,044 | 0,278 | 0,205 |
| 217 | 0 | 0 | 0,08 | 0,196 | 0,098 |
| 218 | 3 | 0 | 0,053 | 0,399 | 0,105 |
| 219 | 0 | 0 | 0,045 | 0,168 | 0,13 |
| 238 | 0 | 0 | 0,05 | 0,287 | 0,175 |
| 252 | 3 | 0 | 0,035 | 0,156 | 0,16 |
| 954 | 0 | 0 | 0,045 | 0,178 | 0,144 |
| MEAN | 0,8 | 0 | 0,048 | 0,240 | 0,147 |
As IFN-γ production towards PPD bovine was the only test showing significant differences between coinfected and tuberculous groups, results of this test were furthermore compared among groups with positive and negative responses towards bovine PPD (group 1 and 2 vs. 3 and 4), a significant difference was found (p<0.001). Similar outcomes were observed by ELISA-CFPE among reactive and non-reactive groups (p< 0.01).
No significant differences (p=0.29) were observed after comparing FhSE ELISA results between the parasitized groups (1 and 3) with and without tuberculosis, Figure 1. And if there were differences with the behavior of this test with that of the tuberculous and control group (group 4).
Discussion
The diagnosis of an infectious disease is critical for its control and the treatment of the affected individuals. Mostly, the latter is based on the host immunologic response towards the etiologic agent. However, some pathogens, particularly helminths, notably impair the host immune response thus promoting susceptibility towards other infections and it affects the diagnostic sensitivity for other implicated diseases. Regarding bovine tuberculosis, it has been identified that different situations may affect sensitivity of the most commonly used immunodiagnostic tests. These situations include host immunosuppression derived from coinfections by several pathogenic agents or parasites. This leads to severe epidemiologic consequence and affects the control of the disease 16. Thus, in order to assess the effect of fascioliasis on the commonly used immunodiagnostic bTB tests (SICTT, IFN-γ and ELISA), this study was carried out within an endemic zone with high prevalence of both diseases, as most of the previous reports in this regard have been based on experimental conditions 13, 15. A few field research studies have been conducted in order to define the influence of parasitic coinfections on the results obtained by the commonly used diagnostic tests to detect such diseases. By applying a descriptive and observational analysis, this study shows different IFN-γ production abilities by T-lymphocytes obtained from coinfected animals when stimulated in vitro with bovine PPD. However, no differences were observed regarding the SICTT reactivity when this group was compared to non-parasitized tuberculous cows. The different IFN-γ production levels displayed by the different groups may be explained by the high IL-4 and IL-10 concentrations previously detected in bovines with chronic fascioliasis. Even though they were not contemplated in this study, the supporting evidence points out that during helminth infections, specially by F. hepatica, important amounts of the above cytokines are produced in order to stimulate the chronical phase and the parasite survival within the host 16, 27, 28. Besides the high IL-4 and IL-10 expression levels, a low expression of the IFN-γ specific antigen has been observed. This indicates that the immune response is polarized towards a Th2 type in animals with chronic infection by F. hepatica. This study also identified a low IFN-γ production stimulated by F. hepatica secretion/excretion antigens in whole blood cultures obtained from both groups either coinfected an infected by this parasite (Data not shown). Significant levels of this cytokine are detected in the early stages of the infection, although the source may be NK cells, the underlying mechanism of this production needs further clarification 29. Based on the of the immune response polarization triggered by the IL-4 and IL-10 cytokines during the chronic stage of the parasitic infection, their effect on the negative regulation of IFN-γ expression and production by T-lymphocytes specific M. bovis antigens is elucidated within the coinfected group. The lack of differences observed regarding reactivity extent to the SICTT between groups 1 and 2 (tuberculous and tuberculous coinfected by F. hepatica) may be explained by the fact that hypersensitivity towards tuberculin is a local inflammatory response induced by cells, in which IFN-γ as well as other cytokines and chemokines participate. Within cytokines, they are primarily pro-inflammatory and IL-2, IL-3, IL-6, TNF-α and TGF-β have been mentioned. On the other hand, the chemokines involved in delayed-type hypersensitivity (DTH) reaction are RANTES, MCP-1, MIP-1α and CXCL8 (IL-8) 30. The latter occurs in significant levels on the cutaneous reaction site along with a number of neutrophils that promote selectin and integrin expression mediating the interaction with both epithelial and endothelial cells. Thus, during the regulation of the DTH reaction, the generalized IFN-γ decrease occurring in infected cows may not significantly participate. This would explain the low effect observed in the SICTT for this group. Nevertheless, the experimental studies conducted by Flynn., et al. (2007) 13 point out that the SICTT extent is decreased in animals coinfected by M. bovis and F. hepatica, unlike the results obtained by this study. The analysis by these authors is based on an infection model consisting of calves inoculated