Abstract
Fetal surgery is the newest surgical specialty with a compelling history. The development of fetal surgery began in primates and lambs and, in its most basic form, was first performed in humans in 1965. Since its introduction, the field has expanded and changed dramatically. Several of these changes have involved the ethical aspect of fetal surgery. This field conflicts with the Hippocratic oath mantra of “first do no harm” as one of the patients, the mother, receives no benefit from these procedures. The ethical dilemma resulted in stringent inclusion and exclusion criteria for fetal operations. Initially, fetal surgery was only indicated for life-threatening conditions of the fetus but is now offered in some disease processes to improve quality of life for the child. As the field has matured, it has grown to encompass numerous different types of fetal interventions. Similar to other areas of surgery, the trend has been to migrate from more invasive to less invasive procedures. Currently, some of these therapies are performed entirely percutaneously. Theoretically, this trend would improve outcomes for both the mother and fetus. While this has generally proven true, there are some important exceptions to this rule. Finally, as the field continues to evolve, much research is being performed looking at possible new types of fetal interventions. Some of these procedures, such as fetal stem cell therapy and fetal gene therapy, could change the face of modern medicine.
Author Contributions
Academic Editor: Ahmed El-Sabbagh, assistant professor of plastic surgery, faculty of medicine,mansoura university, egypt
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2017 Michelle Knezevich, et al
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
History of Fetal Surgery
Amniocentesis paved the way for fetal surgery and intervention. The first report of amniocentesis in the literature was in Germany by Lambl in 1881 where it was used to decompress polyhydramnios1. It was not until decades later that amniocentesis became a diagnostic tool. However, even in 1952, when Bevis utilized amniocentesis, it was to determine the severity of Rh erythroblastosis, not as it is typically thought of today2. Yet, just a few short years later, Fuchs and Riis showed that amniocentesis could be used for antenatal sex determination and detection of hereditary diseases3,4. Once this modern phase of amniocentesis arrived, a wave of antenatal interventions ensued in the 1960s. Most notable was Liley’s contribution of intrauterine transfusion for Rh erythroblastosis in 19655. This methodology was made safer by use of ultrasound guidance in the 1970s but is still used today.
Fetoscopy began as a way to augment amniocentesis by allowing direct visualization into the uterine cavity and to obtain tissue samples (typically blood or skin)6. In 1975, Benzie and Doran used a fetoscope to visualize the intrauterine contents prior to saline abortion7. By 1979, fetoscopy was still considered a risk to the mother and fetus but was deemed appropriate when the fetus was at risk for teratologically induced malformation, inherited blood dyscrasias, chromosomal abnormalities with gross malformations or neural tube defects that could not be diagnosed with amniocentesis8. The field advanced rapidly and by 1986, Rodeck and Nicolaides published a review article detailing techniques for obtaining fetal blood, skin, liver, tumor specimen and chorionic villi9.
In 1981, fetal surgery moved from diagnostic tool to a therapeutic tool in a non-human primate experimental model. Michejda and Hodgen devised what they called the HAVIT (hydrocephalic antenatal vent for intrauterine treatment10. After placement of shunts in these hydrocephalic non-human primates, they found increased survival to delivery, superior postnatal motor skills and improved postnatal weight gain10. Simultaneously in 1981, other groups used an ovine model of congenital diaphragmatic hernia11,12. In this model system, in utero repair of the diaphragmatic defect improved lung histology and size11,12. By 1985, primate models had been established for obstructive uropathy and congenital diaphragmatic hernia13,14. Further research in the primate model, showed worsening preterm delivery rates with increasing manipulation of the uterus15. These studies were able to identify the use of inhaled anesthetics as an important aid in decreasing uterine activity15. Likewise, the primate model revealed many of the maternal complications that could be expected, demonstrated that metal staples markedly decreased subsequent fertility by 50%, and showed that future pregnancies were possible after fetal surgery16. Of importance, 92.6% of primates were fertile and capable of future pregnancies with the use of absorbable sutures16.
Also in 1981, the Dr. Michael Harrison performed the first successful fetal surgery in humans for obstructive uropathy 17and personal communication. By 1987, a review was published of 57 cases of fetal vesicoamniotic shunts18. Nearly half of the cases had complications and only 21% of those fetuses with oligohydramnios survived suggesting the need for a randomized control trial to determine the utility of this fetal procedure 18. In 1988, a similar paper was published regarding fetal intervention for hydrocephalus19. This paper described 40 cases of fetal shunting, the world-wide experience at that time19. Ten percent of the fetuses were thought to have died as a direct complication of the procedure19. Of the remaining viable fetuses, their outcomes were similar to published data for untreated infants, again questioning the utility of the procedure and need for a randomized control trial19. In 1991, UCSF described their 8 year experience with open fetal surgery with 17 cases20. Indications included severe bilateral hydronephrosis, congenital diaphragmatic hernia, sacrococcygeal teratoma, and congenital cystic adenomatoid malformation20. They described their fetal outcomes as disappointing despite having previously performed >1500 operations on fetal lambs and >200 on fetal monkeys20. Similarly, in 1997, the UCSF published their results from a prospective trial on in utero repair of congenital diaphragmatic hernia21. Just as in obstructive uropathy and hydrocephalus, fetal surgery did not improve survival21. While it had been clearly demonstrated that fetal surgery was possible, many questioned the future of fetal surgery20.
Yet, Dr. Michael Harrison, arguably the greatest pioneer in fetal surgery, continued to perform research on fetal surgery in lambs and monkeys and continued to refine criteria for fetal intervention22. Indeed as fetoscopy advanced, its use for twin-twin transfusion syndrome, twin reversed arterial perfusion sequence and tracheal occlusion for congenital diaphragmatic hernia became new potential interventions with optimistic outcomes23,24. Likewise, other indications for fetal surgery showed promise. For example, fetuses with congenital pulmonary airway malformations, whom developed hydrops were deemed candidates for fetal repair as fetal demise was inevitable without repair25. Eight of 13 hydropic fetuses without a dominant cyst were treated with open lobectomies had reversal of hydrops, substantial lung growth and were delivered viable26. Five of six fetuses with dominant cysts underwent fetoscopic thoracoamniotic shunting procedures and survived26. Similarly, two fetuses with hydrops as a result of a large saccrococcygeal teratoma were salvaged by tumor excsion24. With these new successes, the promise of fetal surgery became apparent and the innovation continued.
Ethics of Fetal Surgery
Shortly after the development and implementation of fetal surgery, articles began to emerge regarding the ethics of fetal surgery. These articles first began discussing the time point at which the fetus had rights as a person. These first articles focused on the definition of viability27. The definition of viability continues to change with improved postnatal care. It is unlikely this controversial topic will be resolved in the foreseeable future.
Next, the question of whether a mother has the right to refuse operations for the fetus. This query also prompted the question of the father’s role in this decision making28. In the same article, attention was given to the ethics of fetal pain, which had originally come into the limelight by those opposed to abortions. This was the same era as Roe vs Wade, thus the topic was highly controversial. With regards to fetal surgery, the ethics of fetal pain was highlighted because little mention had been made in descriptions of fetal surgeries regarding fetal analgesics28. It was not until an article published in 1987 showed that premature infants undergoing patent ductus arteriosus ligation had a sizeable stress response to cardiac surgery which was reduced with opioid administration, did analgesic use become the standard of care for neonatal surgery29. The fact that a fetus feels pain was clearly established in the mid-1990s by two groups 30,31. Thus, while most anesthetics cross the placenta to the fetus, direct fetal administration of opioids and muscle relaxants is often added.32
In 1991, UCSF published their experience with 17 open fetal surgery cases. This paper really highlighted what became the current era of fetal surgery ethics20. In this paper it is acknowledged that the goal of fetal surgery is to improve the health of the future child20. To reach this goal, there is risk imposed upon the mother with regard to operative risk and mid-gestation hysterotomy, risk of premature labor, and the risk of compromising further reproductive potential20. They describe the creation of their Fetal Treatment Program which stringently evaluated the fetuses and mothers included in the study20. Over the course of the 8 years of their study, the screening criteria changed20. While it has been shown that women who undergo hysterotomy for fetal surgery can go onto have subsequent children, there continues to be concern for uterine rupture with active labor and thus the ethical dilemma of balancing maternal and fetal health.
