Abstract
Background
Intestinal parasitic infections, especially intestinal protozoan parasites remain significant public health problem in Senegal. Several studies have demonstrated the endemicity of the diseases. The study was carried out with the objective of assessing the epidemiolocal profile of intestinal protozoan infection diagnosed among patients attending to Fann University Hospital in Dakar, Senegal.
Materials and Method
A retrospective study was conducted from 2016 to 2020. Samples were collected from patients attending to the laboratory for parasitological confirmation. Fresh stool samples were observed using direct examination, formal-ether concentration method and modified Zeilh Nielsen staining method. Descriptive analysis was performed using Stata MP 16 software. The significance level was set at 5%.
Results
Among 3825 patients selected in the study, 1009 were found with at least one intestinal protozoan parasite representing an overall prevalence of 26.4% (CI 95% (24.7– 28)). Mono-parasitic and di-parasitic infection represent 81.6% and 18.2% respectively while polyparasitism was observed in 26 patients representing 2,6%. Among positive samples, 16 (8.7%) were associated with helminths. Blastocystis sp. (40.8%), Entamoeba Coli (38.2%), Endolimax nana (8.2%) and Giardia intestinalis (8.1%) were mainly observed. Trophozoites Entamoeba histolytica was observed with 2.3%. Frequency of intestinal protozoa was higher in the 15 – 30 age group (28.3%) and in male group (26.9%). The parasite carriage was most important during the wet season comparing the dry season (p = 0.65). Asymptomatic patients (29.5%) were more infected than symptomatic patients (23.5%) (p <10-3). The main clinical symptoms were diarrhea, abdominal pain, dysenteric syndrome, fever, dyspeptic disorders, and vomiting.
Conclusion
These results showed that intestinal protozoan infections remain prevalent in Senegal with a high proportion of asymptomatic who constitute an important reservoir of parasites. Effective control strategies such as water supply, hands washing, and mass deworming campaign could reduce the prevalence of these diseases.
Author Contributions
Academic Editor: Andrei Alimov, Leading researcher (preclinical studies), Docent (academic teaching) Research Center of Medical Genetics, Moscow, Russia
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2022 Khadime Sylla, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Intestinal parasitic infections (IPIs) are a worldwide public health problem, especially in resources limited countries. Globally, about 3.5 billion people are affected by parasitic infections. The annual morbidities and mortalities due to IPIs are estimated to be over 450 million and 200,000, respectively 1, 2, 3. According to World Health Organization (WHO), children living in endemic are most affected with an estimated number of 270 million preschool children and over 600 million school children 4. This endemicity could be explained by a high population density and low environmental and socio-economic conditions such as poor hygiene conditions, low access to improved water sources, lack of standard sanitary facilities and limited economic resources 5, 6. Morbidity and indirect effects of IPIs have a substantial impact on health and quality of life 4. Two groups of parasites can colonize the digestive tract, protozoa, and helminths. Their pathogenicity is variable from asymptomatic carriage to severe morbidity cases depending on the intensity of the infection 7. Symptoms such as diarrhea, dysentery, vomiting, and anorexia are often observed. Diarrhea, including that of parasitic origin, is among the most common illnesses which cause infant and childhood mortality in developing countries 8, 9. IPIs can also be responsible of anemia, stunting, underweight, physical weakness, and low educational performance in schoolchildren 10, 11.
In Senegal, intestinal parasites are a common cause of outpatient. A study carried out at the Fann University Hospital showed a prevalence of 26.8% with a predominance of protozoa (83%) 12. Other studies carried out in community level have shown a predominance of protozoa over helminths 13, 14.
According to WHO, 10% of the world's population is infected by Entamoeba parasites. Infection with Entamoeba histolytica is the third leading cause of mortalitý by parasitic diseases in the world after malaria and bilharzia. It affects approximately 50 million people, of whom 40,000 to 110,000 each deaths year15. Giardia intestinalis infection is one of the most digestive flagelloses both in terms of frequency and pathogenicity with an estimated number of 280 million cases mainly located in Asia, Africa, and Latin America 16. Other protozoan infections such as Blastocystis sp. are gaining interest with prevalence becoming increasingly high 17
To control morbidity related to intestinal parasitic infection, several strategies are recommended by WHO, including mass deworming campaign with Mebendazole and/or Albendazole. In Senegal, this strategy has been implemented since 2006 by the Ministry of Health (MoH). This strategy has considerably reduced the frequency of intestinal parasite carriage with a decrease of helminths parasites but a persistence of intestinal protozoa parasites 13, 18. In this context, it become relevant to generate new data in order to update the epidemiology of intestinal parasites for better orienting control strategies. The objective of this study was to evaluate the prevalence of intestinal protozoan infection diagnosed in Fann University Hospital.