with F. hepatica metacercariae and a BCG vaccine M. bovis strain in order to assess the impact of helminth infection on the diagnosis of these diseases. However, their observations on SICTT and the IFN-γ test with whole blood (BOVIGAM) were realized thirteen weeks after treatment, which in contrast to a kinetics study conducted on the immune response on vaccinated calves, IFN gamma production has decreased by that time. Furthermore, the reactivity towards the tuberculin test may not occur in immunized calves, and what has also been observed, is that if it develops, it diminishes after a few months of application of the vaccine 31, 32. Regarding the group 3 consisting of Fasciola-infected cows negative for tuberculosis, three of them showed antibodies above the cutoff value determined for M. bovis by ELISA. This may be due to a prime of their immune system by environmental mycobacteria; insomuch as, SICTT and IFN-γ tests they were negative. Further, were evaluated by ELISA using Culture Filtrate Protein Extract (CFPE) of M. avium resulting positives, thus this confirms their sensitization by other mycobacteria. Regarding the results of the diagnostic tests used to evaluate F. hepatica infection, a moderate correlation was observed between the amount of eggs and antibody levels. These observations are in agreement with those obtained by other researchers 33, 34, 35. Furthermore, M. bovis infection had no effect on antibody levels in groups infected by F. hepatica, as previously demonstrated by Flynn., et al (2007 and 2009) 13, 15. Thus, for bovine tuberculosis diagnostic and epidemiologic purposes, the health status of the heard must be considered and a possible parasite infection (particularly by F. hepatica) is to be contemplated within the study zones before performing the tuberculin tests in endemic zones. The inclusion of complementary tests to the already defined antigens may contribute to a better diagnostic of this disease. Considering that F. hepatica prevalence has increased during the last years, being the climate change one of the possible causes, as well as the different use of the soil and modifications to the draining system type. These factors have promoted the development of intermediary hosts and the free life stages of the parasite but without disregarding parasite resistance towards triclabendazol and other fasciolicides 36, 37. Consequently, the infection mediated by F. hepatica is an additional environmental risk for bovine production systems as their presence promotes the establishment of other infectious agents 37, 38. However, contrary to this information on the promotion of infectious diseases, a regulatory role of F. hepatica to infections by pathogens normally controlled by a Th1 or pro-inflammatory response has been argued in other researches. In this respect, it was shown a lower mycobacterial recovery and uptake in blood monocyte‐derived macrophages associated switch to alternative activation of macrophages 39. Likewise, some studies indicate that in co-infected cattle there may be fewer tuberculous lesions, circumscribed to a smaller number of tissues 15, 38, these observations point to a beneficial rather than detrimental effect in F. hepatica coinfections. About this theme, information related to the administration of eggs from the swine parasitic nematode Trichuris suis has been considered as an alternative for the treatment of human inflammatory diseases, such as Crohn’s disease, based on the capacity of helminths to polarize T helper cells to Th2 type which inhibits inflammation 40, 41. Thus, a greater comprehension about the immune mechanisms involved in the regulation of the pro-inflammatory response are necessary to help clarify the possible protective role of this helminth in tuberculous cattle and its consequence on ante-mortem immunodiagnostic tests.
Conclusion
According to the results, there were no important differences for the different diagnostic tests used for both diseases between the co-infected group, with those parasitized or tuberculous, and only the production of interferon gamma towards the PPD-bovine showed to be affected in the co-infected group, but not the intradermal tuberculin test, which is also associated with a cell-type immune response. Unlike what they point out in other studies, as indicated by other studies that the SICTT is likewise affected.
Acknowledgements
Funding for this study came from the Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP) México. Registry number in the Integral System of Institutional Management, 14294534013.
Thanks go also to the farmers that provided the animals and farms for the realization of this study.
References
- 1.González R, R M Pérez, Brito S. (2007) Fasciolosis bovina, evaluación de las principales pérdidas provocadas en una empresa ganadera.Revista de Salud Animal. 29, 167-175.
- 2.Caicedo R R, Calderón M, S VM Badillo. (2011) . Physiopathology effects in bovines (BostaurusxBos indicus) with fascioliasis in México.Actas Iberoamericanas de Conservación Animal 1, 332-335.
- 3.Skuce R A, A R, McDowell S W J. (2011) Bovine tuberculosis (TB): a review of cattle-to-cattle transmission, risk factors and susceptibility. http://www.dardni.gov.uk.
- 4.R J Flynn, Mulcahy G, H M Elsheikha. (2010) Coordinating innate and adaptive immunity inFasciola hepaticainfection: implications for control.Veterinary Parasitology. 169(3), 235-240.
