Abstract
The objective of our study was to evaluate the analgesic and anti-inflammatory activity, as well as possible organ toxicity of 2-3-3-methyl-pentanoic acid (compound 3d), a newly synthesized pyrrolic derivative, structurally similar to Celecoxib. Antinociception was assessed using animal pain models with thermal and chemical stimuli (paw withdrawal, tail-flick and formalin test). Anti-inflammatory activity was measured using the carrageenan-induced paw edema model. Blood samples were collected from the animals to study possible organ toxicity. All experiments were performed on male Wistar rats. The results in our study show that in experimental conditions 2-3-3-methyl-pentanoic acid has analgesic action against thermal and chemical stimuli. This effect is registered after both single and multiple administration of the compound. In the carrageenan model after single administration compound 3d did not inhibit formation of paw edema. After multiple administration all doses of compound 3d significantly suppressed paw edema at second, third and fourth hours. Hematological tests showed that compound 3d did not affect red blood cells and platelets but decreased white blood cell levels and the highest used dose decreased hemoglobin as well. Compound 3d decreased blood sugar levels and liver transaminases, compared to the control. Compound 3d did not affect creatinine levels but the smallest dose used lowered blood urea. We concluded antinociception in the tested compound is most likely mediated by supraspinal, spinal and peripheral mechanisms. Possible tolerance develops towards the analgesic action on spinal level after continuous administration. Anti-inflammatory activity, though significant, is probably not the leading cause for antinociception.
Author Contributions
Academic Editor: Luis Ulloa, Associate Professor, Department of Surgery, Rutgers New Jersey Medical School
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2017 Hristina Zlatanova, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction:
Non-steroidal anti-inflammatory drugs (NSAIDs) are widely prescribed medications, also available as over-the-counter products.1, 2 They are used in the treatment of both acute and chronic pain and inflammation.3 Unfortunately NSAIDs possess many adverse drug reactions (ADRs) – including bleeding from the upper gastrointestinal tract (GIT), damage to the liver and kidneys, allergies.4, 5, 6, 7 Selective cyclooxygenase-2 (COX-2) inhibitors were developed in the hope that aforementioned ADRs will be less severe and clinical studies do show reduced percentage of GIT damage in patients, undergoing treatment with coxibs.8 However, COX-2 inhibitors show various other ADRs, amongst which pro-thrombotic state and cardiotoxicity are the most serious.9, 10
The search for new analgesic and anti-inflammatory drugs with improved drug tolerability and safety profile is a necessity. A possible approach to the design and synthesis of novel drug molecules is based on using the chemical structure of an already existing medication as a prototype.3
The pyrrolic core is chosen due to its relatively low toxicity.11, 12 The pyrrole heterocycle is part of the chemical structure of various drugs with different activities (eg. antimicrobial, antifungal, antiretroviral, inhibitors of dipeptidyl peptidase 4).13, 14, 15, 16 There are several existing NSAIDs with a pyrrole heterocycle in their structure and recent studies have confirmed its role for the anti-inflammatory activity of drugs.17, 18, 19
The newly synthesized derivative discussed in the present study (2-(3-Acetyl-5-(4-chloro-phenyl)-2-methyl-pyrrol-1-yl)-3-methyl-pentanoic acid - compound 3d) is a derivative of N-pyrrolylcarboxylic acid (Figure 1) and its structure is oriented to that of the modern drug Celecoxib (a selective COX-2 inhibitor from the group of coxibs). The chemistry, design, synthesis and characterization by spectroscopy and thin layer chromatography (TLC) are described by Vladimirova et al in “An access to new N-pyrrolylcarboxylic acids as potential COX-2 inhibitors via Paal-Knorr cyclization”.20
Figure 1. Structure of compound 3d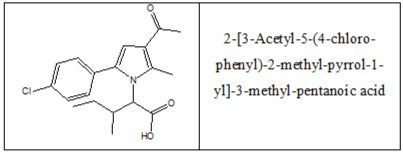
The aims of this study are to evaluate the antinociceptive and anti-inflammatory properties of (compound 3d) after single and multiple (14 days) administration using animal models of pain and inflammation and to assess possible organ toxicity of the substance after multiple (14 days) treatment.
