Abstract
Aim
There is limited published data describing the characteristics of the paediatric population prescribed semi elemental formulas. This retrospective observational audit aimed to describe the characteristics of the paediatric patients who have been prescribed a hydrolysed whey protein, medium chain triglycerides (MCT) based formula, Peptamen Junior® and the nutritional outcomes.
Methods
A retrospective observation audit was completed on a cohort of patients that was prescribed a semi elemental formula between 2016 and 2019 from a single tertiary paediatric medical centre. Data variables were collated such as patient characteristics, indications and modalities of administration, duration and tolerance to the formula.
Results
Data was collated on 375 patients with a median age of 6.2 years. The main underlying medical conditions were haematological/oncology (67%), gastrointestinal disorders (10.7%) and neurological conditions (9.4%). The most common indications for use were chemotherapy related side effects (36.2%), post bone marrow transplant (25.8%) and gastrointestinal symptoms (17.9%). The formula was rarely used as a sole source of nutrition, with 88% patients requiring accompanying forms of nutrition support. The majority of orders prescribed were of standard concentration (80.8%) and tolerance was recorded in 82.8% of patients.
Conclusion
The semi elemental formula Peptamen Junior® appears to be well tolerated in paediatric patients with a variety of medical conditions that have complex pathologies and may have wider scope of use in a more diverse group of medical conditions than currently indicated.
Author Contributions
Academic Editor: Anubha Bajaj, Consultant Histopathology, A.B. Diagnostics, New Delhi, India.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2022 Kristyn Ford, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Paediatric malnutrition is defined as an imbalance between nutrient requirements and intake. 1,2 The failure of the nutritional supply to meet the energy, protein and micronutrient needs in children may negatively affect the growth and development, resulting in faltering growth. Children within the hospital setting are at increased risk of malnutrition due to insufficient nutritional intake, malabsorption or increased needs due to chronic or acute illness. In this nutritionally vulnerable population additional nutrition support may be required in the form of enteral nutrition. 1
When enteral nutrition support is indicated, there are several different types of formula available. They are usually described in accordance with their protein and/or lipid source and prescribed based on clinical condition. 3,4 Polymeric formulas contain whole/intact proteins, carbohydrates and long chain triglycerides, and are generally used in those with a functioning digestive system. Semi elemental formulas contain peptides of varying chain length, carbohydrates and the majority of medium chain triglycerides (MCT) and are usually indicated for digestion and absorption problems or pancreatic insufficiency. Elemental formulas contain amino acids, carbohydrates and a majority of long chain triglycerides (LCT) with some MCT and are indicated in cases of severe food protein allergies. 3,4,5
It has been suggested that the presence of branched chain amino acids (BCAA)-containing dipeptides and tripeptides from whey protein hydrolysates (WHP) are more bioavailable in semi elemental formulas. Advantages of this include enhancing intestinal nitrogen absorption and balance, maintenance of gut integrity, reduction of bacterial translocation, improved protein synthesis and enhanced immune support. This would contribute to an absorptive advantage compared with elemental or polymeric formulas. 4,6
Additionally, the type of lipid influences absorption, as MCTs are passively absorbed in the intestine and do not require the presence of bile salt, pancreatic enzymes or chylomicrons to be transported to the bloodstream. 5,7 In clinical practice, the use of semi elemental or elemental formulas tend to be in situations where the use of polymeric formulas have failed or if failure of polymeric formula’s is expected/predicted because the presence of malabsorption or maldigestion issues.
There is limited published data describing the characteristics of the paediatric population prescribed semi elemental formulas. This retrospective observational audit aimed to describe the characteristics of the paediatric patients who have been prescribed a hydrolysed whey protein, MCT based formula, Peptamen Junior® and the nutritional outcomes.