Materials and Methods
Study Design and Population
A retrospective and descriptive analysis was carried out from January 2016 to December 2020 in the Laboratory of Parasitology-Mycology of Fann University Hospital, which is a public referral hospital, located in the capital city of Dakar. All patients attending to the laboratory for a parasitological examination of stool samples, were included in this study.
Data Collection
Sociodemographic, clinical, and biological data from patients were collected using the laboratory records. The following variables were collected: age, sex, year of sample collection, season, clinical indication, macroscopic aspect of sample and parasitological results. Age was defined in 4 categories: less than 15 years, 15 - 30 years, 30 - 45 years, and more than 45 years. The season was defined in both the dry season (October to June) and rainy season (July to September).
Parasitological Examination
Fresh stool sample was collected into wide mouth for intestinal parasite detection. Stool samples were examined macroscopically for color, consistency, presence of blood, mucus, pus, and large worms. A portion each of the stool samples was processed with a direct examination by light microscopy to detect cysts, trophozoite, eggs and larva. The remaining part of stool samples were examined using a modified Ritchie technique and modified Zielh Neelsen technique.
Data Analysis
After data collection, data were entered in Excel software and the analysis was performed using Stata software version MP 16 software. Quantitative variables were described in terms of means, standard deviation. Inter group comparisons were performed using ANNOVA test or Student t test after checking the conditions of application of these tests. When these tests were not applicable, the non-parametric tests (Man Withney, Kruskall Wallis) were used. For descriptive data, percentage with confidence interval (CI) was used to assess the prevalence of each outcome. Proportions were compared using chi-square test or the Fisher exact test (univariate analysis). The significance level of the different tests was 0.05 two-sided.
Ethics Considerations
This study was conducted according to the Declaration of Helsinki and existing national legal and regulatory requirements. To respect the confidentiality, an identification code was given to each participant. Data are routinely collected from patients who attended to hospital for biomedical testing. Just a permission to used data for publication was requested from the administration of Fann University Hospital which a National Reference Hospital.
Results
General Characteristics of Study Participants
Overall, 3285 patients with completed data were enrolled in the analysis. The age of the patients ranged from 5 to 93 years with an average of 26.2±20.6 years. Study population was mainly represented by subject under 15 years old (41.5%) followed by subjects in the age category 15-30 years with 22.3%. The study population was predominantly male (50.8%). The sex ratio was 1.03. Stratifying on the season, 76.3% of the sample of our study was collected during the dry season against 23.7% during the wet season (Table 1).