Materials and Methods
The experiment was approved by the Ethics Committee on Animals of the Bulgarian Food Safety Agency with permit № 128/09.12.2015 and by decision of the Ethics Committee at the Medical University of Plovdiv, protocol № 2/31.03.2016.
Experimental Animals.
All experiments are performed on 6-weeks old male Wistar rats (n=48) weighing 150±20 grams, randomly divided in 6 parallel experimental groups as described in Table 1. The animals are maintained on a light-dark cycle of 12:12 h in a temperature-controlled environment with food and water available ad libitum.
Table 1. Experimental animal groups.| Experimental group | Tested substance | Number of animals |
|---|---|---|
| 1 | Saline solution | 8 |
| 2 | Metamizole 200 mg/kg b.w. | 8 |
| 3 | Diclofenac 25 mg/kg b.w. | 8 |
| 4 | Compound 3d 10 mg/kg b.w. | 8 |
| 5 | Compound 3d 20 mg/kg b.w. | 8 |
| 6 | Compound 3d 40 mg/kg b.w. | 8 |
Reagents
Metamizole sodium amp. 500mg/ml 2ml (Sopharma AD, Bulgaria); NaCl 0.9% (Sopharma AD, Bulgaria); Diclofenac sodium amp. 75mg/3ml (Hexal AG, Germany); Formalin solution 0.2% (Sigma-Aldrich); Lambda-carrageenan (Sigma-Aldrich); 2-(3-Acetyl-5-(4-chloro-phenyl)-2-methyl-pyrrol-1-yl)-3-methyl-pentanoic acid (Vladimirova S., University of Chemical Technology and Metallurgy, Bulgaria).
All substances are dissolved in 0.9% saline solution. The drugs are administered intraperitoneally.
Plantar Test (Hargreaves Method).
The test animal is placed in an individual compartment and is left unrestrained. After an acclimation period an infrared heat source (Ugo Basile, Italy) is positioned under the glass floor directly beneath the hind paw of the animal and paw withdrawal latency (in seconds) is recorded. The chosen infrared intensity is 50 mW/cm2 and cut-off time is set at 30 seconds to avoid unnecessary overheating of the paw.
Tail Flick Test.
The rat is held by the operator and a radiant heat source (Ugo Basile, Italy) is focused on the underside of the tail approx. 3 cm from its distal end. The time for tail withdrawal (in seconds) is recorded. The chosen infrared intensity is 80 mW/cm2 and cut-off time is set at 15 seconds to avoid tissue damage.
The tests are performed at 1, 2 and 3h after intraperitoneal administration of the saline solution, metamizole sodium and compound 3d for the respective experimental groups. Criterion for analgesic action is increase in the reaction time compared to the animals, treated with saline.
Formalin Test.
0.2% 200µl formalin is injected intradermally in one of the hind paws. Saline solution, metamizole sodium and compound 3d (10, 20 and 40 mg/kg b.w.) are administered intraperitoneally one hour before injection of formalin. The cumulative time spent licking/biting the injected paw (in seconds) is measured during the first 10 minutes and between the 20th and 30th minute after administration of formalin. Criterion for analgesic effect is decreased paw licking time in comparison to the control group.
Carrageenan-Induced Paw Edema.
The volume of the right hind paw of the animals of all experimental groups is measured prior to treatment with a plethysmometer (Ugo Basile, Italy). Edema is induced by injection of 0.1 ml of a 1% suspension of carrageenan in 0.9% saline solution into the right hind paw of the rats. Saline solution, diclofenac sodium and compound 3d (10, 20 and 40 mg/kg b.w.) are administered intraperitoneally immediately after the injection of carrageenan. Paw volume is measured at 2, 3, and 4h after administration of carrageenan. The percentage of paw edema is calculated using the following formula:
Percentage of paw edema = 
Vo – mean paw volume at 0h, Vt – mean paw volume at a particular time interval
Criterion for anti-inflammatory effect is decreased paw edema compared to the control group.
