Abstract
Immunization of cattle with the bacillus Calmette-Guérin (BCG) vaccine, especially neonates, induces protection against Mycobacterium bovis (M. bovis) and has been proposed as a strategy for bovine tuberculosis (bTB) control. Therefore, the aim of this study was to evaluate the immune response induced under field conditions in neonatal calves vaccinated with BCG Phipps, a strain that has rarely been evaluated in the bovine population, using interferon (IFN)-γ and tuberculin tests, flow cytometry and enzyme-linked immunosorbent assay. Two groups (vaccinated and control) of 5 calves were monitored for 12 weeks, and increases in the in vitro IFN-γ production, the percentage of cluster of differentiation (CD)8+ T cells and the activation levels of CD4+ and CD8+ T cells were observed 3 to 4 weeks post-vaccination. Bovine purified protein derivative-specific IFN-γ production was increased about 4.8- and 5.5-fold in vaccinated animals compared to non-vaccinated animals 3 and 4 weeks post-vaccination respectively. CD8+ T cells of the vaccinated group were increased 1.6-, 1.5- and 1.6-fold at weeks 2, 3 and 4 respectively. Levels of activation were 1.7- and 1.9-fold higher for CD4+ T cells and 2.3- and 1.8-fold higher for CD8+ T cells in the vaccinated group at weeks 3 and 4 respectively in response to M. bovis antigens. However, no animals (vaccinated or control) showed positive results for the single intradermal comparative tuberculin test (SICTT). Therefore, our results indicate that vaccination with M. bovis BCG Phipps strain stimulated peripheral blood T cell activity and induced a cell-mediated immune response. In addition, vaccination did not interfere with the SICTT, as previously reported, which indicates that this vaccine could be successfully applied in bTB control campaigns.
Author Contributions
Academic Editor: Yan Tu, Feed Research Institute, Chinese Academy of Agricultural Sciences, Key Laboratory of Feed Biotechnology of the Ministry of Agriculture
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2017 Fernando Díaz Otero, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
The persistence of bovine tuberculosis (bTB) poses a major economic problem and a global human health risk because the etiological agent, Mycobacterium bovis(M. bovis), can also infect humans.Conventionally, control of bTB is based on a test and slaughter strategy, which is a costly method for the developing world. Therefore, vaccination has been proposed as a potential control alternative, and studies have been performed to determine the efficacy of tuberculosis (TB) vaccines 1, 2. The bacillus Calmette-Guérin (BCG) has demonstrated variable efficacy against TB in humans and cattle 3. More recently, the application of alternative vaccination strategies, such as neonatal vaccination 4, and heterologousprime-boost vaccination 5, has significantlyimproved the efficacy of BCG in cattle. However, control of bTB using the BCG vaccine has not been regarded as a suitable option because it can interfere with the tuberculin skin test. Therefore, a vaccine devoid of this shortfall is urgently needed.
BCG vaccination of calves elicits a T helper (Th) 1-biased immune response 6, which is induced mainly by T cell antigen-specific interferon (IFN)-γ secretion 4. In cattle infected with M. bovis, the γδ workshop cluster (WC)1+,the cluster of differentiation (CD) 4+ and the CD8+ T cell subpopulations have been identified as producers of IFN-γ 7. Moreover, changes in the percentages and in the activation levels (CD25+) of these T cell subpopulations were demonstrated in the peripheral blood of experimentally infected and BCG-vaccinated cattle, indicating their involvement in anti-mycobacterial immune response 8. However, under field conditions, the response to M. bovis BCG vaccination is not well understood, as most of these previous experiments were conducted under controlled conditions. Few studies have been performed in a more natural cattle-to-cattle transmission setting, which could significantly influence the results found in controlled experiments.
Any investigation of immunity to an infection or vaccine should take into account the responses that develop under natural conditions. Therefore, the aim of this study was to assess the immune responses induced under field conditions in neonatal calves by vaccination with BCG Phipps, a strain that is not well known as an immunogen, using the tuberculin and IFN-γ tests, enzyme-linked immunosorbent assay (ELISA) and flow cytometry, to provide information for the development of novel vaccines and vaccination strategies.
Materials and Methods
Ethics Statement
Animals used in this study belong to a commercial farm and have been submitted only to the standard clinical practices specifically regulated by the Mexican legislation on TB control program 9, plus vaccination and blood sampling.
Animals and Experimental Design
Holstein-Friesian female calves clinically healthy (between 9-46 days of age, mean age 32 days) were employed in the experiment. The neonatal animals were obtained from a herd with a recent history of bTB and a prevalence of 40 % at the beginning of the study, as determined using the single intradermal comparative tuberculin test (SICTT). The calves (n = 10) provided negative results for the IFN-γ test and antibody levels measured by ELISA (to both avian and bovine antigens). Five calves were chosen randomly and vaccinated with BCG Phipps, and five calves were injected with phosphate-buffered saline (PBS) solution. Then, kinetics of the IFN-γ and antibody responses and changes in T cell subpopulations and cellular activation were evaluated. The SICTT was performed 12 weeks post-vaccination. The experiments were conducted in field conditions and the animals were not segregated from other calves and cattle during the study.