Method
This retrospective observational audit was completed at a single tertiary paediatric medical centre in Australia. All patients who were prescribed Peptamen Junior® during the period 5th May 2016 to 31st December 2019 were included in the audit. The inclusion dates coincided with the implementation of the Electronic Medical Records (EMR) at the centre which enabled electronic extraction of the data. This audit was approved by the research ethics committee of the institution.
Data was collected retrospectively from the EMR using an individualised report that filtered specifically for the use of Peptamen Junior® during the designated time period. It included data variables such as patients characteristics – age, sex, medical condition and feeding regimen.
Data relative to the administration of the formula used for enteral feeding were also recorded: clinical reason or indication for use, route of delivery: oral versus enteral feeding tube (EFT), duration of feeding regime, recipe/concentration: standard or non-standard concentration and any other accompanying nutrition support.
Tolerance to enteral feed was also assessed. A patient was defined as tolerating Peptamen Junior® formula if they remained on this formula or were able to transition to a whole protein feed and/or oral diet or a ready to feed (RTF) alternative brand. Not tolerating was defined as a patient requiring a step down to an elemental formula or parenteral nutrition (PN). Noting that the use of PN did not necessary reflect intolerance to Peptamen Junior® but rather a medical condition that precluded tolerance to enteral feeding.
Patients were excluded from the audit if they were commenced on the formula prior to the inclusion time period or commenced at external institutions.
Data is reported as means or medians (with ranges) for continuous variables and as proportions and percentages for categorical data.
Results
A total of 375 patients were identified using the EMR report extract to have used Peptamen Junior® during the specified time period. Of this total 318 patients were included in the data analysis and 57 patients were excluded as they were commenced on the formula prior to the specified time period, managed at other institutions, ordered and not commenced (requirement of PN or EFT not inserted or change to whole protein formula) or an incorrect order was placed (patient requiring infant peptide formula) (Figure 1).
Figure 1. Inclusion/exclusion criteria for paediatric population of the study 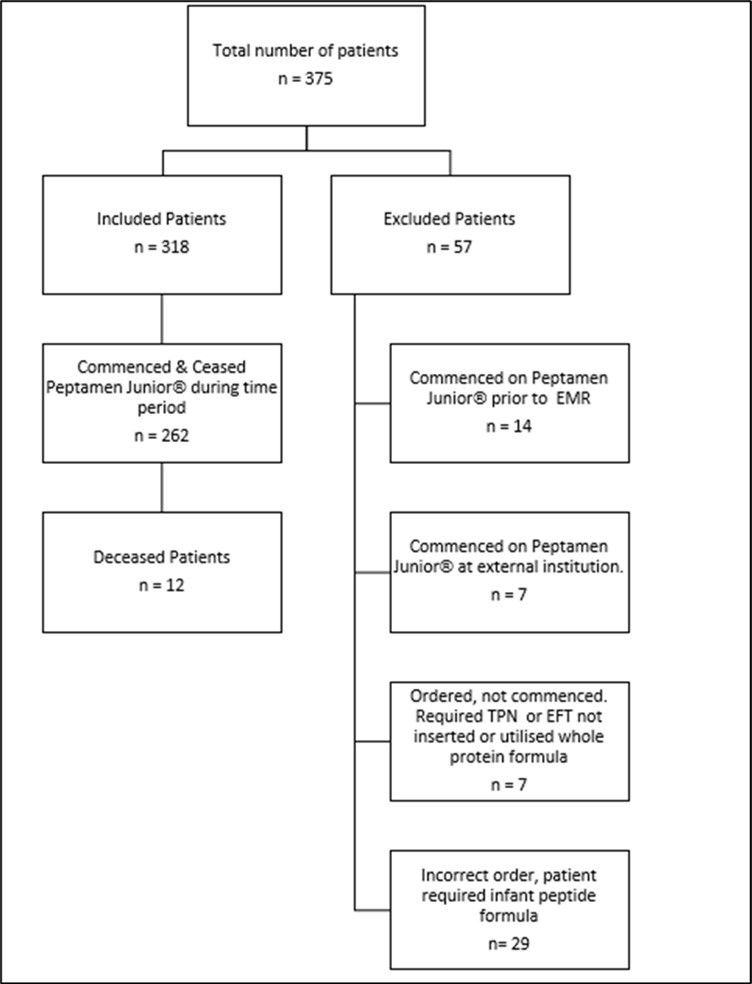
There were 172 males (54%) and 146 females (46%). The median age was 6.2 years (range 1 to 19.3 years). The underlying medical condition was diverse and included 213 (67%) patients with a haematological/oncology, 34 (10.7%) gastrointestinal disorder, 30 (9.4%) neurological disorder including cerebral palsy (CP), 23 (7.2%) other conditions such as faltering growth, nephrology, metabolic and surgical, 11 (3.5%) respiratory disease and 7 (2.2%) with heart conditions (Table 1).