In Vitro Testing of Hematological and Biochemical Parameters.
After continuous (fourteen days) treatment with compound 3d (10, 20 and 40 mg/kg b.w.) blood from each animal is collected in two vials. Analysis of hematological parameters (red blood cells, white blood cells, platelets and hemoglobin)is performed using an automated hematology analyzer Advia – 2120i Siemens Diagnostic. Serum is separated after coagulating at 37oC for 60 min and centrifuged at 3000 rpm for 10 minutes. Serum is used for the estimation of aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatinine, urea and blood glucose. All clinical-chemical analyzes are carried out on Olympus AV 480 Analyzer, Beckman Coulter according to manufacturer’s instructions.
Statistical analysis of the obtained data is evaluated using the Independent Sample t-test with IBM SPSS 20.0 software. Normality of distribution is determined with the Kolmogorov-Smirnov test.21 Results are expressed as arithmetic mean and standard error of the mean (mean±SEM). A p value ≤ 0.05 is considered statistically significant.
Results
Evaluation of Analgesic Activity
The reference analgesic metamizole sodium22 shows significant analgesic effect in all tests after both single and multiple administration.
In the plantar test (Table 2) compound 3d at dose 10 mg/kg b.w. reliably increases withdrawal latency at first and third hours, compared to the control. Doses 20 and 40 mg/kg b.w. significantly prolong paw withdrawal time at first, second and third hours, compared to the saline-treated animals. After multiple administrations, compound 3d at dose 10 mg/kg b.w. significantly increases reaction time at first and second hours, compared to the animals from the saline treated group. Dose 20 mg/kg b.w. reliably increases the observed indicator at first, second and third hours, compared to the control. Dose 40 mg/kg b.w. significantly increases paw withdrawal latency at first and second hours compared to the control group. Differences
Table 2. Comparison of the results between the control group and the groups, treated with Metamizole and compound 3d in doses 10, 20 and 40 mg/kg b.w. in plantar test.| Group | Hour | mean ±S EM | t | p | mean 1 ±S EM 1 | t 1 | p 1 |
| Control | 1st | 6,75±0,48 | - | - | 7,13±1,42 | - | - |
| 2nd | 8,67±2,34 | 7,70±1,72 | |||||
| 3rd | 10,00±2,10 | 7,17±2,22 | |||||
| Metamizole | 1st | 17,11±1,97 | 5,12 | 0,002* | 19,36±2,06 | 4,55 | 0,001* |
| 2nd | 17,10±1,22 | 3,34 | 0,007* | 16,30±2,66 | 2,50 | 0,028* | |
| 3rd | 18,19±2,24 | 2,63 | 0,023* | 13,28±1,37 | 2,47 | 0,030* | |
| 3d 10 mg/kg | 1st | 14,65±1,43 | 5,23 | 0,001* | 18,43±2,40 | 3,87 | 0,003* |
| 2nd | 14,48±1,68 | 2,07 | 0,06 | 15,89±1,03 | 4,22 | 0,001* | |
| 3rd | 17,31±1,79 | 2,70 | 0,021* | 12,64±0,64 | 2,37 | 0,06 | |
| 3d 20 mg/kg | 1st | 16,44±0,99 | 7,86 | <0,001* | 21,18±2,20 | 4,95 | <0,001* |
| 2nd | 15,45±1,17 | 2,81 | 0,016* | 17,91±1,62 | 4,27 | 0,001* | |
| 3rd | 18,54±0,76 | 3,82 | 0,008* | 17,01±2,21 | 3,07 | 0,01* | |
| 3d 40 mg/kg | 1st | 12,44±1,28 | 3,67 | 0,003* | 21,90±2,39 | 5,31 | <0,001* |
| 2nd | 14,64±1,06 | 2,55 | 0,026* | 23,28±1,82 | 6,21 | <0,001* | |
| 3rd | 20,17±1,89 | 3,61 | 0,004* | 12,40±1,92 | 1,78 | 0,11 |
In the tail-flick test (Table 3) compound 3d at doses 10, 20 and 40 mg/kg b.w. significantly prolongs reaction time at second hour compared to the control group. After continuous treatment the registered values of withdrawal latency for doses 10 and 20 mg/kg b.w. are approximately similar to those of the control group. In this instance, metamizole sodium shows significant increase in reaction time, compared to compound 3d (p=0.036, t=2.35 for 10 mg/kg at 2h and p=0.016, t=2.80 for 20 mg/kg b.w. at 1h). After multiple administrations only dose 40 mg/kg b.w. reliably increases tail withdrawal latency at second hour compared to the control.