Vaccination
The animals were injected subcutaneously in the right side of the neck with a 21 g x 38 mm needle containing a 1.0 ml inoculum of 106 colony forming unit (CFU) of BCG Phipps (lot number 001-2013-7H9; kindly gifted by Dr. Clara I. Espitia Pinzón, Instituto de Investigaciones Biomédicas, UNAM, Mexico City, Mexico). This strain was selected because it induced the best protection against challenge in a mouse model where 10 BCG different strains were compared 10. Briefly, bacteria were grown to mid log phase in 7H9 medium (Difco; BD, Oxford, UK) supplemented with 0.05 % Tween 80 (Sigma-Aldrich, Poole, United Kingdom) and ADC (albumin-dextrose-catalase) (BD Diagnostics, Loveton Circle Sparks, USA). Vaccine was then adjusted to contain 106 bacteria per ml and stored in glycerol at -80 ºC until use.
Production and Measurement of IFN-γ
The bovine IFN-γ test was used for two purposes. First, to select animals that were negative for M. avium and M. bovis antigens. Second, to monitor the IFN-γ responses following vaccination with BCG. Briefly, heparinized blood (1.5 ml) was dispensed into three wells of 24-well plates (Nunclon, Roskilde, Denmark), and either avian or bovine purified protein derivative (PPD), both at 20 µg/ml, or PBS was added. The cultures were incubated for 24 h at 37 °C, and the IFN-γ levels in the plasma supernatants were measured using a sandwich ELISA (kit Bovigam®, Prionics AG, Schlieren-Zurich, Switzerland) as described previously 11. The results were interpreted according to the criteria described in the commercial kit instructions.
Detection of IgG Antibodies in Serum
Serological responses were assessed by ELISA, as described previously 12, and were used for two purposes: to select animals that were negative for M. avium and M. bovis antigens and to monitor the antibody responses following BCG vaccination. Briefly, the plates (Nunclon, Roskilde, Denmark) were coated overnight at room temperature with 1 µg/well of culture filtrate protein extract (CFPE) from M. avium or M. bovis in 0.1 M carbonate buffer and blocked with skim milk powder (3 % in PBS) for 1 h at 37 °C. Subsequently, the plates were incubated with sera (1:100) and then with peroxidase-labeled G protein (1:10,000; Sigma–Aldrich, St. Louis, MO, USA) both during 1 h at 37 °C. The chromogenic substrate, 0.04% o-phenylenediamine (Sigma–Aldrich, St. Louis, MO, USA) and hydrogen peroxide (0.04% in citrate buffer) were added, and the plates were incubated at 37 °C for 5 min. The reaction was stopped with 2 M sulfuric acid solution and the optical density (OD) values were read at 492 nm in a spectrophotometer (Benchmark Plus Microplate Spectrophotometer, Bio-Rad Laboratories, Hercules CA, USA).
An analysis of the receiver operator characteristic curve for the OD492nm cut-off point was performed from SICTT data using Win Episcope 2.0 software (Learning Technology Section, College of Medicine & Veterinary Medicine, The University of Edinburgh, Edinburgh, Scotland, UK). The results showed an OD492nm cut-off point of 0.370. A serum containing high levels of antibody from a cow with disseminated lesions of bTB was used as a positive control and a negative control was obtained from a cow on a farm without a recent history of bTB; the cow tested negative for SICTT, IFN-γ and polymerase chain reaction.
Flow Cytometry
The lymphocyte subpopulations were evaluated by flow cytometry as described previously 13. Heparinized blood was obtained from animals, and the red blood cells were lysed. To evaluate cellular activation, blood samples were previously incubated for 24 h at 37°C with either M. bovis CFPE (7 μg/ml) or PBS 14. Aliquots of 5 × 105 cells suspended in PBS containing 0.2% bovine serum albumin and 0.2% sodium azide (cell staining buffer) were incubated for 20 min at room temperature using mouse monoclonal antibodies (mAbs) to bovine WC1-N3 (clone CACTB32A, isotype IgG1), CD4 (clone CACT138A, isotype IgG1), CD8 (clone BAT82A, isotype IgG1), and CD25 (clone CACT108, isotype IgG2a), all obtained from VMRD Inc. (Pullman, WA, USA). Then, the cells were washed twice with cell staining buffer, and stained either with a fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgG1 or a phycoerythrin (PE)-conjugated anti-mouse IgG2a antibody (Jackson ImmunoResearch Laboratories, Inc., West Baltimore Pike, PA, USA) for 20 min at room temperature in the dark. After incubation, the cells were washed twice, suspended in FACSflow solution, and analyzed on a FACSCalibur flow cytometer equipped with 488-nm argon-ion laser and the CellQuest software (BD Biosciences, San José, CA, USA). To analyze the staining of cell surface markers, 10 000 events were acquired and the lymphoid cells were gated by their physical properties (size and internal complexity). After that, a second gate was done based on immunofluorescent characteristics of the gated cells, evaluating fluorescence by quadrant graphics in logarithmic scale. FITC-positive, and PE-positive cells were observed by FL1 channel (filter 530/30 SP) and FL2 channel (filter 585/42 BP), respectively. To established differences of the stained and unlabeled cell populations, the compensation was adjustment as follow: FL1 0.8% FL2 and FL2 24.3 % FL1. To analyze cell staining, the quadrants for positive and negative fluorescence of gated cells were established manually based on the distribution of cells stained with secondary antibodies alone. Data were analyzed using FlowJo 7.6.1 software (Tree Star Inc., Ashland, OR, USA).