With maternal health in mind, Dr. Michael Harris first proposed that fetal surgery should only be considered for conditions which could not be fixed after birth and for which the true mortality rate was defined. To that end, in 1982, a group of perinatal obstetricians, surgeons, ultrasonographers, pediatricians, bioethicists and physiologists convened to draft a consensus statement regarding criteria that must be met for fetal surgery to ethically be performed, regardless of the invasiveness of the procedure 33 (Table 1).
Table 1. Ethical considerations for fetal surgery| The ability to accurately diagnose and stage the illness, with exclusion of associated anomalies |
|---|
| The course of the disease is well understood, has been documented, and the prognosis is established |
| There are no known effective postnatal interventions |
| In utero surgery is proven viable in animal models, correcting the damaging effects of the diagnosis |
| Interventions have been performed in specialized multidisciplinary fetal treatment centers abiding by stringent protocols and with the approval of the local Ethics Committee with informed consent of the mother or parents. |
Types of Fetal Surgery
Open Fetal Surgery
Open fetal surgery is the most invasive form of fetal surgery. It involves both a maternal laparotomy and a hysterotomy. Currently, the indications for open fetal surgery fall in two main categories: those that are life threatening and myelomeningocele. Life threatening indications historically included fetuses with hydrops. However, a recent study by Cass et al demonstrated that hydrops alone should not be an indication for open fetal surgery in congenital lung masses or vascular tumors (this included sacrococcygeal teratomas, cervical teratomas, and hemangioendotheliomas)34. Rather, hydrops with evidence of worsening cardiac function confirmed by cardiac echocardiogram is required to be a candidate for open fetal surgery34. Those with congenital lung masses and hydrops with normal echocardiograms all survived without fetal surgery34. Whereas, in the group of fetuses with vascular tumors, the presence of hydrops and high-output cardiac failure best predicted fetal demise and need for open fetal surgery34. It is important to note that the numbers were small in this study and further investigation will be necessary to verify and document these results.
Myelomeningocele is the only accepted indication for open fetal surgery in a non-lethal condition. Similar to the criteria that was developed for fetal surgery, when the Management of Myelomeningocele Study (MOMS) was incepted at Children’s Hospital of Philadelphia, Vanderbilt University, and the University of California, San Francisco; stringent inclusion and exclusion criteria were identified (Table 2)35.
Table 2. Inclusion and exclusion criteria for surgical management of Myelomeningocele| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Singleton pregnancy | Other fetal anomalies |
| Lesion between T1 and S1 | Acute kyphosis in the fetus |
| Indication of hindbrain herniation | Risk of preterm birth |
| Gestational age 19-25.9 weeks | Placental abruption |
| Typical karyotype | BMI greater than 35 kg/m2 |
| Mother is at least 18 years of age | Surgery is inadvisable |
| History of fetal surgery or hysterotomy |
Once open fetal surgery is deemed appropriate and ethical, informed consent is the next pivotal step. Full disclosure includes a clear explanation that the mother’s health will take precedence during the procedure. It must be transparent that if the mother’s health is in jeopardy, the operation will abate or the fetus may be delivered regardless of gestational age or likelihood of survival.
Likewise, risks to the mother and fetus must be discussed. Risks to the mother include those risks of open abdominal surgery, tocolytic therapy, and bed rest36. This includes surgical site infection, chorioamnionitis, bleeding requiring transfusion, pulmonary edema, thromboembolic events, uterine dehiscence and risk of fetal demise 36,37. In addition to intraoperative and postoperative maternal risks, potential complications with subsequent pregnancies should be discussed. Placental accreta and uterine rupture are life-threatening complications of which the mother should be made aware. She should be thoroughly informed that any subsequent pregnancy will require cesarean section at 37 weeks to avoid the risk of uterine rupture 38,39.
Once maternal risks have been established, attention can turn to fetal risks. The most common fetal risks are preterm premature rupture of membranes and preterm labor. Figure 1 Greater than 1/3 of open fetal myelomeningocele repair cases were complicated by preterm labor despite multimodal therapy with bedrest, tocolytics and analgesics40. Research is being conducted on additional ways to decrease the incidence of preterm premature rupture of membranes and preterm labor (see Research and Future directions below).
After informed consent is obtained, the surgical intervention may proceed. The mother is placed on the operating room table with a left tilt, sequential compression devices in place and invasive blood pressure monitoring. General anesthesia is induced with high concentrations of inhaled anesthetic to maintain uterine relaxation with the goal of minimizing fetal cardiac dysfunction during surgery41,42. Supplementing desflurane with intravenous anesthesia has been shown to reduce fetal cardiac dysfunction during open fetal surgery. High doses of inhalational anesthesia can decrease maternal cardiac output with associated significant decreases in uterine blood flow. Diminished uterine blood flow results in decreased oxygen delivery to the placenta leading to subsequent fetal hypoxia. Supplemental intravenous anesthesia with desflurane may cause less acute fetal cardiac dysfunction compared to traditional high-dose inhalation anesthesia during fetal surgery. The fetus is continuously monitored intraoperatively. This can include pulse oximetry, ultrasound, echocardiogram, and/or electronic fetal monitoring41,43. This allows prompt detection of bradycardia which often precedes hypoxemic events.
Once monitoring devices are in place, a Pfannensteiel, Maylard or lower midline maternal laparotomy is made followed by a hysterotomy. The hysterotomy is made as far from the placental edge as possible (at least 4cm), in order to minimize bleeding44. This may require exteriorizing the uterus to perform a posterior hysterotomy, if the placenta is anterior45. The fetus is positioned such that the tumor, defect, head and neck, or chest is delivered through the hysterotomy. Fetal analgesics and/or paralytics may be administered prior to the fetal procedure. The intervention is then performed and once complete, warmed lactated Ringer’s solution and antibiotics are instilled into the uterus just prior to completion of a two-layer uterine closure45. Tocolytic therapy is initiated as the hysterotomy is being closed. Next, the maternal laparotomy is approximated in layers. Finally, a plan is established for postoperative multimodal analgesia, as maternal pain can also initiate uterine contractions46
Figure 1.Exposure for Open Fetal Surgery for Myelomeningocele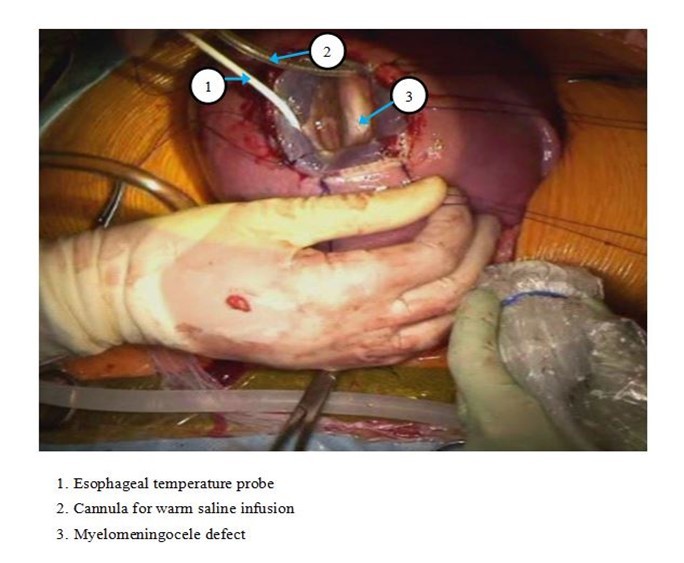
Intravenous tocolysiis, which is begun in the operating room with a magnesium bolus and infusion, is continued for 24 hours. Oral or rectal indomethacin is also often administered for 24-48 hours following surgery. After this point, the mother is maintained on oral nifedipine until delivery. The fetus is sonographically monitored on a daily basis while the mother is recovering in the hospital and on a weekly basis once discharged. The fetus is then delivered at 37 weeks via cesarean section or at which time preterm premature rupture of membranes or preterm labor ensues.