Table 3. Comparison of the results between the control group and the groups, treated with Metamizole and compound 3d in doses 10, 20 and 40 mg/kg b.w. in tail-flick test.| Group | Hour | mean ±S EM | t | p | mean 1 ±S EM 1 | t 1 | p 1 |
| Control | 1st | 3,33±0,22 | - | - | 3,50±0,70 | - | - |
| 2nd | 2,58±0,10 | 2,70±0,25 | |||||
| 3rd | 3,17±0,45 | 3,93±0,48 | |||||
| Metamizole | 1st | 5,14±0,70 | 2,47 | 0,042* | 5,80±0,67 | 2,37 | 0,039* |
| 2nd | 3,79±0,39 | 2,77 | 0,018* | 4,42±0,65 | 2,47 | 0,033* | |
| 3rd | 4,19±0,79 | 1,08 | 0,31 | 4,32±0,97 | 0,36 | 0,73 | |
| 3d 10 mg/kg | 1st | 3,64±0,30 | 0,77 | 0,46 | 5,35±1,20 | 1,21 | 0,25 |
| 2nd | 3,54±0,25 | 3,12 | 0,009* | 2,94±0,25 | 0,65 | 0,53 | |
| 3rd | 3,29±0,11 | 0,26 | 0,80 | 4,81±0,58 | 1,11 | 0,29 | |
| 3d 20 mg/kg | 1st | 5,20±1,13 | 1,40 | 0,19 | 3,61±0,45 | 0,14 | 0,89 |
| 2nd | 3,55±0,34 | 2,73 | 0,025* | 3,63±0,50 | 1,50 | 0,16 | |
| 3rd | 3,81±0,22 | 1,39 | 0,19 | 3,18±0,30 | 1,42 | 0,18 | |
| 3d 40 mg/kg | 1st | 5,39±1,05 | 1,66 | 0,12 | 4,73±0,91 | 1,08 | 0,31 |
| 2nd | 4,16±0,35 | 4,38 | 0,002* | 5,00±0,48 | 4,23 | 0,002* | |
| 3rd | 3,84±0,55 | 0,93 | 0,37 | 3,82±0,25 | 0,22 | 0,83 |
In the formalin test (Table 4) compound 3d at doses 10, 20 and 40 mg/kg b.w. significantly reduces the time spent licking/biting the paw in the first and second phases of the formalin test, compared to the control. After multiple administrations, compound 3d at doses 10 and 20 mg/kg b.w. significantly reduces paw licking time in the first and second phases of the formalin test, compared to the saline-treated animals. Dose 40 mg/kg b.w. significantly reduces the observed indicator in the first phase of the test compared to the control. Comparison of the results between metamizole sodium and compound 3d shows that after single administration and during first phase of the formalin test, dose 40 mg/kg b.w. significantly reduces the time spent licking the paw, compared to the positive control (p=0.026, t=2.62).