Table 1. General characteristic of study participant (N=3825)| Variable | Frequency (n) | Percentage (%) | 95% CI |
|---|---|---|---|
| Year | |||
| 2016 | 817 | 21.4 | 19.9 – 22.8 |
| 2017 | 928 | 24.3 | 22.7 – 25.8 |
| 2018 | 905 | 23.6 | 22.1 – 25.1 |
| 2019 | 722 | 18.8 | 17.5 – 20.3 |
| 2020 | 454 | 11.8 | 10.8 – 13.1 |
| Age group | |||
| < 15 years | 1586 | 41.5 | 37.9 - 42 |
| (15-30) | 851 | 22.3 | 20.7 – 23.8 |
| (30-45) | 645 | 16.9 | 15.6 – 18.3 |
| ≥45 years | 743 | 19.4 | 18 – 20.8 |
| Sex | |||
| Female | 1880 | 49.2 | 46.9 – 51.5 |
| Male | 1945 | 50.8 | 48.6 – 53.2 |
| Season | |||
| Wet | 909 | 23.7 | 22.2 – 25.4 |
| Rainy | 2916 | 76.3 | 73.5 – 79.1 |
| Total | 3825 | 100 |
Clinical Characteristics of Participant
Among 3825 participants, 2017 (52.7%) were symptomatic. The main clinical symptoms were (i) acute febrile diarrhea 2.33%, (ii) acute non-febrile diarrhea 8.3%, (iii) chronic diarrhea on HIV1 terrain 4.6% and (iv) chronic diarrhea without notion of HIV1 10.6%. Abdominal pain was found in 11.1% of cases. Dyspeptic disorders, fever and dysenteric syndrome were observed with respectively 4.8%, 4.7% and 1.6% (Table 2)
Table 2. Clinical symptoms of study participants (N=2017)| Symptom | Frequency (n) | Percentage (%) |
|---|---|---|
| Weight loss | 30 | 0.8 |
| Bronchopneumonia | 17 | 0.4 |
| Constipation | 21 | 0.6 |
| Acute febrile diarrhea | 89 | 2.3 |
| Acute non-febrile diarrhea | 313 | 8.2 |
| Chronic diarrhea (HIV positive) | 409 | 10.7 |
| Chronic diarrhea (HIV negative) | 176 | 4.6 |
| Abdominal pain | 424 | 11.1 |
| Inflammatory stomach disease | 03 | 0.1 |
| Malnutrition | 06 | 0.2 |
| Clinical anemia | 28 | 0.7 |
| Anal pruritus | 21 | 0.5 |
| Rectorrhagia | 11 | 0.3 |
| Dysenteric syndrome | 63 | 1.6 |
| Fever | 180 | 4.7 |
| Dyspeptic disorders | 186 | 4.8 |
| Urticaria | 22 | 0.6 |
| Dizziness | 02 | 0.1 |
| Vomiting | 14 | 0.1 |
| Total | 2017 | 100 |
Prevalence of Intestinal Protozoan Infection
Overall, 1009 samples were positive, which represents a prevalence of intestinal protozoan infection of 26.4%. Mono-parasitic infection defined as presence of one parasite was noted in 81.6%. Bi-parasitism (presence of two parasites) and poly-parasitism (more than two parasites) were found in 18.2% and 2.6% respectively. Association between protozoan and helminth was observed in 8.7% (Table 3). The most prevalent parasites were Blastocystis sp. (40.8%), Entamoeba Coli (38.2%), Endolimax nana (8.2%) and Giardia intestinalis (8.1%). Entamoeba histolytica was observed with 2.3%. Oocyst Isospora belli was observed with a frequency of 0.8% (Table 4). The main co-infections detected in di-parasitism were Blastocystis sp. + E. Coli (20.1%), E. Coli + E. nana (18.5%), Trophozoite E. Coli + Cyst E. Coli (15.2%), E. nana + Blastocystis sp. (8.7%), Blastocystis sp. + G. intestinalis (8.2%).
In poly-parasitism, the most prevalent association were: Blastocystis sp. + E. Coli + E. histolytica (7.7%), Blastocystis sp. + E. Coli + Pseudolimaxbutschili (7.7%).
Table 3. Global prevalence of intestinal protozoan infection| Frequency (n) | Percentage (%) | 95% CI | |
| Parasitological results | |||
| Negative | 2816 | 73.6 | 70.9 – 76.3 |
| Positive | 1009 | 26.4 | 24.7 – 28.0 |
| Parasitism | |||
| Mono- parasitism | 824 | 81.7 | 75.8 - 87 |
| Di- parasitism | 184 | 18.2 | 16.1 – 21.5 |
| Poly- parasitism | 26 | 2.6 | 1.8 - 4 |
| Type of association | |||
| Protozoa - protozoa | 168 | 91.3 | 78 – 99.9 |
| Protozoa - Helminth | 16 | 8.7 | 4.9 – 14.1 |
| Protozoan parasite | Frequency (n) | Percentage (%) | 95% CI |
|---|---|---|---|
| Blastocystis sp | 412 | 40.8 | 36.9 – 44.9 |
| Entamaeba coli | 385 | 38.2 | 34.4 – 42.2 |
| Endolimax nana | 83 | 8.2 | 6.5 – 10.2 |
| Giardia intestinalis | 82 | 8.1 | 6.5 – 10.1 |
| Entamoeba histolytica | 23 | 2.3 | 1.4 – 3.4 |
| Pseudolimax butschili | 8 | 0.8 | 0.3 – 1.7 |
| Oocyste Isospora belli | 8 | 0.8 | 0.3 – 1.7 |
| Trichomonas intestinalis | 4 | 0.4 | 0.1 – 1.1 |
| Chilomastix meslini | 2 | 0.2 | 0.02 – 0.7 |
| Entamoeba hartmani | 2 | 0.2 | 0.02 – 0.7 |
| Total | 1009 | 100 |
Prevalence of Intestinal Potozoan Infection According to Study Participant Characteristics
Stratifying by year of sample collection, the results showed that the frequency of intestinal protozoa was higher in 2017 and 2020 with 29.7% and 31.9%, respectively. The lowest positivity rate was noted in 2018 (20.9%). The difference was statistically significative between the year of sample collection (p<10-3) (Figure 1).