EXIT Procedure
Ex-utero intrapartum therapy (EXIT) serves as a modification of cesarean delivery to allow a near term fetal intervention before the neonate is delivered. The main anesthetic goals are preservation of placental blood flow until other means of gas exchange can be established for the neonate, uterine relaxation, and fetal anesthesia46.There are four main types of EXIT procedures: 1) EXIT to airway, 2) EXIT to resection, 3) EXIT to ECMO (Extracorporeal Membrane Oxygenation) and 4) EXIT to separation48. The EXIT to airway procedure is further classified into extrinsic, intrinsic and iatrogenic (Table 3)48. EXIT procedures are most commonly used to treat lymphatic malformations48.
Table 3. Ex-utero intrapartum therapy (EXIT) treatment procedures| Category | Subcategory | Example(s) |
|---|---|---|
| EXIT to airway | Intrinsic | Congenital high airway obstruction syndrome (CHAOS) |
| Extrinsic | Severe micrognathia, lymphatic malformation, vascular malformation, cervical teratoma | |
| Iatrogenic | Tracheal clip or balloon | |
| EXIT to resection | Thoracic, pulmonary, or mediastinal masses | |
| EXIT to ECMO | Severe congenital heart disease or severe congenital diaphragmatic hernia | |
| EXIT to separation | Conjoined twins |
An EXIT procedure may be considered if the fetus has undergone prenatal imaging indicating the potential for severe airway obstruction or hypoxia. A multidisciplinary team, composed of a pediatric surgeon trained in fetal surgery, maternal-fetal medicine obstetrician, pediatric otolarynologist, fetal cardiologist, social worker, radiologist, neonatologist, and anesthesiologist must then determine whether the mother and fetus are appropriate candidates 49. The team performs a thorough evaluation of the fetus’ condition through the use of ultrasounds, MRI, fetalechocardiogram, and/or karyotyping 49. Though inclusion and exclusion criteria have not yet been defined, specific indications have been proposed; diagnosis of a head/neck teratoma, mass size >5cm, polyhydramnios or absence of fetal gastric fluid, or signs of intrinsic airway obstruction (hyperinflated lungs or flattened diaphragm)48. Contraindications for the procedure include excessive risk such as instances with minimal tracheal involvement, or when little benefit is expected from the procedure, such as when there is complete tracheal agenesis or concomitant anomalies that make survival unlikely48. Placenta previa and subchorionic hemorrhage pose an increased maternal risk of complications and are therefore relative contraindications for the procedure 48. The final decision to utilize an EXIT procedure requires consensus from the multidisciplinary team that a normal delivery would result in a tenuous airway that would be difficult to secure emergently.
Once the decision is made that it is appropriate to proceed with the EXIT procedure, informed consent should address the potential risks to the both the mother and fetus. It is crucial to emphasize that the health and safety of the mother will take precedence for all treatment considerations. Maternal complications to be discussed should include endometriosis and impact on subsequent pregnancies (uterine rupture). To date, no maternal deaths have been reported during an EXIT procedure48. The mother should also be consented for transfusion and a preoperative type and cross should be performed as maternal blood loss tends to ranging from 150-1800ml during these procedures50.
Fetal outcomes have been highly variable depending on the indication for EXIT procedure. This makes the consent more difficult. The discussion of fetal risks must be tailored to the specific patient and caution should be used when comparing outcomes of a particular study to a presenting patient as the selection criteria for each study is highly specific.
One of the most common indications for EXIT to airway is congenital high airway obstruction syndrome (CHAOS), which is caused by several different etiologies including laryngeal atresia, subglottic stenosis or laryngeal. Fetuses with CHAOS must be monitored for the development of hydrops and degree of airway obstruction preoperatively. It is essential to determine the degree and location of the occlusion prior to the procedure. The case by Vaikunth et al illustrates this point well. The fetus involved had complete tracheal agenesis which required neck and mediastinal exploration to secure an airway51. Without careful preoperative planning, the patient may have died in the operating room despite the EXIT procedure because a tracheostomy would have been unsuccessful in securing the airway.
EXIT to resection is beneficial for thoracic, pulmonary or mediastinal48. Typically to be considered for EXIT to resection, the fetus must be considered high risk such as the presence of fetal hydrops or a congenital cystic adenomatoid malformation-volume ratio >1.652. Cass et al studied this group of patients and who also had persistent mediastinal compression near birth. They were divided into an EXIT procedure group (n=9) or standard delivery (n=7)52. All patients who underwent EXIT procedures had favorable outcomes and survived to discharge52. All patients in the standard delivery group developed respiratory distress requiring urgent operations and two did not survive to discharge.
While EXIT to ECMO has been utilized for several conditions such as severe congenital diaphragmatic hernia, currently, the benefits are unclear. Stoffan et al performed a study on patients with severe congenital diaphragmatic hernia. Severe congenital diaphragmatic hernia was defined by an observed-to-expected lung area to head circumference ratio that was less than 15%. In this cohort, there were worse outcomes in the EXIT to ECMO group and thus it could not be recommended53.
EXIT to separation has been reported in the literature. Bouchard et al described a case of thoracoomphalopagus conjoined twins wherein Twin B had a rudimentary heart and was receiving blood flow from Twin A. Due to concerns for the demands on Twin A’s heart after cessation of placental circulation, EXIT to separation was performed with ECMO on standby54. Since cases of conjoined twins which would share the particular anatomy that would benefit from the EXIT procedure is exceedingly rare, it is uncertain whether this procedure will remain.
Typically, EXIT procedures are recommended at 37-39 weeks gestational age, however certain considerations are made for individuals that would benefit from earlier deliveries50. Maternal conditions such as a history of preterm delivery, short cervix or fetal conditions (polyhydramnios), may indicate the need for earlier delivery50. In all cases, the timing of delivery requires careful consideration and discussion.
After the timing of the EXIT procedure has been determined, the entire EXIT team should assemble and simulate the procedure48. The team will include a maternal anesthesiologist, fetal anesthesiologist, scrub nurses, circulators, anesthesia technician, maternal-fetal medicine specialist, fetal cardiologist, pediatric surgeons, and the ECMO team at minimum. This team of 15-30 people is required for a successful EXIT procedure48. Attention to detail and thoroughness are essential. Each individual must know their assigned roles and each task needs to be assigned to a specific individual 48.
Model 1. Operating Room Set Up for Open Fetal Surgery
On the day of operation, two adjacent operating rooms are prepared and the team assembles to review key details of the procedure. As for open fetal surgery, the mother is positioned appropriately and monitoring devices for both the mother and fetus are applied. Prior to making a low transverse abdominal skin incision, ultrasonography is used to determine placental and fetal position, followed by increased anesthetic inhalation. The abdomen is then entered via a muscle dividing or midline fascial incision to expose the uterus. A sterile ultrasound is repeated to verify the position of the fetus and placenta and place full-thickness stay sutures in the uterine wall at the location of the planned hysterotomy at least 4 cm away from the placental edge. A bloodless hysterotomy, which involves isolating and clamping off all blood vessels to temporarily stop blood flow to the area, is established using a uterine stapler. The fetus is partially delivered and given intramuscular analgesics, paralytics and atropine. Fetal monitoring continues throughout the case with pulse oximetry and intermittent echocardiograms. A catheter is placed within the uterus and warmed Ringer lactate or normal saline is infused to maintain amniotic fluid volume. Fetal interventions then typically begin with direct laryngoscopy followed by rigid bronchoscopy. If an airway is unable to be established, a tracheostomy may be necessary48. These steps may be altered depending on the indication for surgery and in some situations the initial intervention may be tracheostomy as anatomy would not permit the preceding steps, such as in congenital high airway obstruction syndrome. In other cases (associated severe congenital heart disease) ECMO cannulation may be the first intervention required.