Table 4. Comparison of the results between the control group and the groups, treated with Metamizole and compound 3d in doses 10, 20 and 40 mg/kg b.w. in formalin test.| Group | Phase | mean ±S EM | t | p | mean 1 ±S EM 1 | t 1 | p 1 |
| Control | 1st | 70,17±16,81 | - | - | 42,00±9,36 | - | - |
| 2nd | 47,83±11,12 | 35,00±5,22 | |||||
| Metamizole | 1st | 23,83±4,35 | 2,67 | 0,039* | 7,38±3,05 | 3,96 | 0,002* |
| 2nd | 18,83±4,07 | 2,45 | 0,048* | 11,25±3,90 | 3,73 | 0,003* | |
| 3d 10 mg/kg | 1st | 17,71±4,28 | 3,02 | 0,025* | 18,33±2,20 | 2,46 | 0,032* |
| 2nd | 15,29±5,23 | 2,78 | 0,018* | 10,00±3,11 | 4,12 | 0,002* | |
| 3d 20 mg/kg | 1st | 22,50±4,00 | 2,76 | 0,036* | 8,50±2,32 | 3,48 | 0,006* |
| 2nd | 11,83±2,61 | 3,15 | 0,022* | 9,67±3,58 | 4,00 | 0,003* | |
| 3d 40 mg/kg | 1st | 10,50±2,64 | 3,51 | 0,016* | 12,17±4,78 | 2,84 | 0,018* |
| 2nd | 14,83±2,60 | 2,89 | 0,03* | 21,50±5,21 | 1,83 | 0,97 |
Evaluation of Anti-Inflammatory Activity
In the carrageenan inflammation model (Table 5), diclofenac sodium (used as a reference substance with anti-inflammatory action)23 significantly reduces the swelling of the paw at all tested hours compared to the control group. After single administration, compound 3d at any tested dose does not reduce paw edema, compared to the saline-treated animals. After repeated administration (14 days), compound 3d at doses 10, 20 and 40 mg/kg b.w. significantly reduces carrageenan-induced edema at second, third and fourth hours compared to the saline-treated animals. Examination of the results between the positive control and compound 3d shows that after multiple administrations, doses 20 (p=0.003, t=3.62) and 40 (p=0.012, t=3.30) mg/kg b.w. significantly reduce paw swelling at second hour, compared to diclofenac.
Table 5. Comparison of the results between the control group and the groups, treated with Diclofenac and compound 3d in doses 10, 20 and 40 mg/kg b.w. in carrageenan-induced paw edema test.| Group | Hour | mean ±S EM | t | p | mean 1 ±S EM 1 | t 1 | p 1 |
| Control | 2nd | 47,08±4,06 | - | - | 45,77±3,79 | - | - |
| 3rd | 35,70±6,88 | 43,67±3,88 | |||||
| 4th | 41,55±3,75 | 49,24±4,33 | |||||
| Diclofenac | 2nd | 21,86±2,33 | 5,57 | <0,001* | 14,46±0,89 | 8,05 | <0,001* |
| 3rd | 14,41±2,93 | 2,98 | 0,011* | 11,19±1,39 | 7,88 | <0,001* | |
| 4th | 16,54±3,02 | 5,25 | <0,001* | 7,70±1,25 | 9,21 | <0,001* | |
| 3d 10 mg/kg | 2nd | 47,09±5,53 | 0,002 | 1,00 | 15,96±4,56 | 5,03 | <0,001* |
| 3rd | 52,28±4,04 | 2,15 | 0,051 | 20,08±6,66 | 3,06 | 0,009* | |
| 4th | 55,79±8,74 | 1,50 | 0,17 | 26,80±5,33 | 3,27 | 0,006* | |
| 3d 20 mg/kg | 2nd | 38,32±6,46 | 1,11 | 0,29 | 3,75±2,82 | 8,90 | <0,001* |
| 3rd | 48,24±6,06 | 1,37 | 0,19 | 10,62±4,39 | 5,64 | <0,001* | |
| 4th | 42,01±4,33 | 0,08 | 0,94 | 9,54±3,96 | 6,76 | <0,001* | |
| 3d 40 mg/kg | 2nd | 33,28±6,63 | 1,71 | 0,11 | 6,07±2,39 | 8,56 | <0,001* |
| 3rd | 38,80±6,23 | 0,33 | 0,74 | 11,24±3,82 | 5,92 | <0,001* | |
| 4th | 52,13±8,48 | 1,14 | 0,28 | 13,19±4,57 | 5,72 | <0,001* |