Single Intradermal Comparative Tuberculin Test
The SICTT was conducted by intradermal injection of the cattle with 0.1 ml (1 mg/ml) of bovine PPD and 0.1 ml (0.5 mg/ml) of avian PPD (PRONABIVE, Mexico City, Mexico). The skin thickness was measured before the intradermal injection and at 72 h post-injection. The results were recorded as the increase in skin thickness at 72 h compared to the thickness pre-injection. Values of both, avian and bovine PPD were plotted and the intersection point gave the result according to the official graphic of the Mexican Official Norm 9.
Statistical Analysis
Significant differences between groups for all the tests conducted in this study were determined using student t-tests with JMP 5.0.1 software for Windows (SAS Institute Inc., Cary, NC, USA). A probability of P < 0.05 was considered significant.
Results
IFN-γ Production
The production of IFN-γ prior to and at intervals ranging from 1 to 12 weeks post-vaccination was measured in antigen-stimulated blood cultures Table 1. No animal from either group showed an IFN-γ response to avian or bovine PPD prior to the beginning of the study (weeks -2 and 0). Significant increases in PPD-specific IFN-γ production in response to vaccination were evident at 4 weeks for avian PPD (0.961 ± 0.535 vs. 0.114 ± 0.126, P= 0.0087) and 3 (0.638 ± 0.365 vs. 0.134 ± 0.141, P = 0.0239) and 4 (0.987 ± 0.611 vs. 0.180 ± 0.197, P = 0.0229) weeks for bovine PPD. By week 8, the cytokine levels had waned significantly compared to week 4. No significant increase was detected in the control group. The individual avian and bovine PPD-specific IFN-γ OD values varied widely among vaccinated animals at week 4 post-vaccination Figure 1 compared to the control group Figure 2.
Table 1. Levels of IFN-γ detected in PPD-stimulated whole blood cultures of BCG Phipps-vaccinated and control calves.| Weeks post-vaccination | Vaccinated group a | Control group a | |||
| Avian PPD | Bovine PPD | Avian PPD | Bovine PPD | ||
| -2 | 0.039 ± 0.041 | 0.034 ± 0.060 | 0.006 ± 0.009 | 0.001 ± 0.002 | |
| 0 | 0.064 ± 0.054 | 0.041 ± 0.054 | 0.057 ± 0.086 | 0.084 ± 0.026 | |
| 1 | 0.428 ± 0.387 | 0.190 ± 0.183 | 0.203 ± 0.201 | 0.104 ± 0.093 | |
| 2 | 0.733 ± 0.881 | 0.407 ± 0.405 | 0.145 ± 0.165 | 0.030 ± 0.019 | |
| 3 | 0.471 ± 0.311 | 0.638 ± 0.365b | 0.130 ± 0.123 | 0.134 ± 0.141 | |
| 4 | 0.961 ± 0.535b | 0.987 ± 0.611b | 0.114 ± 0.126 | 0.180 ± 0.197 | |
| 8 | 0.322 ± 0.339 | 0.381 ± 0.349 | 0.150 ± 0.257 | 0.077 ± 0.121 | |
| 12 | 0.023 ± 0.022 | 0.048 ± 0.026 | 0.011 ± 0.012 | 0.015 ± 0.021 | |
Figure 1.Kinetics of IFN-γ production in avian (a) and bovine (b) PPD-stimulated and non-stimulate (c) whole blood cultures from individual calves vaccinated with BCG Phipps.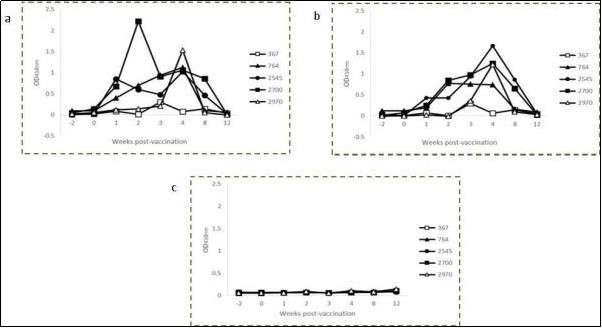
Figure 2.Kinetics of IFN-γ production in avian (a) and bovine (b) PPD-stimulated and non-stimulated (c) whole blood cultures from individual control calves.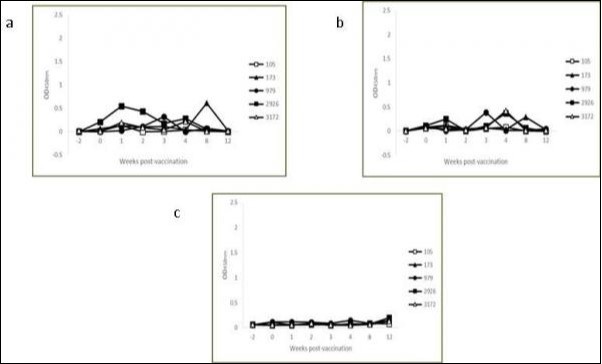
IgG Antibody Levels
An ELISA utilizing M. avium or M. bovis CFPE as the capture antigen was used to measure the IgG antibody levels in serum prior to and at intervals ranging from 1 to 12 weeks post-vaccination. No significant differences were observed at any time point between the groups (data not shown).