Reports have indicated that the fetus can be maintained on placental circulation for up to 59 minutes, given that the uterus remains quiescent50. It is crucial to note that if the mother begins to hemorrhage, the fetus must be delivered at this time, regardless of if a definitive airway has been secured55. Prior to clamping the cord, all members of the team should be alerted. Inhaled anesthetics should be reduced, oxytocin bolus administered and the uterus massaged to allow the uterus to contract and avoid maternal hemorrhage. The cord is then clamped and the fetus and placenta delivered. If the uterus remains atonic, methylergonovine and carboprost or mistoprostol can be administered to support uterine contraction and decrease maternal blood loss. A B-Lynch procedure can be utilized if there is continued maternal blood loss and uterine atony56. The B-Lynch procedure is performed by compressing the uterus with a large absorbable suture to control uterine hemorrhage or atony without hysterectomy. In rare and very severe cases, hysterectomy may be required to control uterine hemorrhage. After control of uterine bleeding has been established, the hysterotomy and laparotomy are closed 49.
The most common complication from an EXIT procedure is maternal anemia, even in the case of an uncomplicated EXIT procedure. In this event, the mother requires transfusions as clinically indicated48. Other possible postoperative complications include endometritis and wound infections 57, both of these conditions can be treated with antibiotic therapy. As with open fetal surgery, if the hysterotomy must be extended onto the muscular, active portion of the uterus, there is a risk of uterine rupture in following pregnancies49. In these scenarios, the mother should be counseled to have a scheduled cesarean section in any subsequent pregnancies, prior to the onset of labor 49.
Closed Fetal Surgery
Several fetal procedures can be performed without the need for a hysterotomy and these are collectively referred to as closed fetal surgery58. This class of fetal interventions includes a wide array of procedures ranging from those that are entirely percutaneous technique to fetoscopic surgery requiring multiple trocars. And, although the procedures are minimally invasive for the fetus/uterus, there is still risk to the mother58,59.
Twin-twin transfusion syndrome (TTTS), twin reversed arterial perfusion (TRAP) sequence, multifetal reduction, and discordant structural anomalies are common indications for closed fetal surgery. Prenatal imaging as early as 10-14 weeks gestation age can be used to determine chorionicity as only 20% of twins are monochorionic and at risk for TTTS and TRAP60. Ultrasonic indications of monochorionicity include a single placenta, concordant sex by external genitalia and the shape of the intertwin membrane61. A “T” sign will be identified if there is a single chorion and two aminiotic sacs, while a “λ” sign will be seen if there are two amniotic sacs and 2 chorions. If a “T” sign is encountered, these fetuses must be monitored for TTTS and TRAP at regular intervals (as frequent as every 2 weeks) 60.
TTTS occurs in 9-15% of monochorionic twins62. All pregnancies complicated by TTTS have placental vascular anastomoses and it is theorized that there is unbalanced flow between the twins which causes TTTS62. Prenatal ultrasound diagnostic criteria for TTTS includes monochorionicity; polyhydramnios and enlarged bladder in the recipient twin; oligohydramnios and small bladder in the donor twin, and weight discordance62.
Approximately 5% of pregnancies complicated by TTTS will develop fetal hydrops in one or both twins62, 63. These pregnancies are at risk for the development of mirror syndrome which is potentially left-threatening to both the mother and fetus. Symptoms of mirror syndrome include edema, hypertension, proteinuria, mild transaminases, headache or visual changes63. These symptoms closely resemble those of preeclampsia and are best distinguished by anemia in mirror syndrome but slight polycythemia in preeclampic patients63. Mothers affected by mirror syndrome may experience postpartum hemorrhage, pulmonary edema, congestive heart failure, DIC and acute renal failure 63. They are also at increased risk of severe complications after undergoing interventions that increase blood flow across the hydropic placenta63. Therefore, closed fetal surgical interventions for TTTS should not be performed in cases of mirror syndrome as it may increase maternal morbidity and mortality.
TRAP sequence is a rare phenomenon occurring in 1:100 monozygotic pregnancies64. One “twin” has acardia and depends on the other, the “pump twin” for blood flow64. These pregnancies are often complicated by polyhydramnios of the “pump twin” and may result in demise of the “pump twin” if heart failure develops 64. TRAP sequence can be identified in monochorionic twins via prenatal ultrasound by identification of acardia in one twin, placental vascular anastomosis on Doppler with reverse flow on artery-artery anastomosis64.
If TTTS or TRAP sequence develops, preoperative planning must include consideration of maternal and fetal factors related to the type of procedure and location of the intervention. There are a variety of fetal interventions that can be used to treat TTTS. There are unique risks and benefits to each type of procedure and fetoscopes are only approved to treat TTTS between 16-26 weeks of gestation limiting some indications 62.
Serial Amnioreduction
Serial amnioreduction and serial amnioreduction with micorseptostomy are perhaps the least invasive of the fetal procedures to treat TTTS. These are palliative measures which simply reduced the amount of amniotic fluid around the recipient twin to a normal amniotic fluid volume62. The goal of these procedures is merely to decrease the risk of preterm labor and premature rupture of membranes 62. This goal must be tempered with the knowledge that removal of more than 5 liters at once increases the risk of placental abruption and fetal bradycardia62. Adding microseptostomy to amnioreduction allows equilibration of intra-amniotic pressure between the two gestational sacs. Moise et al performed a randomized control trial of amnioreduction versus amnioreduction with microseptostomy. Their data demonstrated that infant survival was similar between interventions with 50% and 60% of both infants surviving or 78% and 80% of at least one infant surviving in the amnioreduction versus amnioreduction with microseptostomy respectively65. They did note that the amnioreduction group did require statistically more interventions than the amnioreduction with microseptostomy group65. The risks of microseptostomy include amniotic bands and disruption of the intertwin membrane creating a monoamniotic pregnancy with risk of cord entanglement and double demise.
Amnioreduction is performed under ultrasound guidance where in the needle transverses only the recipient twin’s sac and amniotic fluid is removed until the amniotic fluid volume is within the normal range or 5 liters have been remove. Microseptostomy is similarly performed under ultrasound guidance but a single passage of the needle is made through the amniotic sac of the donor twin. It is important to note, that only a single passage should be performed through the donor sac as multiple punctures can result in monoamnionicity, cord entanglement and fetal demise62.
Laser Ablation
Like serial amnioreduction, with laser ablation, there is the potential for survival of both twins62. As mentioned above, monochorionic twin pregnancies complicated by TTTS have abnormal placental vascular anastomoses. Unlike amnioreduction, which treats the symptoms of TTTS, laser ablation ablates the placental anastomoses and can lead to resolution of TTTS. Correction of the TTTS has been shown to have superior outcomes to amnioreduction in randomized control trials. Furthermore, the procedure has been modified over the years to improve outcomes. The most recent variation on this procedure, the Solomon technique (described below), has been shown to have improved outcomes in a randomized control trial66. These improved outcomes include a decreased risk of TRAP and recurrent TTTS. Recurrent TTTS and large intertwin anastomoses are also more likely to occur if the cord is short. In fact, cord insertion sites <5cm apart makes the procedure more challenging but cord insertion sites <1cm is a relative contraindication to laser ablation62.
Also like serial amnioreduction, laser ablation is generally performed under local anesthesia62. Under ultrasound guidance, the fetoscope is inserted into the recipient twin’s amniotic sac percutaneously or via a minilaparotomy with a 10-12 mm cannula62. The dividing membrane is identified and each vessel inspected to determine its endpoint62. If there is no endpoint, it is determined to be an intertwin anastomosis and 20-40W from a diode or Yttrium Aluminum Garnet (YAG) laser is delivered to the aberrant vessel with a 400-600um laser fiber62. This portion of the procedure is referred to as selective fetoscopic laser photocoagulation. The Solomon technique involves the addition of laser photocoagulation in a line from one edge of the placenta to the other66. This supplementary portion of the procedure effectively dichorionizes the placenta. Prior to completion of the procedure, amnioreduction is also performed62.