Evaluation of Organ Toxicity
Hematological and biochemical parameters are evaluated as preliminary screening for possible organ toxicity. Blood from metamizole and diclofenac-treated animals is not obtained, as these drugs have been on the market for decades and their toxicities are well-known. A significant decrease in the levels of AST, urea and blood glucose is observed in the animals treated with compound 3d at dose 10 mg/kg b.w., compared to the control group. Compound 3d at dose 20 mg/kg b.w. significantly lowers ALT and blood glucose compared to saline-treated animals. Dose 40 mg/kg b.w. reliably reduces AST, ALT and blood glucose levels compared to controls. Compound 3d at doses 10 and 40 mg/kg b.w. reliably reduces the number of leukocytes compared to control animals. Dose 40 mg/kg b.w. significantly decreases hemoglobin levels compared to the control group. (Table 6 and Table 7)
Table 6. Comparison of biochemical test indicators between the control group and the groups, treated with compound 3d in doses 10, 20 and 40 mg/kg b.w.| Group | Biochemical test panel indicators | mean±S EM | t | p |
| Control | AST | 217,63±8,92 | - | - |
| ALT | 63,50±5,78 | |||
| CREA | 37,25±0,94 | |||
| UREA | 6,45±0,26 | |||
| GLUC | 10,18±0,22 | |||
| 3d 10 mg/kg | AST | 180,50±12,76 | 2,39 | 0,032* |
| ALT | 56,50±4,00 | 1,00 | 0,34 | |
| CREA | 38,25±0,70 | 0,85 | 0,41 | |
| UREA | 5,43±0,30 | 2,60 | 0,021* | |
| GLUC | 7,80±0,33 | 5,98 | <0,001* | |
| 3d 20 mg/kg | AST | 187,50±16,13 | 1,64 | 0,12 |
| ALT | 43,38±3,18 | 3,05 | 0,009* | |
| CREA | 38,13±1,16 | 0,59 | 0,57 | |
| UREA | 5,91±0,52 | 0,93 | 0,37 | |
| GLUC | 8,03±0,30 | 5,79 | <0,001* | |
| 3d 40 mg/kg | AST | 163,14±10,64 | 3,96 | 0,002* |
| ALT | 30,71±2,55 | 4,93 | <0,001* | |
| CREA | 37,14±2,01 | 0,05 | 0,96 | |
| UREA | 7,51±0,61 | 1,62 | 0,14 | |
| GLUC | 7,83±0,72 | 3,30 | 0,006* |
| Group | Full Blood Count indicators | mean±S EM | t | p |
| Control | RBC | 6,33±0,25 | - | - |
| HCT | 0,33±0,01 | |||
| HGB | 124,50±4,69 | |||
| WBC | 10,78±0,78 | |||
| PLT | 659,75±70,52 | |||
| 3d 10 mg/kg | RBC | 5,80±0,17 | 1,75 | 0,10 |
| HCT | 0,31±0,01 | 1,29 | 0,22 | |
| HGB | 114,25±3,01 | 1,84 | 0,09 | |
| WBC | 7,93±0,78 | 2,59 | 0,02* | |
| PLT | 533,43±59,70 | 1,32 | 0,22 | |
| 3d 20 mg/kg | RBC | 5,77±0,23 | 1,64 | 0,12 |
| HCT | 0,32±0,02 | 0,65 | 0,53 | |
| HGB | 112,86±4,36 | 1,82 | 0,09 | |
| WBC | 7,94±1,15 | 2,05 | 0,06 | |
| PLT | 473,75±54,96 | 2,01 | 0,07 | |
| 3d 40 mg/kg | RBC | 5,50±0,32 | 2,08 | 0,06 |
| HCT | 0,30±0,02 | 1,23 | 0,24 | |
| HGB | 106,43±6,29 | 2,34 | 0,036* | |
| WBC | 5,11±1,01 | 4,50 | 0,001* | |
| PLT | 450,00±127,96 | 1,44 | 0,19 |
Discussion
The results in our study show that in experimental conditions 2-(3-Acetyl-5-(4-chloro-phenyl)-2-methyl-pyrrol-1-yl)-3-methyl-pentanoic acid has analgesic action against thermal and chemical stimuli. This effect is registered after both single and multiple administration of the compound.