Figure 1.Prevalence of Intestinal Protozoa Infection according to the year of sample collection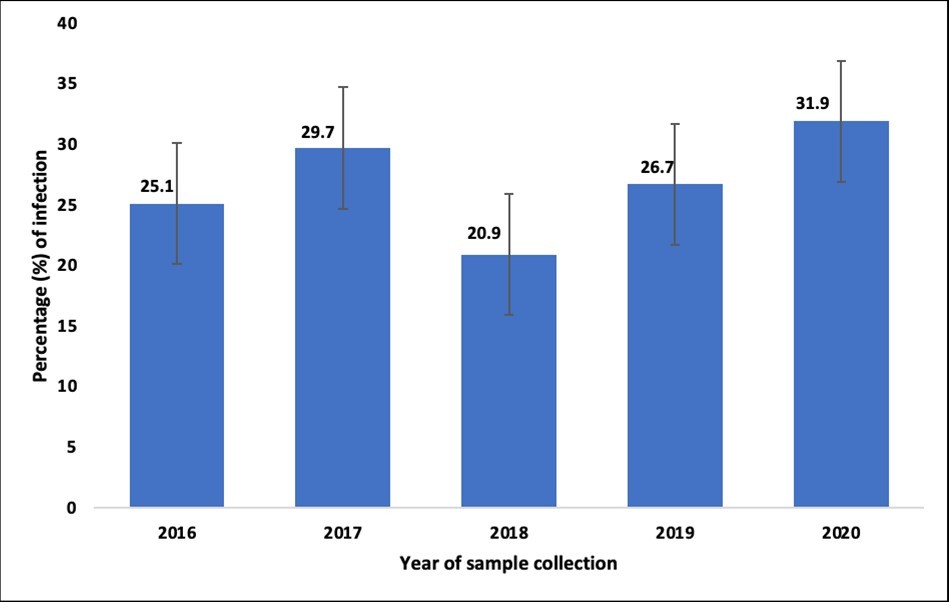
According to age category, intestinal protozoa carriage was more important in patients aged 15 - 30 years with 28.3%. The prevalence in subject under 15 years old was 25.9%. The difference between the age group was not significative (p=0.52). Intestinal protozoa prevalence was higher among male participants (29.9%) compared to female participants (25.7%) (p=0.57). During the rainy season, patients were more infected (26.9%) compared to dry season where the frequency of intestinal protozoan infection lower with no significant difference (p=0.65). According to symptoms, the prevalence was more important in asymptomatic subjects (29.5%) compared to symptomatic ones (23.5%) (Table 5).