Radiofrequency Ablation
Unlike serial amnioreduction and laser ablation where there is the possibility of dual twin survival, other treatments of TTTS, such as radiofrequency ablation (RFA) and bipolar cord occlusion, involve selective fetal reduction. Indications include TTTS, severe Intrauterine Growth Restriction (IUGR), and discordant lethal anomalies. Both of these procedures require complete ablation of blood flow in the umbilical cord67. If complete cord occlusion is not achieved, there can be a shift in the blood flow from the healthy to the dying twin which results in demise of both fetuses67. As a result, ultrasounds are often performed on postoperative day one to look for increased middle cerebral artery pressures in the surviving twin which are indicative of fetal anemia68.
Radiofrequency ablation is generally performed on fetuses ranging in age from 16-27 weeks estimated gestational age (EGA) and outcomes do not seem to be related to the gestational age at which the procedure is performed61,68. As with other fetal procedures, RFA presents a 9-22% risk of premature labor61,69. Radiofrequency ablation also includes complications specific to the procedure itself. The procedure can cause thermal injury to the surviving twin61,68,69. Kumar et al reported their experience with 100 cases of RFA. In their cohort there were two cases of presumed thermal injury to the surviving twin. Both cases resulted in significant injury, as one resulted in a severe limb abnormality and the other required skin grafting68.
As with most fetal procedures, RFA is performed under ultrasound guidance. The cord insertion site of the target twin is identified and a trajectory for the RFA needle identified to avoid the placenta. The RFA needle is only 8cm in length, therefore maternal obesity or a posterior placenta may be a may pose a challenge for accessibility or be an absolute contraindication61. Local anesthetic is instilled followed by a 3 mm incision in the maternal abdominal wall68. The RFA (17-gauge) needle is inserted, positioned at the cord insertion of the fetal abdominal wall, and the tines are extended. Heat is applied for 3 minutes until a temperature of 100-110°C is reached 61,68. One cycle is often adequate but if further cycles are required, the tines are allowed to cool for 1-2 minutes between cycles 61, 68, 69. Cycles are continued until there is cessation of flow in the umbilical cord by pulsed wave and color Doppler and cardiac asystole is confirmed68.
Bipolar Cord Occlusion
Bipolar Cord Occlusion (BCO) is another form of selective fetal reduction utilized to treat TTTS. To date, there has not been a randomized control trial comparing RFA with BCO but there has been a recent metaanalysis reported67. This study revealed that there was no statistical difference between RFA and BCO with regards to live birth rate, neonatal death rate or overall survival. RFA was associated with a significantly lower rate of preterm premature rupture of membranes (17.7% vs 28.2%), however, this may not be clinically significant as the mean gestational age at delivery was 34.7 ± 1.7 weeks in the RFA group and 35.1 ± 1.6 weeks in the BCO group67. Thus, the authors appropriately concluded that one procedure is not clearly superior.
To perform this procedure, a free cord loop of the umbilical cord is identified near its abdominal or placental insertion site70. A cannula is inserted into the uterine cavity perpendicular to the cord such that a cross-sectional view of all three vessels is visible70. A 5mm endoscopic bipolar coagulation forceps is inserted, the cord grasped and bipolar coagulation initiated at 20 Watts70. If needed, the power can slowly be increased until Doppler flow demonstrates complete occlusion of the vessels70. This is repeated at two additional cord segments70.
Fetoscopic Surgery
Occasionally there are indications for fetoscopic surgery wherein multiple trocars are required in the uterus. For example, fetoscopic surgery with multiple trocars has been used for tracheal occlusion in severe congenital diaphragmatic hernias, to release amniotic bands if the fetus or limb is threatened71 and in patients with myelomeningocele (MMC)72.
Preoperative counseling and imaging are similar to other forms of fetal surgery. A multidisciplinary team should meet with the mother to discuss risks, benefits, expected outcomes, and perinatal care. The fetus should undergo thorough imaging with a complete sonographic anatomic survey, echocardiogram, amniocentesis and fetal MRI to rule out other anatomic and chromosomal anomalies.
Complications of fetoscopic surgery are also similar to other forms of fetal surgery including premature labor and premature rupture of membranes. Unfortunately, despite the less invasive nature of these operations, they have similar rates of premature rupture of membranes (40-100%)73.
Fetoscopic MMC Repair
Currently, fetoscopic repair of MMC is controversial. It has been reported to require longer operative times in comparison to open fetal surgical repair and presents technical difficulty as the result of the layered closure of the defect. Interestingly, open myelomeningocele repair is associated with lower rates of preterm delivery < 34 weeks, premature rupture of membranes (PROM), oligohydramnios and perinatal death whereas fetoscopic repair had lower rates of hysterotomy dehiscence or thinning74. These findings are unexpected and be indicative of the need for multiple trocars and longer operative times for fetoscopic repair. In spite of this, Pedreira et al recently completed a Phase I trial suggesting that fetoscopic repair can be safe and effective using the technique described below75. Further research will be necessary to directly compare the outcomes of these two interventions.
Maternal antibiotics are administered within one hour prior to incision. Next the mother is administered local, epidural, or general anesthesia. The gravid abdomen is imaged through ultrasound to determine the position of the fetus and placenta. To avoid injury to the fetus, bleeding, or inducting PPROM; an anterior placenta may require maternal laparotomy with exteriorization of the uterus, but laparoscopic-assisted fetoscopy or the use of curved trocars can be utilized to avoid maternal laparotomy. Once proper positioning is established to triangulate the fetal pathology, Seldinger technique is used to insert a camera port. Additional trocars are placed. Amnioinfusion or partial amniotic Carbon Dioxide (CO2) insufflation can improve visibility 72, 75. The fetus is monitored by sonography throughout the procedure. For MMC repair, the fetus is positioned and the neural placode is released circumferentially at the transition zone75. The placode can be covered with a patch and then skin is closed over the defect. A two-layer closure is performed. A skin substitute may be used if the defect does not allow midline approximation75. Although not required, uterine closure devices may be used to seal the puncture sites if the patient is believed to benefit from their use75.
Fetoscopic Tracheal Occlusion
Fetoscopic tracheal occlusion (FETO) has been shown to improve outcomes in severe congenital diaphragmatic hernias in multiple studies. The theory behind the surgery emerged from the knowledge that congenital tracheal stenosis/atresia results in lung overgrowth. Since the most profound influence on outcomes in congenital diaphragmatic hernia is due to pulmonary hypoplasia and the resulting pulmonary hypertension, Dr. Harrison theorized that tracheal occlusion would stimulate lung growth. After developing an ovine model and proving this theory in sheep, it was translated to humans. Currently, only patients with isolated congenital diaphragmatic hernias, singleton pregnancies, gestational age between 24 and 30 weeks, with a lung-to-head ratio ≤ 1 or observed-to-expected lung-to-head ratio less than 25% and liver herniation are considered for this procedure76,77. With careful selection of the patient population, randomized control trials have demonstrated increased survival in isolated congenital diaphragmatic hernias treated with FETO77. However, studies have also shown that the mortality for is greater for a subset of this population who deliver prior to 35 weeks gestation and that these patients require significantly longer duration of mechanical ventilation, supplementary oxygen and have longer hospital stays78.
The FETO procedure begins as fetoscopic MMC repair above. Once trocars are placed and the fetoscope inserted, the fetus is given an intramuscular injection of anesthetic and paralytic. The cannula containing the fetoscope is advanced into the fetal mouth and down through the vocal cords into the trachea. A catheter is inserted along the scope containing a detachable balloon and positioned just above the carina. The balloon is inflated with 0.8ml saline and detached from the catheter. The position of the balloon just above the carina is confirmed by ultrasound. The procedure is concluded as above.