T Cell Subpopulations
The T cell subpopulations (WC1+, CD4+ and CD8+) were investigated using flow cytometry from 1 to 12 weeks post-vaccination Figure 3. No differences were observed in the percentages of WC1+ and CD4+ T cells. Nevertheless, there was a significant increase in CD8+ T cells of the vaccinated group at weeks 2 (21.09 ± 4.05 % vs. 12.89 ± 3.74 %, P = 0.0105), 3 (22.94 ± 2.32 % vs. 14.89 ± 3.97 %, P = 0.0047) and 4 (24.21 ± 4.37 % vs. 14.95 ± 4.32 %, P = 0.0098) post-vaccination. By week 8, these percentages had decreased, although the difference was still significant compared to the control group (19.59 ± 2.20 % vs. 14.87 ± 2.16 %, P = 0.0091). CD8+ T cells of the vaccinated group were increased 1.6-, 1.5- and 1.6-fold at weeks 2, 3 and 4 respectively.
Figure 3.Kinetics of WC1+ (a), CD4+ (b) and CD8+ (c) T cells in the peripheral blood of BCG Phipps-vaccinated and control calves. Each square represents the group mean ± SD of the group. * Statistically significant difference (P < 0.05).
T Cell Activation Levels
Blood samples from both groups of animals were stimulated with PBS or M. bovisCFPE for 24 h, and the T cell activation (CD25+) was evaluated from 1 to 12 weeks post-vaccination. Both groups showed similar activation levels with little variation between weeks post-vaccination in the cultures without antigen stimulation (PBS). The mean percentages during the study were 63.29 ± 12.16 %, 11.92 ± 3.10 % and 5.06 ± 2.60 % for WC1+, CD4+ and CD8+ T cells, respectively (data not shown). In relation to the cultures stimulated with M. bovisantigens, no significant differences were observed in the activation of WC1+ T cells Figure 4a. Very high percentages were detected in both groups during the entire experiment, with almost all of the cells showing an activated phenotype. However, the levels of activation were significantly higher in the vaccinated group at weeks 3 and 4 for CD4+ T cells (17.95 ± 5.71 % vs. 10.55 ± 2.20 %, P = 0.0308 and 28.18 ± 8.03 % vs. 14.55 ± 3.71 %, P = 0.0113, respectively) and CD8+ T cells (7.09 ± 1.77 % vs. 3.08 ± 0.87 %, P = 0.0019 and 8.70 ± 2.46 % vs. 4.88 ± 1.27 %, P = 0.0149, respectively) in response to M. bovis CFPE (Figure 4b, c). Levels of activation were 1.7- and 1.9-fold higher for CD4+ T cells and 2.3- and 1.8-fold higher for CD8+ T cells in the vaccinated group at weeks 3 and 4 respectively in response to M. bovis antigens. Examples of individual data of flow cytometry evaluating T cells activation in whole blood stimulated with a CFPE of M. bovis from a vaccinated animal are shown in Figure 5.
Figure 4.Kinetics of WC1+ (a), CD4+ (b) and CD8+ (c) activated (CD25⁺) T cells in M. bovis CFPE-stimulated peripheral blood of BCG Phipps-vaccinated and control calves. Each square represents the group mean ± SD of the group. * Statistically significant difference (P < 0.05).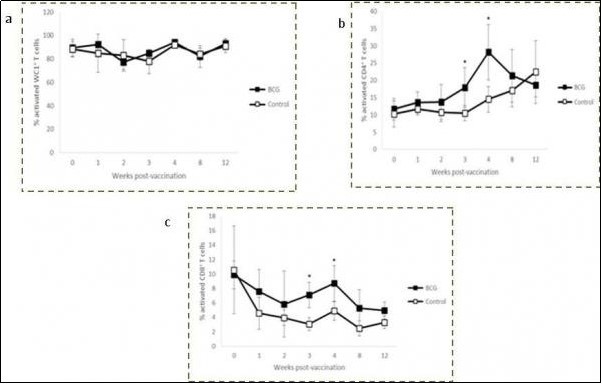
Figure 5.Representative graphs of flow cytometry evaluating the activation cellular marker CD25+ on different T cell subpopulations (WC1+ (a), CD4+ (b) and CD8+ (c)) in whole blood from one animal vaccinated with BCG after 24 h of culture with a CFPE from M. bovis.