The reason for commencement of the hydrolysed whey protein, MCT based formula Peptamen Junior® is outlined in Table 1. The most common reasons for commencing Peptamen Junior® was due to chemotherapy related side effects, 115 (36.2%) and post bone marrow transplant (BMT) 82 (25.8%). 57 (17.9%) sited gastrointestinal symptoms such as vomiting, reflux or diarrhoea and 45 (14.2%) malabsorption conditions including 30 (9.4%) liver disease, 7 (2.2%) cystic fibrosis (pancreatic insufficiency), 5 (1.6%) short gut and 3 (0.9%) generalised fat malabsorption. 9 (2.8%) cow’s milk protein intolerance and 8 (2.5%) had an unclear clinical reason for commencing Peptamen Junior®.
The most common route of delivery was via an enteral feeding tube (EFT). The most common EFT being nasogastric 231 (72.6%), followed by gastrostomy, 58 (18.2%), jejunostomy 11 (3.5%) and nasojejunal 7 (2.2%). Very few patients drank the formula orally 11 (3.5%) (Table 1).
Table 1. Documented indication for commencing Peptamen Junior®| Gender | Number (n=318) | Percentage (%) |
| Male | 172 | 54.1 |
| Female | 146 | 45.9 |
| Medical Condition | ||
|---|---|---|
| Haematological/Oncology Conditions | 213 | 67.0 |
| Gastrointestinal Disorders | 34 | 10.7 |
| Neurological Disorders | 30 | 9.4 |
| Other (including Faltering Growth, Nephrology, Metabolic, surgical) | 23 | 7.2 |
| Respiratory Disease | 11 | 3.5 |
| Heart Conditions | 7 | 2.2 |
| Reason/Indication | ||
| Chemotherapy | 115 | 36.2 |
| Bone Marrow Transplant (including Graft vs Host Disease) | 84 | 26.4 |
| Gastrointestinal Symptoms (Vomiting, Reflux, and Diarrhoea) | 57 | 17.9 |
| Malabsorption Conditions | ||
| Liver Disease | 30 | 9.4 |
| Cystic Fibrosis (Pancreatic Insufficiency) | 7 | 2.2 |
| Short Gut | 5 | 1.6 |
| Generalised Fat Malabsorption | 3 | 0.9 |
| Cow’s Milk Protein Intolerance | 9 | 2.8 |
| Unclear | 8 | 2.5 |
| Route of Delivery | ||
| Nasogastric Feeding Tube | 231 | 72.6 |
| Gastrostomy Feeding Tube | 58 | 18.2 |
| Jejunostomy Feeding Tube | 11 | 3.5 |
| Oral | 11 | 3.5 |
| Nasojejunal Feeding Tube | 7 | 2.2 |
The majority of patients had accompanying sources of nutrition (Figure 2). Parental nutrition was used in 153 (48%) of patients in addition to Peptamen Junior® and/or oral diet, with only 38 (11.9%) of patients receiving Peptamen Junior® as their sole source of nutrition.