- 5.WelshM D, R T Cunningham, D M Corbett, R M Girvin, McNair J et al. (2005) Influence of pathological progression on the balance between cellular and humoral immune responses in bovine tuberculosis.Immunology. 114, 101-111.
- 6.C R Falcón, Masih D, Gatti G, M C Sanchez, C et al. (2014) Fasciola hepatica Kunitz type molecule decreases dendritic cell activation and their ability to induce inflammatory responses.PLoSOne. 9(12), 114505.
- 7.Charlier J, L De Meulemeester, Claerebout E, Williams D, Vercruysse J. (2008) Qualitative and quantitative evaluation of coprological and serological techniques for the diagnosis of fasciolosis in cattle.Veterinary Parasitology. 153, 44-51.
- 8.Gormley E, M B Doyle, Fitzsimons T, McGill K, J D Collins. (2006) Diagnosis ofMycobacterium bovisinfection in cattle by use of the gamma-interferon (Bovigam) assay.Veterinary Microbiology. 112, 171-179.
- 9.de la Rua-Domenech, Goodchild R, A T Vordermeier, H M, R G Christiansen et al. (2006) Ante mortem diagnosis of tuberculosis in cattle: a review of the tuberculin tests, gamma-interferon assay and other ancillary diagnostic techniques.Research in Veterinary Science. 81, 190-210.
- 10.Elias D, Wolday D, Akuffo H, Petros B, Bronner U et al. (2001) Effect of deworming on human T cell responses to mycobacterial antigens in helminth-exposed individuals before and after bacille Calmette-Guerin (BCG) vaccination.Clinical and Experimental Immunology. 123, 219-225.
- 11.Farid A, Al-Sherbiny M, Osman A, Mohamed N, Saad A et al.(2005).Schistosomainfection inhibits cellular immune response to core HCV peptide.Parasite Immunology. 27, 189-196.
- 12.Elias D, Akuffo H, Pawlowski A, Haile M, Schon T et al. (2005) reduces the protective efficacy of BCG vaccination against virulentMycobacterium tuberculosis.Vaccine. 23, 1326-1334.
- 13.R J Flynn, Mannion C, Golden O, Hecariz O, Mulcahy G. (2007) ExperimentalFasciola hepaticainfection alters responses to test used for diagnosis of bovine tuberculosis.Infection and Immunity. 75, 1373-1381.
- 14.R J Flynn, Mulcahy G. (2008) The roles of IL-10 and TGF-beta in controlling IL-4 and IFN-gamma production during experimentalFasciola hepaticainfection.International Journalfor Parasitology. 38, 1673-1680.
- 15.R J Flynn, Mulcahy G, Welsh M, J P Cassidy, Corbett D et al. (2009) Co-Infection of cattle withFasciola hepaticaandMycobacterium bovis- immunological consequences.Transboundary and Emerging Diseases. 56, 269-274.
- 16.Claridge J, Diggle P, M C, Mulcahy G, Flynn R et al. (2012) hepaticais associated with the failure to detect bovine tuberculosis in dairy cattle.Nature Communications. 3(853), 1-8.
- 17.Gilbert M, Mitchell A, Bourn D, Mawdsley J, Clifton-Hadlry R et al. (2005) . Cattle movements and bovine tuberculosis in Great Britain.Nature.435 491-496.
- 18.Pajuelo-Camacho G, Luján-Roca D, Paredes-Pérez B, Tello-Casanova R. (2006) Aplicación de la técnica de sedimentación espontánea en tubo en el diagnóstico de parásitos intestinales.Revista. , Biomédica 17, 96-101.
- 19.Espino A A, E B Duménigo, Huesva N, C M Finlay. (1998) Mantenimientoin vitrode adultos deFasciolahepatica: Obtención de antígenos de excreción-secreción.Revista de Salud Animal. 10, 287-293.
- 20.Almeida M A De, M B Ferreira, Planchart S, Terashima A, Maco V et al. (2007) Preliminary antigenic characterization of an adult worm vomit preparation ofFasciola hepaticaby infected human sera.RevistadoInstitutodeMedicina Tropical. 49, 31-35.
- 21.Bradford M M A. (1976) Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding.Analytical. , Biochemistry 72, 248-254.
- 22.Bernardelli A. (2007) Producción y control de tuberculina bovina y aviar. Derivado Proteico Purificado (DPP). Servicio Nacional de Sanidad y Calidad Agroalimentaria. Buenos Aires. , Argentina
- 23.O F Díaz, R V Banda, M L Jaramillo, D C Arriaga, S D González et al. (2003) Identificación de bovinos portadores deMycobacteriumbovisaplicando técnicas inmunológicas y moleculares.Veterinaria México. 34, 13-26.