SICCT
The SICCT skin test was conducted at 12 weeks post-vaccination in all calves Table 2. No positive reactions were observed in any of the vaccinated or control animals.
Table 2. SICTT skin test responses to PPD from BCG Phipps-vaccinated and control calves at 12 weeks post-vaccination.| Groups | SICTT (mm) ª | |
|---|---|---|
| Avian PPD | Bovine PPD | |
| BCG | ||
| 367 | 0 | 0 |
| 764 | 0 | 1 |
| 2545 | 1 | 1 |
| 2700 | 0 | 0 |
| 2970 | 0 | 0 |
| Control | ||
| 105 | 0 | 0 |
| 173 | 0 | 0 |
| 979 | 1 | 1 |
| 2926 | 0 | 0 |
| 3172 | 0 | 0 |
Discussion
In the present study, the immune responses induced in calves by vaccination with BCG Phipps, a strain that has rarely been studied in humans, mice and bovines, was evaluated. To avoid any interference with environmental mycobacteria in the development of BCG-induced immunity, we vaccinated the calves neonatally. Additionally, the study was performed on a farm managed under routine husbandry conditions, which allowed the evaluation of the immune responses induced by the vaccine, including all the environmental factors present in a dairy herd setting.
BCG vaccination of neonate calves is associated with a Th1-type immune response, which is important for the induction of protection 4. IFN-γ is an essential component of protective immune response to M. bovis, and vaccines that fail to prime this recall memory response invariably fail to protect against virulent challenge 15. In our experiment, the protection induced by the vaccine could not be assessed through challenge/slaughter strategy due to establishment constraints and may be investigated in future studies. However, the IFN-γ production was evaluated as surrogate marker of immunogenicity. It has been reported that IFN-γ levels increase by 2-4 weeks post-vaccination and then decrease after week 5 4, which is similar to our results. Indeed, we observed significant increases at week 4 to avian PPD and at weeks 3 and 4 to bovine PPD, with a decrease at week 8. The induction of IFN-γ to both PPD after vaccination with BCG has been observed with Pasteur strain as well 16. Therefore, vaccination stimulated the production of a surrogate marker of protection.
Interestingly, an experience demonstrated that not all animals were protected to the same extent after vaccination with BCG. In the study, they divided the animals into protected and not-protected by considering all BCG vaccinates with CFU counts lower than the animal presenting the lowest CFU counts in the non-vaccinated group as protected; all other BCG vaccinates could be considered as not protected. Using this criterion, 6/12 animals would have been protected by BCG vaccination after 3 weeks 17. In the present work, the individual avian and bovine IFN-γ OD values varied widely among vaccinated animals. We observed that not all animals developed an IFN-γ recall response post-vaccination. Consequently, we suggest that these calves were probably not protected by the vaccine, however, more investigations are needed to support these results.
There is little evidence that the induction of antibodies post-vaccination plays an important role in bTB resistance 18. Moreover, it has been reported that it may contribute to the dissemination of extra-thoracic disease in calves. In our study, vaccination of calves with BCG Phipps did not induce an antibody response to mycobacterial antigens early after vaccination, as previously reported 19.
Few data exist regarding the changes in circulating T cells after vaccination of cattle with BCG. A study detected three phases in BCG Danish-vaccinated cattle that were similar to those observed in infected animals 8. The first phase was observed two weeks post-vaccination and showed an increase in γδ WC1+ T cells. The second and third phases showed increases in CD4+ and CD8+ T cells 4-6 and 8-10 weeks after vaccination, respectively. Other studies performed in BCG-vaccinated calves demonstrated that the animals developed a cellular-mediated immunity (CMI) response; however, no differences in peripheral T cell subpopulations were observed 20. In the present study, no significant changes were observed in γδ WC1+ and CD4+ T cells, while a significant increase was detected in CD8+ T cells 2-8 weeks post-vaccination. Antigenic presentation of intracellular bacteria is achieved mainly through major histocompatibility complex (MHC)-I molecules, leading to the activation of CD8+ T cells 21. The increase in CD8+ T cells observed in this study could indicate that BCG antigens induced the MHC-I pathway, and the activation and proliferation of these cells, leading to a CMI-biased immune response.
CD25 expression was evaluated in T cell subpopulations. As previously observed 22, no differences were detected between groups in unstimulated blood (data not shown). However, in blood stimulated with mycobacterial antigens, increased activation levels of T cell subpopulations have been reported in M. bovis-infected and BCG-vaccinated animals 23. In our experiment, antigen stimulation led to pronounced activation of γδ WC1+ T cells in both groups during all the study, which prevented the observation of any effect of vaccination. These high levels of cellular activation have been observed in tuberculous and BCG-vaccinated cattle 24, however, lower levels were observed in control animals 14. This potent activation may have been a consequence of cross-reactivity between PPD and environmental mycobacteria antigens. It has been demonstrated that γδ T cells can be primed in response to mycobacterial antigens, resulting in more rapid and robust responses in vitro, without the need for prior selection and expansion 25. Moreover, CD25 expression on γδ WC1+ T cells is not dependent on exposure to exogenous antigen, although it is further up-regulated following stimulation of the cells. This is in contrast with α/β T cells, on which CD25 is only expressed as a transient event following antigenic or mitogenic stimulation 26. It has been demonstrated, in bovine γδ WC1+ T cells that had not been exposed to Theileriaannulata, an up-regulation of CD25 on the majority of these cells after stimulation with parasite antigens, indicating that there must be a high frequency of precursors capable of responding 27. In addition, the high proportion of γδ WC1+/CD25+ T cells observed in unstimulated blood showed that these cells present high levels of activation even without stimulation. Otherwise, vaccination significantly increased the expression of CD25 among CD4+ and CD8+ T cells by weeks 3 and 4, indicating that both subpopulations were activated simultaneously and play a role in immunity induced by BCG Phipps.