Figure 2. Use of Peptamen Junior® as a source of Nutrition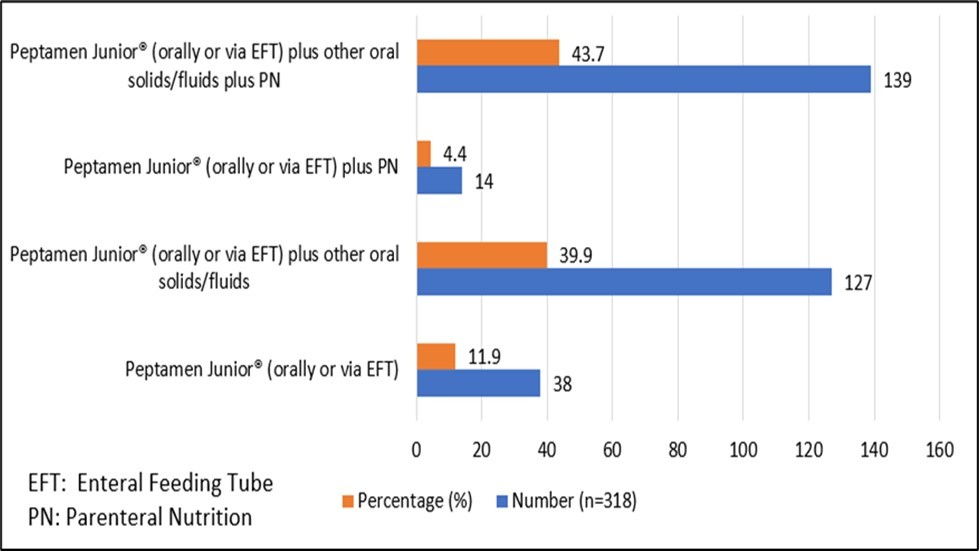
Of the 318 patients prescribed Peptamen Junior®, 262 (82.5%) patients commenced and ceased the use of Peptamen Junior® during the time period. The number of orders prescribed, concentration and tolerance were assessed in this group.
Of the 262 patients within this group, 236 (90.1%) were prescribed Peptamen Junior® whilst an inpatient indicating that it is used in periods of active medical treatment when gut insult is more likely. Only 12 (4.6%) patients had Peptamen Junior® as an outpatient and 14 (5.3%) in both the outpatient and inpatient setting (Table 2).
Within this group the median number and average number of different formula concentrations that each patient was prescribed was 1 and 1.7 (range 1 to 7) respectively.
Table 2. Clinical setting and orders for each concentration of Peptamen junior® for those patients who commenced and ceased the formula during the study period (n=262).| Clinical setting | Number (n=262) | Percentage (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Inpatient | 236 | 90.1 | |||||||||
| Inpatient and Outpatient | 14 | 5.3 | |||||||||
| Outpatient | 12 | 4.6 | |||||||||
| Total | Standard Concentration (kJ/100ml) | Non-Standard Concentration(kJ/100ml) a | Transition b | Other c | |||||||
| 429 | 536 | 643 | <429 | 429-536 | 536-643 | >645 | |||||
| Number of patients prescribed each formula concentration (%) | 262 | 238 (52.4) | 65 (14.3) | 64 (14.1) | 4 (0.9) | 18 (4.0) | 5 (1.1) | 14 (3.1) | 45(9.9) | 1(0.2) | |
| Total Number of Days | 15593 | 6814 | 1962 | 3295 | 68 | 2072 | 297 | 903 | 95 | 87 | |
| Median Number of Days | 23 | 15 | 11 | 11 | 9 | 30 | 4 | 11 | 1 | 87 | |
| Average Number of Days | 60 | 29 | 30 | 51 | 17 | 115 | 59 | 65 | 2 | 87 | |
| Minimum Number of Days | 1 | 1 | 1 | 1 | 2 | 1 | 2 | 1 | 1 | 0 | |
| Maximum Number of Days | 897 | 335 | 538 | 411 | 49 | 897 | 278 | 413 | 10 | 87 | |
Table 2 indicates the number of orders prescribed and the number of days on each of the different formula concentration for those patients who commenced and ceased the use of Peptamen Junior® during the study period.