Long Term Follow-up
Little can be said regarding the long-term outcomes for children whom have undergone fetal surgery. Many have theorized that neonatal exposure to anesthetics could lead to neurocognitive deficits. Thus, similar concerns are held for survivors of fetal surgery but studies are lacking. Tosello et al have looked at short and medium-term outcomes for survivors of fetoscopic laser therapy for TTTS79,8. They found increased survival for their cohort but demonstrated a 5.7% rate of major neurological abnormality which is similar to published data for laser ablation79. Van Klink et al also published a meta-analysis on long-term neurodevelopmental outcomes in twins after fetal therapy80. Not surprisingly, they found decreased gestational age to be an independent risk factor for neurodevelopmental impairment80. Their data also suggested an overall rate of cerebral palsy of 7.1% and neurodevelopmental impairment of 13.3% in twins with TTTS treated with fetoscopic laser surgery80
More information is available for outcomes on mothers involved in fetal surgery. As mentioned above, fetal surgery offers the mother no benefits and has long term implications. There is a risk of maternal death during or shortly after surgery. Likewise, there is a risk of postoperative pulmonary edema that may require intensive care and intubation81. Perhaps the most extensively studied aspect of maternal outcomes is the impact on her future reproductive health. Fortunately, normal conception occurs in 98-100% of patients after fetal surgery. However, the reported risk of uterine rupture is 6-20% after open fetal surgery compared to <0.5-6.2% with prior cesarean section39,40. In order to decrease these risks, it is recommended that mothers wait two years after open fetal surgery to attempt a subsequent pregnancy to allow the uterus to heal. Likewise, she is not allowed to labor during subsequent pregnancies and thus requires a cesarean section prior to the onset of labor at 37 weeks. Subsequent pregnancies are also at risk of placental accreta. The incidence of placental accreta is rare and the incidence after fetal surgery has not been calculated at this point, but when the placental vasculature invades the wall of the uterus too deeply, the effects can be life threatening82.
Research and Future Directions
As repeatedly noted in this review, one of the greatest complications of all types of fetal surgery is preterm labor (occurrence rates ranging as high as 40-100% for open and fetoscopic surgery73) and premature rupture of membranes. Identifying potential modifications of the procedures to decrease these risks could greatly expand the indications for these procedures by decreasing the associated risks. Cervical length shortening is an identified risk factor for preterm labor. Cervical pessary and cervical cerclage have been proposed to minimize the risk of preterm delivery after fetal surgery. In a retrospective study of 16 patients, delivery at greater gestational ages was achieved through the use of the Arabin cervical pessary83. Further research is required to verify these data given the modest sample size and retrospective nature of this study. Inconsistent findings have been reported with cervical cerclage. One retrospective study of 14 patients treated with cervical cerclage demonstrated a greater mean gestational age at delivery in patients with a cervical length <15mm at the time of fetal surgery84. Conversely, there is another retrospective multi-center study of 164 patients that finds no significant difference between the use of cerclage or no cerclage after laser surgery85. In a subgroup analysis, patients with cervical length less than 15mm with or without cerclage did not show any discrepancy in group outcomes. However, in mothers with a cervical length between 16 and 20mm, there was a significant prolongation of pregnancy and improved survival to 28 days in the cerclage group85. A randomized control trial is likely necessary to determine if cervical cerclage is beneficial.
Other groups have proposed modifying the surgical technique to improve the incidence of preterm labor and premature rupture of membranes following fetal surgery. Chang et al, used a gelatin sponge to seal the defect created by fetoscopic surgery. In their cohort of 24 patients, they reported improved outcomes with placement of the gelatin plug. However, use of the gelatin plug was associated with a high rate of dual fetal demise (34.6%) which could artificially decrease the rate of preterm premature rupture of membranes73. Two larger studies that included 79 and 72 patients by Papanna et al. failed to show a decreased rate of preterm premature rupture of membranes with the use of chorioamniotic plugs at the trocar site86, 87. Bennett et al described a method of entering the uterus that involved a lower midline laparotomy and suturing the amniotic membrane to the uterus to stabilize the membrane. This technique resulted in lower rates of premature rupture of membranes (22% vs 46%), chorioamnion separation (0% vs 26%), uterine dehiscence at the repair site (3% vs 13%), and maternal transfusion at delivery (0% vs 9%)45. They also reported a higher percentage of infants born at 37 weeks gestational age (39% vs 21%) while maintaining similar rates of oligohydramnios (24% vs 21%) and mortality (5% vs 3%). These data suggest that improving the surgical technique may improve outcomes for both the mother and fetus.
In addition to research dedicated to improving outcomes of current procedures, there also extensive research being done to expand the types of fetal interventions. Two such examples of this are in utero stem cell therapy and in utero gene therapy. Studies have shown that chimeras can spontaneously develop in monochorionic, dizygotic twins demonstrating that the principles of in utero stem cell therapy are achievable88. Forming chimeras through in utero stem cell therapy could be used to treat hemoglobinopathies, inborn errors of metabolism and immunological defects prior to the development of the majority of associated complications89. Moreover, in utero administration of stem cell therapy encompasses other potential benefits including high levels of proliferation in the fetus, naturally occurring stem cell migration, and the immature immune system of the fetus may be tolerable of foreign antigens88, 89. Furthermore, because the fetus is small, it allows for a greater relative dose of stem cells per gram88, 89. Most attempts to perform stem cell transplantation in human fetuses have resulted in minimal or no engraftment88,89. The exception to these findings is in utero stem cell therapy for X-linked severe combined immunodeficiency disorder (XSCID). XSCID is characterized by low T-cell counts and absent T- and B-cell function. It is thought that the low T-cell counts allows additional space and the nonfunctional T- and B-cells do not pose competition or inhibit transplanted cells, placing the transplanted cells in an advantageous state. In contrast, other diseases states present challenges such that there is a lack of space for donated cells, competition from native cells and incomplete fetal tolerance88. Research is ongoing to resolve these challenges.
In utero gene therapy also has the potential to eradicate disease. The potential benefits of in utero gene therapy mirror those of in utero stem cell therapy; there is incomplete fetal tolerance and the possibility of a greater dose per gram ratio given the small size of the fetus90. Specific to in utero gene therapy, there are unique risks relating to normal organogenesis and germ-line transmission90. Current research continues to examine the most suitable timing and methods of gene transfer. What is more, research of a novel technology that allows sequence of a gene at its native locus, called gene editing91 has the potential to treat and correct genetic abnormalities and circumvent problems of incomplete fetal tolerance once this technology has been mastered.
Conclusion
Despite being established over 50 years ago, fetal surgery is still in its infancy. It is evolving in to an exciting and diverse discipline with procedures ranging from relatively minor outpatient, entirely percutaneous procedures to invasive operations involving both a maternal laparotomy and hysterotomy as well as open fetal surgery. Rapidly accumulating research will continue to change the face of fetal surgery with expounding indications and new procedures. All the while, it will be important to maintain the stringent inclusion and exclusion criteria, established early in the development of fetal surgery, in order to preserve ethical use of these procedures and minimize maternal risk. It is certainly a field to watch as it has the potential to change how we treat a plethora of diseases.
Summary
Fetal surgery is an exciting, new and diverse discipline with procedures ranging from relatively minor outpatient, entirely percutaneous procedures to invasive operations involving both a maternal laparotomy and hysterotomy as well as open fetal surgery. Continued research is expounding indications and procedures in the field while maintaining stringent inclusion and exclusion criteria to minimize maternal risk.
References
- 2.BEVIS D C. (1952) The antenatal prediction of haemolytic disease of the newborn. , Lancet 1(6704), 395-398.
- 4.FUCHS F, FREIESLEBEN E, KNUDSEN E E, RIIS P. (1956) Antenatal detection of hereditary diseases. Acta Genet Stat Med. 6(2), 261-263.
- 5.LILEY A W. (1965) The use of amniocentesis and fetal transfusion in erythroblastosis fetalis. Pediatrics. 35, 836-847.
- 6.Elias S, Verp M S. (1983) Prenatal diagnosis of genetic disorders. Obstet Gynecol Annu. 12, 79-102.
- 7.Benzie R J, Doran T A. (1975) The “fetoscope”--a new clinical tool for prenatal genetic diagnosis. , Am J Obstet Gynecol 121(4), 460-464.
- 8.Gohari P, Spinelli A. (1979) Fetoscopy in the practice of perinatology and obstetrics. Obstet Gynecol Annu. 8, 179-202.
- 9.Rodeck C H, Nicolaides K H. (1986) Fetal tissue biopsy: Techniques and indications. Fetal Ther. 1(1), 46-58.