Table 5. Prevalence of intestinal protozoan infection according to characteristics of study participant (N=1009)| Variable | Frequency (n) | Percentage (%) | 95% CI | P value |
|---|---|---|---|---|
| Age group | ||||
| < 15 years | 412 | 25.9 | 36.9 – 44.9 | |
| (15-30) | 241 | 28.3 | 20.9 – 27.1 | |
| (30-45) | 168 | 26.1 | 14.2 – 19.4 | |
| ≥45 years | 188 | 25.3 | 16.1 – 21.5 | 0,52 |
| Sex | ||||
| Female | 484 | 25.7 | 48.8 – 52.4 | |
| Male | 525 | 26.9 | 47.6 – 56.7 | 0.38 |
| Season | ||||
| Wet | 245 | 26.9 | 21.3 – 27.5 | |
| Rainy | 764 | 26.2 | 70.4 – 81.3 | 0.65 |
| Symptom | ||||
| Asymptomatic | 534 | 29.5 | 48.5 – 57.6 | |
| Symptomatic | 475 | 23.5 | 42.9 – 51.5 | <10 -3 |
In symptomatic subjects, patients with acute febrile diarrhea were more infected 29.2%. The prevalence in patients with acute non-febrile diarrhea was 25.5%. In patients with chronic diarrhea (HIV positive), the prevalence of intestinal protozoan infection was 21.2%. Among patients with dysenteric syndrome and dyspeptic disorders, intestinal protozoa carriage was 23.8% and 21.8% respectively (Figure 2).
Figure 2.Prevalence of Intestinal Protozoa Infection according to the clinical symptom
Prevalence of Intestinal Protozoan Infection According to According to the Aspect of Stool Samples and the Presence of Cellular Elements in Stool Samples
According to the aspect stool samples, intestinal protozoa parasites were more frequent when the stool appearance was consistent (26.9%). The prevalence of intestinal protozoa parasites in patients with watery and watery+mucus stools was 20.8% and 19.8% respectively. Patients with watery stools with blood and mucus had a positivity rate of 21.5%. The prevalence of intestinal protozoan parasite in patients with yeast on microscopic examination was 12.5%. Parasites carriage in patients with filaments on microscopy was 10% and 7.1% in those with red blood cells in the stool. No parasites were observed in patients with leukocytes in the stool (Table 6).
Table 6. Intestinal protozoan carriage according to the aspect of stool samples and the presence of cellular elements in stool samples| Variable | Total samples (N) | Positive samples (n) | Percentage (%) | 95% CI | P value |
| Aspect of stool samples | |||||
| Consistent | |||||
| Yes | 1486 | 401 | 26.9 | 24.4 – 29.7 | |
| No | 2339 | 608 | 25.9 | 23.9 – 28.1 | 0.49 |
| Watery+Mucus | |||||
| Yes | 384 | 80 | 20.8 | 16.5 – 25.9 | |
| No | 3441 | 929 | 27 | 25.3 – 28.8 | 0,01 |
| Watery only | |||||
| Yes | 342 | 68 | 19.8 | 15.4 – 25.2 | |
| No | 3483 | 941 | 27.02 | 25.3 – 28.8 | <10 -3 |
| Watery+Blood+Mucus | |||||
| Yes | 65 | 14 | 21.5 | 11.7 – 36.1 | |
| No | 3760 | 995 | 26.5 | 24.8 – 28.2 | 0.37 |
| Cellular elements in the stool samples | |||||
|---|---|---|---|---|---|
| Yeast | |||||
| Yes | 104 | 13 | 12.5 | 6.5 – 21.4 | |
| No | 3721 | 996 | 26.7 | 25.1 – 28.4 | <10 -3 |
| Filament | |||||
| Yes | 20 | 2 | 10 | 16.5 – 25.9 | |
| No | 3805 | 1007 | 26.5 | 25.3 – 28.8 | 0.09 |
| Red blood cells | |||||
| Yes | 14 | 1 | 7.1 | 0.2 – 3.9 | |
| No | 3811 | 1008 | 26.5 | 28.8 – 28.1 | 0.1 |
| Leukocytes | |||||
| Yes | 19 | 0 | 0.0 | ||
| No | 3805 | 1009 | 26.5 | 24.5 – 28.2 | 0.01 |
Discussion
Intestinal parasitic infections are major cause of morbidity and mortality worldwide especially in developing countries. With the implementation of mass deworming campaign with Mebendazole/Albendazole, there is a rarefaction of helminths and a persistence of intestinal protozoa.