The majority of the orders prescribed were the standard concentration of 429kJ/100ml, accounting for 238 (52.4%) of the orders, followed by 536kJ/100ml for 65 (14.3%) orders, and 636kJ/100ml for 64 (14.1%) orders. Non-standard concentrations that are not reflective of usual clinical practice were less common, however were used in situations to ensure nutritional adequacy. There were only 45 (9.9%) orders that were included in the transition from Peptamen Junior® to standard polymeric formula. Interestingly, the median number of days of Peptamen Junior® feeding duration was highest in the non-standard 429-536kJ/100ml group with 30 days, however the range was great from 1 to 897 days. This was largely due to one patient with short gut syndrome requiring Peptamen Junior® for a prolonged period. Excluding this outlier, the medium number of days on this concentration of formula was 26 days.
The tolerance to Peptamen Junior® was defined as previously outlined and was assessed for each patient (Figure 3). According to our definition for tolerance, 217 patients (82.8%) tolerated Peptamen Junior® and were transitioned to either a whole protein formula or oral diet or a convenient ready to feed (RTF) option. 43 patients (16.5%) did not tolerate Peptamen Junior®, with 13 patients (5%) requiring an elemental formula, 18 (6.9%) requiring cessation of enteral nutrition and 12 (4.6%) were deceased. These outcomes are indicative of the severity this cohort’s clinical condition. PN was utilised in 130 (49.6%) of patients, including 109 (41.6%) who were defined as tolerating Peptamen Junior® indicating that PN was required to ensure nutritional adequacy.
Figure 3. Tolerance of Peptamen Junior® and next transition feeding step
Discussion
This was a large retrospective observational audit that describes the characteristics of paediatric patients and their associated medical conditions that were prescribed the hydrolysed whey protein formula, Peptamen Junior® for their nutritional requirements.
It was observed that the largest patient group for which the use of Peptamen Junior® was most commonly prescribed for was the haematology/oncology patient group for which there is currently no clinically recognised guideline regarding the type of formula that should be prescribed for this cohort. Although not described in detail in this report, the vast majority of the patients within this haematology/oncology group were changed from a polymeric formula to Peptamen Junior® when significant gastrointestinal symptoms such as mucositis and diarrhoea were evident or if significant gut toxicities were anticipated in treatment modalities such as post bone marrow transplant (BMT). The rationale for this is the theoretical benefit of semi elemental formulas being more easily digested, providing improved nutrient absorption, which is supported by the tolerance data reported in this study.
Other significant medical conditions that were identified in our cohort of patients prescribed Peptamen Junior® included liver disease, crohn’s disease, short gut, cystic fibrosis and cerebral palsy. Studies have shown use of high MCT and BCAA formulas in paediatric patients with liver disease have shown some potential benefit by improving lean body mass and protein retention, however the evidence is quite limited.7 Similarly, some potential benefit has been reported for the use of semi elemental formulas in paediatric patients with crohn’s disease such as increased weight and height velocity, improve nutritional status and a decrease in disease activity.8,9 In conditions such as short bowel syndrome, it has been suggested that there may be nutritional benefits in the use of semi elemental formulas due to enhanced micronutrient and nitrogen absorption however, there is limited randomised control trials to support the use of semi elemental or amino acid based formulas in these patients. 4,10,11,12
In patients with cystic fibrosis, there is limited evidence to the efficacy of elemental or semi elemental feeds over polymeric feeds. However, it is believed that semi elemental and elemental formulas may be more easily absorbed with the benefits of not requiring out pancreatic enzyme replacement therapy due to the high proportion of MCT fat. 13,14,15
Studies in paediatric patients with cerebral palsy and/or gastrointestinal dysfunction have shown some benefit with the use of semi elemental whey protein hydrolysate with reports of significant reduction in degree of regurgitation, gastric emptying times and gagging. 16,17,18
The clinical reason or indication for use in our cohort of patients was vast. Peptamen Junior® was predominantly prescribed in patients that had chemotherapy related side effects or BMT treatments. The clinical indication of fat malabsorption (due to liver disease, short gut, cystic fibrosis or general fat malabsorption) for which Peptamen Junior® is currently scheduled for on the Pharmaceutical Benefits Scheme (PBS) only made up 14% of our cohort.