The Hargreaves test is developed to deliver a more localized heat on unrestrained animals and offers the advantage of minimizing the role of subjective factors.24 Compared to the widely used hot plate method which is very susceptible to learning phenomena that result in a progressive shortening of the reaction time, the plantar test provides no such unwanted clues.25 In this test compound 3d shows significant analgesic effect, after both single and multiple administration. The behavioral reflex response in this test is mediated by supraspinal structures,25 hence we can assume that supraspinal pathways are involved in the analgesia induced by compound 3d. After continuous administration the observed analgesic effect in the plantar test does not weaken which could indicate lack of tolerance development on this level.
In tail-flick test after single administration all tested doses of compound 3d show analgesic effect. This test is considered precise since it is measured electronically and there is small interanimal variability in reaction times.24 In this test, the observed reflex response to the nociceptive stimulus occurs on a spinal level,25 therefore we can theorize that spinal pathways are implicated in the analgesic mode of action of compound 3d. After continuous administration, however, only the highest tested dose shows analgesic action. This could suggest possible development of tolerance towards the analgesic effect on spinal level. Since tolerance, antinociception on spinal level and increased latencies in tail-flick test are very typical of opioids,26 we propose involvement of the endogenous opioidergic system in the analgesia induced by compound 3d. Involvement of this system in the antinociception caused by NSAIDs is supported by data from recent studies, which propose that GABA-containing synapses act as a site where NSAIDs converge with endogenous opioids. Multiple intraperitoneal administrations of non-opioid analgesics in rats induce antinociception and cause tolerance, as well as tolerance to morphine.27 Furthermore, administration of Naloxone (a specific µ-receptor opioid antagonist) significantly decreases the antinociceptive effects of NSAIDs.27 Further investigations of possible involvement of opioidergic mechanisms are necessary to establish the specific mechanisms of antinociceptive action of compound 3d.
Intraplantar administration of formalin leads to biphasic pain response. The initial phase results from direct activation of peripheral nociceptors and last approx. 10 minutes. The second phase begins after a ten minute quiescent period. It is attributed to the release of local endogenous pro-inflammatory mediators, causing peripheral inflammatory processes and subsequent sensitization of nociceptive spinal neurons.28 In our study compound 3d after both single and multiple administration shows significant antinociceptive effect in both phases of the formalin test. We can speculate that peripheral mechanisms are also part of the mode of antinociceptive action of compound 3d, which would be in line with the expected cyclooxygenase inhibiting activity of this substance. The analgesia produced by the compound during the second phase of the test could result from either suppression of pro-inflammatory mediators or a modulation of pain transmission on spinal level. After single administration, compound 3d increases latencies in tail-flick test – a sign that antinociception on spinal level does occur. However, for the smaller doses this effect is lost after continuous administration and since the compound continues to show analgesic effect in the second phase of formalin test, we suggest that suppressing inflammation plays a role in the antinociception induced by compound 3d.
To further clear the possible anti-inflammatory effect of compound 3d, we chose carrageenan-induced model of inflammation. According to literary data diclofenac or indomethacin are the most commonly used reference drugs in experimental models of inflammation.23,29,30 We use diclofenac sodium as a positive control because it has greater selectivity towards COX-2 compared to other NSAIDs (with the exception of coxibs) and the compound tested in our study is also expected to show such selectivity. The experimental edema induced by carrageenan in rats and the acute exudative inflammation in humans possess similarities in vascular and cell reactions. Carrageenan-induced inflammation possesses two distinct phases – vascular and cellular.31 Bradykinin, serotonin, histamine and several pro-inflammatory cytokines (TNF-α, IL-1β) are responsible for the vasodilation and initial extravasation during the first couple of hours. Prostaglandins play a major role in the cellular phase, which occurs approx. 4 hours after intraplantar administration of carrageenan.32 Suppression of this last phase is typical of NSAIDs and correlates to a big extent with their therapeutic efficacy.33 This makes the model particularly suitable for registering anti-inflammatory effect of novel substances with suspected COX-inhibiting mode of action. After single administration compound 3d does not inhibit formation of paw edema. After multiple administrations all doses of compound 3d significantly suppress paw edema at second, third and fourth hours. This suggests a possible need for accumulation of the compound in the tissues in order to bind stably to COX. Since inhibition of paw edema is pronounced during all tested hours, this suggests compound 3d influences both vascular and cellular phases of inflammation.