The results of our study showed an overall prevalence of 26.4% of intestinal protozoan infection. Sylla et al when studying the epidemiological aspects of intestinal parasitic infection in the same hospital between, have found a prevalence of 26.8% with a predominance of protozoa (83%) 19. Similar trends were previously described by Tine et al and Faye et al who reported 29.6% and 23.7% prevalence of intestinal protozoa parasites respectively14, 19 . Other studies conducted in worldwide have showed the endemic characters of intestinal protozoa parasites. In Côte d’Ivoire, Ouatara et al when assessing the prevalence and polyparasitism of intestinal protozoa in school aged children have noted a high intestinal protozoa prevalence 98.5% (4398/4466) 20. Afshar et al in Iran reported high prevalence of protozoan parasites 32.3% (95% CI 28.4 to 36.5) compared helminthic parasites 3.2% (95% CI 2.1 to 4.7) 21.
The species most frequently found in our series were Blastocystis sp. (40.8%), E. coli (38.2%), E. nana (8.2%), G. intestinalis (8.1%), E. histolytica (2.3%).These species are therefore mostly non-pathogenic except G. intestinalis. Diongue et al in 2017 have found́ in their series Entamoeba coli (51.5%), E histolytica (17%), G. intestinalis (10.5%) and Blastocystissp (5.6%) 22. The high frequency of intestinal protozoa parasites was described in other countries. In Benin by Nicolas et al have showed 76.03% intestinal parasite prevalence with high proportion of protozoa parasites (87.5%). The main protozoa species were Giardia lamblia (39.42%), E. histolytica (25.96%), E. coli (11.54%), Trichomonas intestinalis (10.58%)23. Dorkenoo et al in Togo when studying the prevalence of Soil-Transmitted Helminths and Intestinal Protozoa among School Children in Lomé have observed high prevalence of intestinal protozoa (52.2%) with the following main species: E. coli (16.5%), E. histolytica (2.3%), G. lamblia (11.5%), Blastocystis sp. (10.9%) 24. Similar species were noted in Marocco by Hafida et al with Blastocystis sp. (30.2%), E. coli (25.6%), G. intestinalis (17.4%) and E. histolytica (10.5%)25. Au Tchad, Emmanuel et al have showed 39.5% prevalence of intestinal parasites. The parasitism was mainly due to protozoa with 97%. The main parasites identified are G. intestinalis (27%), E. histolytica (21%), E. coli (18%) T. intestinalis (15%) 26.
The results from this study showed that intestinal protozoa carriage was more important in patients aged 15 - 30 years with a percentage of 28.3%. The prevalence in subject under 15 years old was 25.9%. The lower frequency observed in the 0-15 years category (25.9%) is probably the result of the action of the Ministry of Health which since 2006 has implemented regular systematic deworming campaigns with Mebendazole/Albendazole in children in all health districts of Senegal.
Our results showed that the frequency of protozoa parasite was higher in males (26.9%) than in females (25.7%). Similar results showing predominance of intestinal protozoan infection in male patients were observed in Yemen and Malaysia 27, 28. Other studies conducted in Senegal have demonstrated that the prevalence of intestinal protozoa parasites was more important among female participants14, 23. This is in line with previous study conducted in Kenya where the prevalence of intestinal parasitic infection was higher in female participant (42.5%) compared to male participants (41.6%) 29.
Regarding the season, the results from our study showed that intestinal protozoa carriage was is more frequent during the rainy season (26.9%). This situation could be explained by a degradation of hygiene conditions observed during the period. Similar results were obtained by Sylla et al with a higher frequency in rainy season (27.27%) than in the dry season 26.5% 12.
According to the aspect of stool samples, the results of this study showed that intestinal protozoa parasite carriage was observed in patients with watery stool and watery+mucus stools was 20.8% and 19.8% respectively.Patients with watery stools with blood and mucus had a positivity rate of 21.5% This was previously described in Rwanda by Emilie et al who noted that intestinal parasites were found in patients having watery stool (78.9%), watery+mucus (42.2%). Low parasite carriage (8.3%) was noted in patients with watery+mucus+blood in their stool samples 30. In patients with consistent stool sample, similar results were noted in Rwanda.
Therefore, our results point out that in developing countries intestinal parasitic infection is still endemic despite the implementation of mass deworming campaign in children and other age groups. Intestinal protozoa parasites are frequent with a predominance of non or low pathogenic forms.