In our cohort the vast majority of patients received Peptamen Junior® via an EFT, with very few patients drinking the formula orally. Peptamen Junior® was often prescribed in addition to other forms of nutrition support, such as oral diet or PN. Of our 318 patients, nearly half (48%) were receiving PN simultaneously, which is likely to reflect the severity of gastrointestinal dysfunction in these particular groups of paediatric patients and requirement of PN to ensure nutritional adequacy. It also supports the premise of the importance of trophic feeds during periods of gastrointestinal dysfunction to maintain gut mucosal structure, encourages adaptation and reduces the risk of PN associated liver disease.19 Consideration of an enteral formula that can be tolerated as a trophic enteral feed is also of significance.
Additionally, 82% commenced and ceased the use of Peptamen Junior® in our specified time period, whilst 90% had only inpatient orders, indicating that it is a formula that is used temporarily, in periods where other forms of nutrition support are unable to be tolerated or able to meet nutritional needs of patients. 83% of patients were defined as tolerating Peptamen Junior®. It was interesting to note that 42% of patients that were defined as tolerating Peptamen Junior®, also required PN.
The concentration of Peptamen Junior® ordered was varied. It was noted that 81% of the orders were considered standard concentrations as per the company’s recommendations. There was a proportion of non-standard concentrations ordered in our cohort, indicating that clinical practice was adapted to the individual requirements in this vulnerable group. The duration on which patients were receiving Peptamen Junior® was great, ranging from 1 to 897 days, with the median total number of days being 23. This vast range may be explained by the daily transition from a lower concentration to progressively higher concentrations to meet the patients desired requirements. In addition, to a patient’s slow clinical recovery.
Strengths of this large retrospective observational audit include the time period of observation (over 3 ½ years), the use of EMR to retrieve the data and the inclusivity of capturing all patients who were solely managed at a single tertiary paediatric medical centre and who were commenced on Peptamen Junior® over this period. Retrospective data collection also ensures no bias in the formula being prescribed to measure the true efficacy of its use in a variety of different medical conditions and patient groups. Retrospective data collection is also a limitation of the audit when data is missing or poorly recorded, resulting in outcome measures of total caloric intake and nutritional markers such as weight, height and body mass index (BMI) unable to be reported.
In conclusion, Peptamen Junior® is prescribed for a variety of medical conditions with complex pathologies that are predominantly related to gastrointestinal tract diseases/disorders. Currently, there is limited evidence for the use of semi elemental formulas in paediatric patients. This audit suggests a population of wider scope of use in a more diverse group of medical conditions than currently indicated on the PBS schedule. Further randomised controlled studies would better define the role of semi elemental formulas in a variety of paediatric medical conditions.
Statement of Authorship
K. Ford and Dr H. Gilbertson contributed equally to the concept and design of the research. K. Ford contributed to the acquisition, analysis and interpretation of the data and drafted the manuscript. All authors revised the manuscript and agreed to be fully accountable for ensuring the integrity and accuracy of the work and read and approved the final manuscript.
Financial Disclosure
Project officer received Funding to complete this project by Nestle Health Science.
Ethics Statement
Ethical Approval received from The Royal Children’s Hospital Melbourne Human Research Ethics Committee (HREC). Reference number QA/54022/ RCHM-219).