The tuberculin skin test remains the immunodiagnostic tool most commonly applied for detecting bTB 28. However, BCG-vaccinated cattle can react positively to the test, invalidating the diagnosis 29. In the present study, the SICTT was performed at 12 weeks post-vaccination, and no animals, vaccinated or control, tested positive, demonstrating that BCG vaccination does not always interfere with the tuberculin test. The absence of a tuberculin response could be due to the strain used (Phipps), as the majority of studies in which reactivity was observed were performed using the Danish or Pasteur strain of BCG 18, 28. It has been shown that the genetic diversity of different BCG strains influences the expression of genes, immunogenicity, virulence and viability, which may affect the animals’ immune response to the tuberculin test 30, 31, 32, 33.
Few studies have evaluated the BCG Phipps strain. In humans, a study showed that the BCG Phipps strain had the lowest level of vaccine efficacy compared to BCG Pasteur, demonstrating differences in protection 34. Another study conducted in mice with 10 different strains of BCG also demonstrated differences in protection 10. Moreover, each strain showed a particular Th1 and Th2 response profile, exhibiting differences in immunogenicity. BCG Phipps induced the highest protection level and moderate delayed hypersensitivity responses post-challenge. In cattle, a study demonstrated that BCG Phipps induced significant efficacy against M. bovis 35. Results from that study showed that 9/10 vaccinated animals reacted to the tuberculin test; however, this test was performed on 5- to 6-month-old animals, and the study did not mention which tuberculin test was applied. In our study, the lack of reaction to the SICTT could demonstrate that the Phipps strain induced an immune profile different from the Pasteur and Danish strains in neonatal calves.
The reason why the vaccine did not induce a delayed hypersensitivity reaction is not known. However, the vaccine induced a specific IFN-γ production in vitro, demonstrating two separate responses. The dissociation of these responses post-vaccination has been described in newborns 36. Moreover, in neonatal cattle vaccinated with BCG, it has been shown that positive IFN-γ responses are observed in both tuberculin-positive and -negative animals 28. It remains unclear whether the independence of these responses is due to greater sensitivity of the IFN-γ assay or a specific subpopulation of T cells present in the blood only. In a TB mouse model, it has been demonstrated that the T cell subpopulations involved in the delayed hypersensitivity reaction differ markedly from those participating in protection 37, 38. Therefore, the mechanisms involved in these responses remain an interesting topic for future research.
Conclusion
The results from this study provide information on the immune responses induced in neonatal calves under field conditions following vaccination with BCG Phipps, a strain rarely evaluated in the bovine population. The vaccination stimulated peripheral blood T cell activity and induced a CMI response that was detectable 3-4 weeks post-vaccination, characterized by specific IFN-γ production, increases in the percentages of CD8+ T cells and activation levels of CD4+ and CD8+ T cells, indicating their role in BCG-induced immunity. Moreover, an important finding was that the BCG Phipps vaccine did not interfere with the SICTT performed 12 weeks post-vaccination, offering an attractive tool for bTB control programs. Greater understanding of the immune responses associated with vaccination against M. bovis in cattle will help in the development of strategies for prevention of the disease, and further studies are required to evaluate the protective efficacy of this BCG strain, especially in a natural transmission setting.
Author Contributions
Eve-Lyne Quevillon: PhD student (This study was part of her PhD research work).
Fernando Díaz-Otero: As the project manager, he realized the experimental design and found the financial support for the project.
Laura Jaramillo-Meza: She contributed in the planning of the project and in the realization of laboratory activities.
José A. Gutiérrez-Pabello: He participated in the planning of the project and in the analysis of the results.
Ricardo Lascurain: He provided the flow cytometer and participated in revising the manuscript.
Camila Arriaga: She participated in the planning of the project.
Felipe A. Castañeda and Xochitl E. González: As veterinarians, they were involved in the sampling of animals and in the realization of laboratory techniques.
Funding Source
This work was support by theInstituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias, grant number 142 945 340 13.
Acknowledgements
We thank the farmers who kindly provided the animals for this study and the Instituto Nacional de Enfermedades Respiratorias that allowed us to use the cytometer.
References
- 1.Buddle B M. (2010) Tuberculosis vaccines for cattle: the way forward. , Expert. Rev. Vaccines 9, 1121-1124.