- 10.Michejda M, Hodgen G D. (1981) In utero diagnosis and treatment of non-human primate fetal skeletal anomalies. , I. hydrocephalus. JAMA 246(10), 1093-1097.
- 11.Harrison M R, Ross N A, de Lorimier AA. (1981) Correction of congenital diaphragmatic hernia in utero. III. development of a successful surgical technique using abdominoplasty to avoid compromise of umbilical blood flow. J Pediatr Surg. 16(6), 934-942.
- 12.Adzick N S, Outwater K M, Harrison M R. (1985) Correction of congenital diaphragmatic hernia in utero. IV. an early gestational fetal lamb model for pulmonary vascular morphometric analysis. J Pediatr Surg. 20(6), 673-680.
- 14.Adzick N S, Harrison M R, Flake A W. (1985) Experimental studies on prenatal treatment of congenital anomalies. Br J Hosp Med. 34(3), 154-159.
- 15.Nakayama D K, Harrison M R, Seron-Ferre M, Villa R L. (1984) Fetal surgery in the primate II. uterine electromyographic response to operative procedures and pharmacologic agents. J Pediatr Surg. 19(4), 333-339.
- 16.Adzick N S, Harrison M R, Glick P L. (1986) Fetal surgery in the primate. III. maternal outcome after fetal surgery. J Pediatr Surg. 21(6), 477-480.
- 17.Russell S. (2005) First fetal surgery survivor finally meets his doctor / 24 years ago, UCSF surgeon saved his life in mom's womb. http://www.sfgate.com/health/article/SAN-FRANCISCO-First-fetal-surgery-survivor-2348923.php. Updated
- 18.Elder J S, Duckett JW Jr, Snyder H M. (1987) Intervention for fetal obstructive uropathy: Has it been effective? Lancet. 2(8566), 1007-1010.
- 19.Abramowicz J, Jaffe R. (1988) Diagnosis and intrauterine management of enlargement of the cerebral ventricles. J Perinat Med. 16(3), 165-173.
- 20.Longaker M T, Golbus M S, Filly R A, Rosen M A, Chang S W et al. (1991) Maternal outcome after open fetal surgery. A review of the first 17 human cases. JAMA. 265(6), 737-741.
- 21.Harrison M R, Adzick N S, Bullard K M. (1997) Correction of congenital diaphragmatic hernia in utero VII: A prospective trial. J Pediatr Surg. 32(11), 1637-1642.
- 24.Albanese C T, Harrison M R. (1998) Surgical treatment for fetal disease. the state of the art. , Ann N Y Acad Sci 847, 74-85.
- 26.Kitano Y, Flake A W, Crombleholme T M, Johnson M P, Adzick N S. (1999) Open fetal surgery for life-threatening fetal malformations. Semin Perinatol. 23(6), 448-461.
- 28.Arant B S. (1987) Prevention of hereditary nephropathies by antenatal interventions. ethical considerations. Pediatr Nephrol. 1(3), 553-560.
- 29.Anand K J, Sippell W G, Aynsley-Green A. (1987) Randomised trial of fentanyl anaesthesia in preterm babies undergoing surgery: Effects on the stress response. Lancet. 1(8527), 243-248.
- 30.Teixeira J, Fogliani R, Giannakoulopoulos X, Glover V, Fisk N M. (1996) Fetal haemodynamic stress response to invasive procedures. , Lancet 347(9001), 624.
- 31.Giannakoulopoulos X, Sepulveda W, Kourtis P, Glover V, Fisk N M. (1994) Fetal plasma cortisol and beta-endorphin response to intrauterine needling. , Lancet 344(8915), 77-81.
- 32.Bellieni C V, Tei M, Stazzoni G, Bertrando S, Cornacchione S et al.Use of fetal analgesia during prenatal surgery. , J Matern Fetal Neonatal Med 26(1), 90-95.
- 33.Harrison M R, Filly R A, Golbus M S. (1982) Fetal treatment. , New England Journal of Medicine 307(26), 1651-1652.
- 34.Cass D L, Olutoye O O, Ayres N A. (2012) Defining hydrops and indications for open fetal surgery for fetuses with lung masses and vascular tumors. J Pediatr Surg. 47(1), 40-45.
- 35.Adzick N S, Thom E A, Spong C Y. (2011) A randomized trial of prenatal versus postnatal repair of myelomeningocele. , N Engl 364(11), 993-1004.
- 36.RA Moreira de Sa, PR Nassar de Carvalho, Kurjak A. (2015) Is intrauterine surgery justified? report from the working group on ultrasound in obstetrics of the world association of perinatal medicine (WAPM). J Perinat Med.
- 37.Wu D, Ball R H. (2009) The maternal side of maternal-fetal surgery. Clin Perinatol. 36(2), 247-53.
- 38.Scott J R.Intrapartum management of trial of labour after caesarean delivery: Evidenceand experience. , BJOG 121(2), 157-162.
- 39.Al-Zirqi I, Stray-Pedersen B, Forsen L, Daltveit A K, Vangen S. (2015) Uterine rupture: Trends over 40 years. BJOG.
- 40.Moldenhauer J S, Soni S, Rintoul N E. (2015) Fetal myelomeningocele repair: The post-MOMS experience at the children's hospital of philadelphia. Fetal Diagn Ther. 37(3), 235-240.
- 41.Boat A, Mahmoud M, Michelfelder E C. (2010) Supplementing desflurane with intravenous anesthesia reduces fetal cardiac dysfunction during open fetal surgery. Paediatr Anaesth. 20(8), 748-756.
- 42.Johnson M D, Birnbach D J, Burchman C, Greene M F, Datta S et al. (1989) Fetal surgery and general anesthesia: A case report and review. , J Clin Anesth 1(5), 363-367.
- 43.Jalilian L, Delgado Upegui C, Ferreira R, Simmons L, Ciliberto C. (2016) Intraoperative treatment of fetal asystole after endovascular repair of aortic coarctation in a pregnant woman with mitral stenosis. A A Case Rep. 6(6), 150-153.
- 44.Bennett K A, Carroll M A, Shannon C N. (2014) Reducing perinatal complications and preterm delivery for patients undergoing in utero closure of fetal myelomeningocele: Further modifications to the multidisciplinary surgical technique. J Neurosurg Pediatr. 14(1), 108-114.
- 46.Marques M V, Carneiro J, Adriano M, Lanca F. (2015) Anesthesia for ex utero intrapartum treatment: renewed insight on a rare procedure. Rev Bras Anestesiol. 65(6), 525-8.
- 47.Santolaya-Forgas J, Romero R, Mehendale R. (2006) The effect of continuous morphine administration on maternal plasma oxytocin concentration and uterine contractions after open fetal surgery. , J Matern Fetal Neonatal Med 19(4), 231-238.
- 48.Walz P C, Schroeder JW Jr. (2015) Prenatal diagnosis of obstructive head and neck masses and perinatal airway management: The ex utero intrapartum treatment procedure. Otolaryngol Clin North Am. 48(1), 191-207.
- 50.Lazar D A, Olutoye O O, Moise K J. (2011) Ex-utero intrapartum treatment procedure for giant neck masses--fetal and maternal outcomes. J Pediatr Surg. 46(5), 817-822.
- 51.Vaikunth S S, Morris L M, Polzin W. (2009) Congenital high airway obstruction syndrome due to complete tracheal agenesis: An accident of nature with clues for tracheal development and lessons in management. Fetal Diagn Ther. 26(2), 93-97.
- 52.Cass D L, Olutoye O O, Cassady C I. (2013) EXIT-to-resection for fetuses with large lung masses and persistent mediastinal compression near birth. J Pediatr Surg. 48(1), 138-144.
- 53.Stoffan A P, Wilson J M, Jennings R W, Wilkins-Haug L E, Buchmiller T L. (2012) Does the ex utero intrapartum treatment to extracorporeal membrane oxygenation procedure change outcomes for high-risk patients with congenital diaphragmatic hernia? J Pediatr Surg. 47(6), 1053-1057.