Conclusion
The study showed that intestinal protozoan infection is a public health concern in health facilities in Senegal. Adults and male subjects are more infected. In the rainy season which is associated with degradation of socio-environmental factors, the prevalence intestinal protozoan infection is high. Therefore, there is a need to improve access to safe water, quality of sanitary facilities and also to sensitize populations on the respect of hands washing in order to limit the spread of parasites. Additional studies should be conducted with advanced microscopic and molecular techniques that can be helpful for a better diagnosis of intestinal parasites and to update the epidemiology of the disease in order to take appropriate prevention and control measures.
Funding
This study was not funded. Funding for data collection was covered by the Laboratory of Parasitology-Mycology, of Fann teaching Hospital.
Acknowledgment
We would like to acknowledge the entire study participant and the staff of the laboratory of parasitology at Fann Teaching Hospital.
Authors’ Contributions
KS conceived and designed the study. KS supervised the data collection. KS analyzed the data and wrote the first draft of the manuscript. All authors read and approved the final manuscript.
Availability of Data and Material
The data used for this research article are available from the corresponding author upon request.
References
- 1.Abbaszadeh Afshar MJ, Barkhori Mehni M, Rezaeian M, Mohebali M, Baigi V et al. (2020) Prevalence and associated risk factors of human intestinal parasitic infections a population-based study in the southeast of Kerman province southeastern Iran. , BMC Infect 20(12), 1-8.
- 2.Abdullah I, Tak H, Ahmad F, Gul N, Nabi S et al. (2016) Predominance of gastrointestinal protozoan parasites in children. a brief review. , J Health Educ Res Dev 4, 4.
- 3.Akhlaghi L, Mafi M, Oormazdi H, Meamar A, Shirbazou S et al. (2013) Frequency of intestinal parasitic infections and related factors among primary school children in Abyek township of Qazvin province (2011-2012). , AnalBiol.Res 4, 22-26.
- 4.Al-Mekhlafi A M, Abdul-Ghani R, Al-Eryani S M, Saif-Ali R, Mahdy M A. (2016) School-based prevalence of intestinal parasitic infections and associated risk factors in rural communities of Sana'a Yemen. , Acta Trop 163, 135-41.
- 5.Alum A, Rubino J R, Ijaz M K. (2010) The global war against intestinal parasites-should we use a holistic approach. , Int J Infect Dis 14(9), 732-738.
- 6.Ayeh-Kumi P F, Quarcoo S, Kwakye-Nuako G, Kretchy J P, Osafo-Kantanka A et al. (2009) Prevalence of intestinal parasitic infections among food vendors in Accra. , Ghana. J Trop Med Parasitol 32(1), 1-8.
- 7.Diongue K, Ndiaye M, Seck M C, Diallo M A, Ndiaye Y D et al. (2017) Diseases due to intestinale protozoa diagnosed at Le Dantec University Hospital in Dakar. , Senegal, from 27(4), 431-434.
- 8.Dorkenoo M A, Agbeko F, Dokoto H, Plate D, Fiawoo M et al. (2021) . Prevalence of Soil-Transmitted Helminths and Intestinal Protozoa among School Children in Lome , Togo, Open J Pediatr 11, 313-328.
- 9.Emile N, Bosco N J, Karine B. (2013) Prevalence of intestinal parasitic infections and associated risk factors among Kigali Institute of Education students in Kigali. , Rwanda. Trop Biomed 30(4), 718-726.
- 10.Emmanuel I.Abdelrazzack AF, Ameyapoh YB (2021). Place of intestinal parasites in diarrhoea in children aged 0 to 5 years in the eastern Logone province (Doba-Chad). , Int J Med Res Pharm Sci 894, 1-9.
- 11.Faye N K. (2021) Protozooses intestinales diagnostiquées au laboratoire de parasitologie-mycologie du CHU Le Dantec de Dakar de 2016 à 2020. Pharm D Thesis. Université Cheikh Anta Diop. , Dakar, Senegal
- 12.Fischer Walker CL, Aryee M J, Boschi-Pinto C, Black R E. (2012) Estimating diarrhea mortality among young children in low and middle income countries. PLoS One. 7(1), 29151.
- 13.Hafida H, Khadija E K, Driss B. (2017) . Prospective Study of Intestinal Parasites in Children Hospitalized in the Pediatric Department at the Hospital in El Eldrissi (Kenitra City - Morocco). Eur Sci 13(18), 520.