- 2.N A Parlane, Shu D, Subharat S, D N Wedlock, B H Rehm et al. (2014) Revaccination of cattle with Bacille Calmette-Guérin two years after first vaccination when immunity has waned, boosted protection against challenge withMycobacteriumbovis. PLoS One.;9(9):e106519
- 3.Buddle B M, Wards B J, Aldwell F E, Collins D M, Lisle G W de. (2002) Influence of sensitisation to environmental mycobacteria on subsequent vaccination against bovine tuberculosis. , Vaccine 20, 1126-1133.
- 4.J C Hope, M L Thom, McAulay M, Mead E, H M Vordermeier et al. (2011) Identification of surrogates and correlates of protection in protective immunity againstMycobacteriumbovisinfection induced in neonatal calves by vaccination withM.bovisBCG Pasteur andM.bovisBCG Danish. , Clin. Vaccine Immunol 18, 373-379.
- 5.H M Vordermeier, Villarreal-Ramos B, P J Cockle, McAulay M, S G Rhodes et al. (2009) Viral booster vaccines improveMycobacteriumbovisBCG-induced protection against bovine tuberculosis. , Infect. Immun 77, 3364-3373.
- 6.S H Kaufmann. (2006) Envisioning futures strategies for vaccination against tuberculosis. , Nat. Rev. Immunol 6, 699-704.
- 7.H E Kennedy, Welsh Bryson, D G Cassidy, J P Forster, F I Howard et al. (2002) Modulation of immune responses toMycobacteriumbovisin cattle depleted of WC1+ γδ T cells. , Infect. Immun 70, 1488-1500.
- 8.Buza J, Kiros T, Zerihun A, Abraham I, Ameni G. (2009) Vaccination of calves withMycobacteriumbovisBacilli Calmete Guerin BCG induced rapid increase in the proportion of peripheral blood gamma delta T cells. , Vet. Immunol. Immunopathol 130, 251-255.
- 9.NormaOficial Mexicana NOM-031-ZOO-1995. 27 de agosto1998.Campaña Nacional Contra la Tuberculosis Bovina (Mycobacteriumbovis). Secretaria de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación. Servicio Nacional de Sanidad, Inocuidad y Calidad Agro alimentaria. http://www.gob.mx/senasica/documentos/norma-oficial-mexicana-nom-031-zoo-1995-campana-nacional-contra-la-tuberculosis-bovina-Mycobacterium-bovis,September26,2016
- 10.A I Castillo-Rodal, A M Castañón, P R Hernández, J, D E Sada et al. (2006) substrains confer different levels of protection againstMycobacterium tuberculosisinfection in a BALB/c model of progressive pulmonary tuberculosis. , Infec. Immun 74, 1718-1724.
- 11.J S Rothel, S L Jones, L A Corner, J C Cox, P R Wood. (1990) A sandwich enzyme immunoassay for bovine interferon-γ and its use for the detection of tuberculosis in cattle. , Aust. Vet. J 67, 134-137.
- 12.Estrada-Chávez C, Mancilla R, D C Arriaga, G R Pérez, O F Díaz. (2001) Determinación de anticuerpos anti-PPD en hatos lecheros con distintas prevalencias de tuberculosis bovina en México. , Vet. Mex 32, 207-211.
- 13.Sopp P, C J Howard, J C Hope. (2006) Flow cytometric detection of gamma interferon can effectively discriminateMycobacteriumbovisBCG-vaccinated cattle from M. bovis-infected cattle. , Clin. Vaccine Immunol 13, 1343-1348.
- 14.A J Smyth, Welsh Girvin, R M Pollock, M J. (2001) In vitro responsiveness of γδ T cells from Mycobacterium bovis-infected cattle to mycobacterial antigens: predominant involvement of WC1+ cells. , Infect. Immunol 69, 89-96.
- 15.P J Hogarth, R G Hewinson, H M Vordermeier. (2006) Development of vaccines against bovine tuberculosis. , J. Pharm. Pharmacol 58, 749-757.
- 16.M L Thom, McAulay M, H M Vordermeier, Clifford D, R G Hewinson et al. (2012) Duration of immunity againstMycobacteriumbovisfollowing neonatal vaccination with bacillus Calmette-Guérin Danish: significant protection against infection at 12, but not 24, months. , Clin. Vaccine Immunol 19, 1254-1260.
- 17.Villarreal-Ramos B, Berga S, Chamberlaina L, McShane H, R G Hewinson et al. (2014) Development of a BCG challenge model for the testing of vaccine candidates against tuberculosis in cattle. , Vaccine 32, 5645-5649.
- 18.Ameni G, H M Vordermeier, Aseffa A, D B Young, R G Hewinson. (2010) Field evaluation of the efficacy ofMycobacteriumbovisbacillus Calmette–Guerin against bovine tuberculosis in neonatal calves in Ethiopia. , Clin. Vaccine Immunol 17, 1533-1538.