- 24.Norma Oficial.Mexicana NOM-031-ZOO-1995. Campaña Nacional Contra la Tuberculosis Bovina (Mycobacteriumbovis). Secretaria de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación. Ser-vicio Nacional de Sanidad, Inocuidad y Calidad Agro alimentaria. http://www.senasica.gob.mx/?doc=2189
- 25.J S Rothel, S L Jones, L A Corner, L A Cox, J C Cox et al. (1990) A sandwich enzyme immunoassay for bovine interferon-γ and its use for the detection of tuberculosis in cattle.Australian. , Veterinary Journal 67, 134-134.
- 26.Wood P R, Jones S L. (2001) BOVIGAM: an in vitro cellular diagnostic test for bovine tuberculosis.Tuberculosis(Edinb). 81, 147-55.
- 27.Donnelly S, M S O'Neill, Sekiya M, Mulcahy G, Dalton P J. (2005) Thioredoxin peroxidase secreted byFasciola hepaticainduces the alternative activation of macrophages.Infection and Immunity. 73, 166-173.
- 28.Mendes E A ,, Santos T A ,dos, ,Menezes-Souza D S L, B D Castanheira, Martins V F I et al. (2013) Expression of IL-4,IL-10 and IFN-γ in the liver tissue of cattle that are naturally infected withFasciola hepatica.Veterinary Parasitology. 195, 177-182.
- 29.N J Beesley, Caminade C, Charlier J, R J Flynn, J E Hodgkinson et al. (2017) Fasciola and fasciolosis in ruminants in Europe: identifying research needs. Transboundary and Emerging Diseases. 1-18.
- 30.Domingo-Gonzalez R, Prince O, Cooper A, S A Khader. (2016) Cytokines and chemokines inMycobacterium tuberculosisinfection.Microbiology Spectrum.4(5)TBTB2-0018-2016.
- 31.A O Whelan, Coad M, B L Upadhyay, D J Clifford, R G Hewinson et al. (2011) Lack of correlation between BCG-induced tuberculin skin test sensitization and protective immunity in cattle.Vaccine. 29, 5453-5458.
- 32.Corona-Gómez L, Jaramillo-Meza L, Pérez-González R, Diosdado-Vargas F, Santiago-Cruz J et al. (2017) Effect of a low dose of BCG-Phipps vaccine on the development of reactivity to tuberculin skin test in neonatal calves and adult cows.Journal of Veterinary Medicine Animal Health. 9, 290-299.
- 33.Ibarra F, Montenegro N, Vera Y, Boulard C, Quiroz H et al. (1998) Comparison of three ELISA tests for seroepidemiology of bovine fascioliosis.VeterinaryParasitology. 77, 229-236.
- 34.C H Quiroz, R H Guerrero, M C Ibarra, V F Ochoa, P G. (1999) Cinética de excreción de huevos y títulos de anticuerpos aFasciolahepatica, en ganado bovino tratado con triclabendazol en clima cálido húmedo en México.VeterinariaMéxico. 30, 273-279.
- 35.J A Munguía-Xóchihua, Ibarra-Velarde F, Ducoing-Watty A Montenegro- Cristino, Quiroz-Romero N, H. (2007) Prevalence ofFasciola hepatica(ELISA and fecal analysis) in ruminants from a semi-desert area in the northwest of Mexico.Parasitology Research. 101, 127-130.
- 36.McCann C M, Baylis M, D J Williams. (2010) Seroprevalence and spatial distribution ofFasciola hepatica-infected dairy herds in England and Wales.Veterinary Record166. 612-617.
- 37.N J Fox, P C White, C J Mc, Marion G, Evans A.Hutchings, MR.(2011).Predicting impacts of climate change onFasciolahepaticarisk.PLoSOne.10; 6(1):. 16126.
- 38.Claridge J A. (2012) DoesFasciola hepaticainfection increase the susceptibility of cattle to infection with other pathogens normally controlled by a Th1 or pro-inflammatory response?. Thesis for the degree of Doctor in Philosophy by JA.Claridge of the UniversityofLiverpool,UK.
- 39.Garza-Cuartero L, O’sullivan J, Blanco A, McNair J, Welsh M et al. (2016) reducesMycobacteriumbovisburden and mycobacterial uptake and suppresses the pro-inflammatory response.ParasiteImmunology. 38, 387-402.
Cited by (1)
- 1.Sultana Nazneen, Pervin Munmun, Sultana Sajeda, Mostaree Moutuza, Tamanna Mumu Tanjin, et al, 2022, Fascioliasis may promote tuberculous infectivity in small ruminants, Saudi Journal of Biological Sciences, 29(10), 103402, 10.1016/j.sjbs.2022.103402