- 14.Kavili D M, Simbauni J A, Gicheru M M, Mungiria J N. (2016) Prevalence and Risk Factors ofEntamoeba histolyticaamongst Children Attending Primary Schools in Kyuso Zone, Kyuso District, Kitui County. , Kenya. J. Appl. Life Sci. Int 7(1), 1-12.
- 15.Keogh M B, Castro-Alférez M, Polo-López M I, Fernández Calderero I, Al-Eryani Y A et al. (2015) Capability of 19-L polycarbonate plastic water cooler containers for efficient solar water disinfection (SODIS): field case studies in India, Bahrain and Spain. Sol Energy. 116, 1-11.
- 16.Lobo M L, Augusto J, Antunes F, Ceita J, Xiao L et al. (2014) Cryptosporidium spp., Giardia duodenalis, Enterocytozoon bieneusi and other intestinal parasites in young children in Lobata Province, Democratic Republic of São Tomé and Principe. , PLoS One 9(5), 97708.
- 17.Magne D, Chochillon C, Savel J, Gobert J G. (1996) Giardia intestinalis et giardiose. , J Pediatrie Pueric 9(2), 74-83.
- 18.Mbuh J V, Ntonifor H N, Ojong J T. (2010) The incidence, intensity and host morbidity of human parasitic protozoan infections in gastrointestinal disorder outpatients in Buea Sub Division, Cameroon. , J Infect Dev Ctries 4(01), 038-043.
- 19.Nicolas A, Olounlade P, Degbey C, Theodora A A, Zannou L et al. Zone Saint Jean De Dieu De Tanguieta (Hz Sjdt) Atacora (Republic of Benin, West Africa). Aïkou Nadine LM, Aïkou Arielle NE (2021). Prevalence of Intestinal Parasitosis in the Pediatrics Department at the Hospital of , South Asian Journal of Parasitology 5(1), 17-25.
- 20.Noor Azian MY, San Y M, Gan C C, Yusri M Y, Nurulsyamzawaty Y et al.Vythilingam I (2007).Prevalence of intestinal protozoa in an aborigine community in Pahang Malaysia. Trop Biomed. 24(1), 55-62.
- 21.Okyay P, Ertug S, Berna G, Ozlem O, Erdal B. (2004) Intestinal parasites prevalence and related factors in school children, a western city sample-turkey. , BMC Public Health 4, 64.
- 22.Ouattara M, Silué K D, N’Guéssan A N, Yapi A, Barbara M et al. (2008) Prévalences et polyparasitisme des protozoaires intestinaux et répartition spatiale d’Entamoeba histolytica/Entamoeba dispar et Giardia intestinalis chez des élèves en zone rurale de la région de Man en Côte-d’Ivoire. Cahiers d'études et de recherches francophones/Santé. 18(4), 215-222.
- 23.Sow D, Sylla K, Dieng T, Tine R C, Ndiaye M et al. (2015) Infection par Blastocystis hominis au Sénégal : Aspects épidémiologiques, cliniques et parasitologiques des cas diagnostiqués au CHNU de Fann à Dakar. Rev CAMES Santé. 3, 2.
- 24.Sylla K, Tine R C, Sow D, Dieng T H, Faye B et al. (2013) Aspects épidémiologiques des parasitoses intestinales diagnostiquées au laboratoire de parasitologie-mycologie du centre national hospitalier de fann. , Med Afr Noire 60(7), 339-346.
- 25.Sylla K, Tine R C, Sow D, LA Lelo S Ndiaye, Faye B T et al. (2018) et al. Epidemiological profile of intestinal parasitic infection among preschool and school children living in a rural community in Senegal: a cross sectional survey. , J Bacteriol Parasitol 9(4), 2.
- 26.Tine R C, Sylla K, Sow D, Lelo S, Ngom D et al. (2018) Low prevalence of soil transmitted helminths among children in rural areas in Senegal: A cross sectional survey. , JPVB 10(1), 19-25.
- 27.Tine R C, Faye B, Ndour C T, Sylla K, Sow D et al. (2013) Parasitic infections among children under five years in Senegal: prevalence and effect on anaemia and nutritional status. , ISRN Parasitol 272701, 6.