- 19.D N Wedlock, Bridget V, M A Skinner, Lisle G W de, I M Orme et al. (2000) Vaccination of cattle withMycobacteriumbovisculture filtrate proteins and interleukin-2 for protection against bovine tuberculosis. , Infec. Immun 68, 5809-5815.
- 20.Rizzi C, M V Bianco, F C Blanco, Soria M, M J Gravisaco et al. (2012) Vaccination with a BCG strain overexpressing Ag85B protects cattle againstMycobacteriumbovischallenge. doi: 10.1371/journal.pone.0051396. PLoS. , One 7, 51396.
- 21.Liébana E, R M Girvin, Welsh Neill, S D Pollock, M J. (1999) Generation of CD8+ T-cell responses toMycobacteriumbovisand mycobacterial antigen in experimental bovine tuberculosis. , Infect. Immun 67, 1034-1044.
- 22.B J Nonnecke, W R, M R Foote, M V Palmer, B L Miller et al. (2005) Development of an adult-like cell-mediated immune response in calves after early vaccination withMycobacteriumbovisbacillus Calmette-Guérin. , J. Dairy Sci 88, 195-210.
- 23.Maue A C, Waters W R, Davis W C, Palmer M V, Minion F C et al. (2005) Analysis of immune responses directed toward a recombinant early secretory antigenic target six-kilodalton protein-culture filtrate protein 10 fusion protein inMycobacteriumbovis-infected cattle. , Infec. Immun 73, 6659-6667.
- 24.Welsh M D, Kennedy H E, Smyth A J, Girvin R M, Andersen P et al. (2002) Responses of bovine WC1+γδ T cells to protein and nonprotein antigens of Mycobacterium bovis. , Infect. Immun 70, 6114-6120.
- 25.M A Jutila, Holderness J, J C Graff, J F Hedges. (2008) Antigen-independent priming: a transitional response of bovine gamma delta T-cells to infection. , Anim. Health Res. Rev 9, 47-57.
- 26.D A Cantrell, K A Smith. (1983) Transient expression of interleukin 2 receptors. Consequences for T cell growth. , J. Exp. Med 158, 1895-1911.
- 27.R A Collins, Sopp P, K I Gelder, W I Morrison, C J Howard. (1996) Bovine γδ TcR1 T lymphocytes are stimulated to proliferate by autologous Theileria annulata-infected cells in the presence of interleukin-2. , Scand. J. Immunol 44, 444-452.
- 28.A O Whelan, Coadm M, B L Upadhyay, D J Clifford, R G Hewinson et al. (2011) Lack of correlation between BCG-induced tuberculin skin test sensitization and protective immunity in cattle. , Vaccine 29, 5453-5458.
- 29.Buddle B M, GW de Lisle, Pfeffer A, Aldwell F E. (1995) Immunological responses and protection againstMycobacteriumbovisin calves vaccinated with a low dose of BCG. , Vaccine 13, 1123-1130.
- 30.Brosch R, S V Gordon, Garnier T, Eiglmeier K, Frigui W et al. (2007) Genome plasticity of BCG and impact on vaccine efficacy. , Proc. Natl. Acad. Sci. USA 104, 5596-5601.
- 31.Davids V, W A Hanekom, Mansoor N, Gamieldien H, S J Gelderbloem et al. (2006) The effect of Bacille Calmette–Guerin vaccine strain and route of administration on induced immune responses in vaccinated infants. , J. Infect. Dis 193, 531-536.
- 32.Kröger L, Korppi M, Brander E, Kröger H, Wasz-Höckert O et al. (1995) Osteitis caused by bacille Calmette–Guerin vaccination: a retrospective analysis of 222 cases. , J. Infect. Dis 172, 574-576.
- 33.Gheorghiu M, P H Lagrange. (1983) heat stability and immunogenicity of four BCG vaccines prepared from four different BCG strains. , Ann. Immunol. Paris 134, 125-147.
- 34.N E Aronson, Santosham M, G W Comstock, R S Howard, L H Moulton et al. (2004) Long-term efficacy of BCG vaccine in American Indians and Alaska Natives: a 60-year follow-up study. , JAMA 291, 2086-2091.
- 35.Nava Vargas A, Olvera Ramírez AM, Milian Suazo F, Canto Alarcon GJ, Rubio Venegas Y et al. (2013) Efficacy of a vaccine formula against tuberculosis in cattle. doi: 10.1371/journal.pone.0076418. PLoS One 8:e76418.
- 36.M O Ota, Goetghebuer T, Vekemans J, B J Okoko, M J Newport et al. (2006) Dissociation between tuberculin skin test and in vitro IFN-gamma responses following neonatal BCG vaccination. , J. Trop. Pediatr 52, 136-140.
Cited by (1)
- 1.Sirak Asegedech, Tulu Begna, Bayissa Berecha, Gumi Balako, Berg Stefan, et al, 2021, Cellular and Cytokine Responses in Lymph Node Granulomas of Bacillus Calmette Guérin (BCG)-Vaccinated and Non-vaccinated Cross-Breed Calves Naturally Infected With Mycobacterium bovis, Frontiers in Veterinary Science, 8(), 10.3389/fvets.2021.698800