- 54.Bouchard S, Johnson M P, Flake A W. (2002) The EXIT procedure: Experience and outcome in 31 cases. J Pediatr Surg. 37(3), 418-426.
- 55.Butwick A, Aleshi P, Yamout I. (2009) Obstetric hemorrhage during an EXIT procedure for severe fetal airway obstruction. Can.J Anaesth. 56(6), 437-442.
- 56.B-Lynch C, Coker A, Lawal A H, Abu J, Cowen M J. (1997) The B-lynch surgical technique for the control of massive postpartum haemorrhage: An alternative to hysterectomy? five cases reported. Br J Obstet Gynaecol. 104(3), 372-375.
- 57.Taghavi K, Beasley S. (2013) The ex utero intrapartum treatment (EXIT) procedure: Application of a new therapeutic paradigm. J Paediatr Child Health. 49(9), 420-7.
- 58.Wenstrom K D, Carr S R. (2014) Fetal surgery: Principles, indications, and evidence. Obstet Gynecol. 124(4), 817-835.
- 61.Moise KJ Jr, Johnson A, Moise K Y, Nickeleit V. (2008) Radiofrequency ablation for selective reduction in the complicated monochorionic gestation. , Am J Obstet Gynecol.198(2);198.e1-198.e5
- 62.Johnson A. (2015) Diagnosis and management of twin-twin transfusion syndrome. Clin Obstet Gynecol. 58(3), 611-631.
- 63.Chai H, Fang Q, Huang X, Zhou Y, Luo Y. (2014) Prenatal management and outcomes in mirror syndrome associated with twin-twin transfusion syndrome. Prenat Diagn. 34(12), 1213-1218.
- 64.Buyukkaya A, Tekbas G, Buyukkaya R. (2015) Twin reversed arterial perfusion (TRAP) sequence; characteristic gray-scale and doppler ultrasonography findings. , Iran.J Radiol 12(3), 14979.
- 65.Moise KJ Jr, Dorman K, Lamvu G. (2005) A randomized trial of amnioreduction versus septostomy in the treatment of twin-twin transfusion syndrome. , Am J Obstet Gynecol.193(3Pt1): 701-707.
- 66.Slaghekke F, Lopriore E, Lewi L. (2014) Fetoscopic laser coagulation of the vascular equator versus selective coagulation for twin-to-twin transfusion syndrome: An open-label randomised controlled trial. , Lancet 383(9935), 2144-2151.
- 67.Gaerty K, Greer R M, Kumar S.Systematic review and metaanalysis of perinatal outcomes after radiofrequency ablation and bipolar cord occlusion in monochorionic pregnancies. , Am J Obstet Gynecol 213(5), 637-643.
- 68.Kumar S, Paramasivam G, Zhang E. (2014) Perinatal- and procedure-related outcomes following radiofrequency ablation in monochorionic pregnancy. , Am J Obstet Gynecol.210(5);454.e1-454.e6
- 69.Lee H, Wagner A J, Sy E. (2007) Efficacy of radiofrequency ablation for twin-reversed arterial perfusion sequence.196(5);459.e1-459.e4. , Am J Obstet Gynecol
- 70.Gallot D, Laurichesse H, Lemery D. (2003) Selective feticide in monochorionic twin pregnancies by ultrasound-guided umbilical cord occlusion. Ultrasound Obstet Gynecol. 22(5), 484-488.
- 71.Quintero R A, Morales W J, Phillips J, Kalter C S, Angel J L. (1997) In utero lysis of amniotic bands. Ultrasound Obstet Gynecol. 10(5), 316-320.
- 72.Peiro J L, Carreras E, Guillen G. (2009) Therapeutic indications of fetoscopy: A 5-year institutional experience. J Laparoendosc Adv Surg TechA. 19(2), 229-236.
- 73.Chang J, Tracy TF Jr, Carr S R, Sorrells DL Jr, Luks F I. (2006) Port insertion and removal techniques to minimize premature rupture of the membranes in endoscopic fetal surgery. J Pediatr Surg. 41(5), 905-909.
- 74.Araujo Junior E, Eggink A J, Dobbelsteen J van den, Martins W P, Oepkes D. (2015) Procedure-related complications of open versus fetoscopic fetal surgery for treatment of spina bifida: Systematic review and meta-analysis in the new era of intrauterine myelomeningocele repair. Ultrasound Obstet Gynecol.
- 75.Pedreira D A, Zanon N, Nishikuni K. (2015) Endoscopic surgery for the antenatal treatment of myelomeningocele: The CECAM trial. Am J Obstet Gynecol.
- 76.Ruano R, da Silva MM, Campos J A. (2012) Fetal pulmonary response after fetoscopic tracheal occlusion for severe isolated congenital diaphragmatic hernia. Obstet Gynecol. 119(1), 93-101.
- 77.Al-Maary J, Eastwood M P, Russo F M, Deprest J A, Keijzer R. (2016) Fetal tracheal occlusion for severe pulmonary hypoplasia in isolated congenital diaphragmatic hernia: A systematic review and meta-analysis of survival. Ann Surg.
- 78.Ali K, Bendapudi P, Polubothu S. (2016) Congenital diaphragmatic hernia-influence of fetoscopic tracheal occlusion on outcomes and predictors of survival. Eur.J Pediatr. 175(8), 1071-1076.
- 79.Tosello B, Blanc J, Haumonte J B, D'Ercole C, Gire C. (2014) Short and medium-term outcomes of live-born twins after fetoscopic laser therapy for twin-twin transfusion syndrome. J Perinat Med. 42(1), 99-105.
- 80.van Klink JM, Koopman H M, Oepkes D, Walther F J, Lopriore E. (2011) Long-term neurodevelopmental outcome in monochorionic twins after fetal therapy. Early Hum Dev. 87(9), 601-606.
- 81.DiFederico E M, Burlingame J M, Kilpatrick S J, Harrison M, Matthay M A. (1998) Pulmonary edema in obstetric patients is rapidly resolved except in the presence of infection or of nitroglycerin tocolysis after open fetal surgery. Am J Obstet Gynecol. 179(4), 925-933.
- 82.Silver R M, Fox K A, Barton J R.Center of excellence for placenta accreta. , Am J Obstet Gynecol 212(5), 561-568.
- 83.Carreras E, Arevalo S, Bello-Munoz J C. (2012) Arabin cervical pessary to prevent preterm birth in severe twin-to-twin transfusion syndrome treated by laser surgery. Prenat Diagn. 32(12), 1181-1185.
- 84.Salomon L J, Nasr B, Nizard J. (2008) Emergency cerclage in cases of twin-to-twin transfusion syndrome with a short cervix at the time of surgery and relationship to perinatal outcome. Prenat Diagn. 28(13), 1256-1261.
- 85.Papanna R, Habli M, Baschat A A. (2012) Cerclage for cervical shortening at fetoscopic laser photocoagulation in twin-twin transfusion syndrome. , Am J Obstet Gynecol.206(5);425.e1-425.e7
- 86.Papanna R, Molina S, Moise K Y, Moise KJ Jr, Johnson A. (2010) Chorioamnion plugging and the risk of preterm premature rupture of membranes after laser surgery in twin-twin transfusion syndrome. Ultrasound Obstet Gynecol. 35(3), 337-343.
- 87.Papanna R, Mann L K, Moise K Y, Johnson A, Moise KJ Jr. (2013) Absorbable gelatin plug does not prevent iatrogenic preterm premature rupture of membranes after fetoscopic laser surgery for twin-twin transfusion syndrome. Ultrasound Obstet Gynecol. 42(4), 456-460.
- 88.Flake A W. (2004) In utero stem cell transplantation. Best Pract Res Clin Obstet Gynaecol. 18(6), 941-958.
- 89.Troeger C, Surbek D, Schoberlein A. (2006) In utero haematopoietic stem cell transplantation. experiences in mice, sheep and humans. Swiss Med Wkly.136(31-32):. 498-503.
- 90.McClain L E, Flake A W. (2015) In utero stem cell transplantation and gene therapy: Recent progress and the potential for clinical application. Best Pract Res Clin Obstet Gynaecol.