In controlled clinical studies 6% of patients taking Celecoxib presented with elevated liver enzymes.34 We decided to screen the novel substance for possible hepatotoxicity by evaluating the levels of liver transaminases (AST and ALT) as markers for initial liver damage. The experimental animals, treated with compound 3d for 14 days present with decreased levels of liver enzymes, compared to the control.
Nephrotoxicity can also be observed during treatment with NSAIDs. Renal damage can present with interstitial nephropathy and tubulointerstitial nephritis although kidney injury is usually reversible and patients recover after cessation of NSAIDs use.35,36 Nephrotoxicity is rare in Celecoxib use, although elevated levels of urea and creatinine have been observed.34 Compound 3d does not affect creatinine levels but the smallest dose used – 10 mg/kg b.w., lowers urea. Urea also reflects functioning of the liver since its production occurs primarily in the liver. Since compound 3d decreases levels of not only urea, but AST and ALT we may theorize it suppresses liver functions.
Blood glucose is tested as a part of the standard biochemical blood profile. Compound 3d significantly decreases blood glucose levels, compared to the animals, treated with saline. Studies show that some NSAIDs (aspirin, nimesulide, phenylbutazone) lower blood glucose levels due to inhibition of glucose transport and enzymatic changes in the intestines.37 In vitro studies have shown, however, that Celecoxib does not affect intestinal permeability significantly.38
Hematological tests show the following results: compound 3d does not affect red blood cells and platelets; compound 3d decreases white blood cell levels and the highest used dose decreases hemoglobin as well. Platelets are very sensitive to cyclooxygenase inhibitors since they cannot re-synthesize the enzyme.39 For selective COX-2 inhibitors, however, pro-thrombotic state is typical because they block the production of prostacyclin (PGI2) but not that of thromboxane A2 (since TXA2 synthesis depends on preserved COX-1 activity).9,32 Lack of suppression of platelets in compound 3d could mean it does possess the expected selectivity towards COX-2. Bone marrow suppression and agranulocytosis in particular, are not typical adverse drug reactions for NSAIDs. Metamizole is one of the few COX-inhibitors that show it,40 although it does not belong to the group of NSAIDs but rather to the group of analgesics-antipyretics. The decrease in white blood cells observed in compound 3d could be due to bone marrow suppression. Another possible explanation is left-over anti-inflammatory action since blood samples were obtained approx. 24 hours after injection of carrageenan. This could potentially explain the significant difference between white blood cell levels in the control group and the groups, treated with compound 3d.
Conclusion
Compound 3d (2-(3-Acetyl-5-(4-chloro-phenyl)-2-methyl-pyrrol-1-yl)-3-methyl-pentanoic acid) induces antinociception after single and multiple (14 days) administration in rats. Antinociception in the tested compound is most likely mediated by supraspinal, spinal and peripheral mechanisms. Possible tolerance develops towards the analgesic action on spinal level after continuous administration. Anti-inflammatory activity is probably not the leading cause for antinociception.
Compound 3d shows significant anti-inflammatory activity, inhibiting both vascular and cellular phases of inflammation after multiple (14 days) administration in rats. A possible accumulation of the tested compound in the tissues is needed for stable binding to COX.
After continuous administration compound 3d lowers levels of AST and ALT, blood glucose and urea. It seems to not be nephrotoxic although it might suppress liver functions.
Compound 3d does not affect red blood cell and platelet levels. It suppresses white blood cells after 14-days administration which could result from persisting anti-inflammatory activity.
Acknowledgements
Part of this research is financially supported by a Research grant from Medical University - Plovdiv (NO-7/2015).