Abstract
Disposal of chitin wastes from crustacean shell can cause environmental and health hazards. Chitin is a well known abundant natural polymer extracted after deproteinization and demineralization of the shell wastes of shrimp, crab, lobster, and krill. Extraction of chitin and its derivatives from waste material is one of the alternative ways to turn the waste into useful products. Chitinases are enzymes that degrade chitin. Chitinases contribute to the generation of carbon and nitrogen in the ecosystem. Chitin and chitinolytic enzymes are gaining importance for their biotechnological applications. The presence of surface charge and multiple functional groups make chitin as a beneficial natural polymer. Due to the reactive functional groups chitin can be used for the preparation of a spectrum of chitin derivatives such as chitosan, alkyl chitin, sulfated chitin, dibutyryl chitin and carboxymethyl chitin for specific applications in different areas. The present review is aimed to summarize the efficacy of the chitinases on the chitin and its derivatives and their diverse applications in biomedical and environmental field. Further this review also discusses the synthesis of various chitin derivatives in detail and brings out the importance of chitin and its derivatives in biomedical and environmental applications.
Author Contributions
Academic Editor: Jelena Markovic, Assistant Professor, Department of Biology and Ecology, Faculty of Sciences, University of Novi Sad.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2018 Palanivel Rameshthangam, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Chitin is a natural polymer, first discovered in mushrooms by French Professor, Henrni Braconnot, in 1811. Chitin is the second most abundant biopolymer next to cellulose with an annual production of 1010 to 1011 tons1 . In many respects, chitin is similar to cellulose and is considered to be a derivative of cellulose where the C2 hydroxyl groups were replaced by acetamido residues1,2 In nature, chitin is found as crystalline microfibrils which form the structural components of many organisms. Chitin serves as a structural and functional material wherever reinforcement and strength are required in a number of living organisms2 . The commercial value of chitin has dramatically increased recently due to the beneficial properties of its soluble derivatives, which are suitable for a wide variety of industrial applications in biotechnology, agriculture, food processing, cosmetics, veterinary, medicine, dentistry, environment protection, and paper or textile production3 . Chitin is one of the ubiquitous polymers found in many organisms (Table 1) from cell walls of fungi and algae to cuticle of insect’s, shells of mollusks (endoskeleton of cephalopods) and crustaceans4. Chitin is widely distributed in the invertebrates and in the lower forms of plants. Chitin is a well-known component in the fungi while it is a major component in the exoskeletons of arthropods such as crustaceans and insects. Approximately 75% of the total weight of crustaceans (shrimp, crabs, prawns, lobster, and krill) ending up as waste are mainly used for the isolation of chitin5 . In fact more than 10,000 tons of shell fish waste is available every year, which would provide sufficient raw material for the production of chitin6 Chitin contains amino sugars, compriseing of two monomeric units namely N-acetylglucosamine and glucosamine. Chitin is a linear unbranched chains of β-(1 → 4) linked 2-acetamido-2-deoxy-D-glucose (N-acetyl-D-glucosamine) residues of polysaccharides. The amount of glucosamine present in chitin is very low and hence it is less soluble in solvents and water31 . The β-1,4-linkage between the monomeric units provides a linear structure, stability and rigidity to chitin. The abundant hydroxyl groups and amino groups of the polymer have the tendency for inter and intra molecular hydrogen bonds which resulted in the formation of linear aggregates with extensive crystallinity32 The molecular weight (Mw) of chitin can be as high as 106 Da and the structure of chitin is represented in Figure 1. In nature, chitin exists in three different polymeric forms namely α, β and γ with different physical properties33 . The different forms of chitin differ in their arrangement of the polymeric chain (Figure 2). In α-chitin, the chains are arranged anti-parallel to each other, in β-chitin, they are arranged parallel to each other and in γ-chitin the polymeric chains are arranged randomly in which two parallel chains and one anti-parallel chain forms the polymeric structure.
Table 1. Various sources of chitin.| Groups/Species | References |
|---|---|
| Beetles (Insects) | 7,8 |
| Bombyx mori (Insects) | 7 |
| Honeybees (Insects) | 9 |
| Aedes aegypti (Insects) | 10 |
| Cancer pagurus (Crab) | 11 |
| Carcinus maenas (Crab) | 12 |
| Lithodes aequispinus (Golden king crab) | 13 |
| Chionoecetes opilio (Snow crab) | |
| Erimacrus isenbeckii (Korean hair crab) | |
| Paralithodes platypus (Blue king crab) | |
| Paralithodes camtchaticus (Red king crab) | |
| Chionoecetes bairdi (Tanner crab) | |
| Parapenaeopsis stylifera (Shrimp) | 14 |
| Penaeus carinatus (Shrimp) | 15 |
| Penaeus monodon (Shrimp) | 15,16 |
| Litopenaeus vannamei (Shrimp) | 17 |
| Jasus lalandii (Lobster) | 18 |
| Homarus americanus (Lobster) | 19 |
| Sepia offcinalis (Cuttlefish) | 20 |
| Loligo vulgaris (Squid) | 20 |
| Absidia glauca (Fungi) | 21 |
| Absidia coerulea (Fungi) | 22 |
| Aspergillus niger (Fungi) | 23 |
| Mucor rouxii (Fungi) | 24 |
| Phycomyces blakesleeanus (Fungi) | 25 |
| Gongronella butleri (Fungi) | 26 |
| Absidia blakesleeana (Fungi) | 27 |
| Rhizopus oryzae (Fungi) | 28 |
| Trichoderma reesei (Fungi) | 29 |
| Lentinus edodes (Fungi) | 30 |
The main source of α-chitin is from crustaceans such as crabs and shrimp whereas β-chitin is derived from squids and γ-chitin is from loligo32 . The characteristic features of chitin namely degree of deacetylation (DDA) and molecular mass can vary with the method of isolation, the process and origin of chitin. The degree of deacetylation can be defined as the molar fraction of deacetylated monomer units present in the chitin polymer chain34. The DDA content allow to differentiate between chitin and chitosan. If the DDA is less than 50%, it is then termed as chitin and if the DDA is greater than 50%, it is termed as chitosan35 . DDA is the most important factor which influences the properties of chitin, viz. solubility, flexibility, polymer conformation and viscosity36 .
Figure 1.Chemical structure of chitin.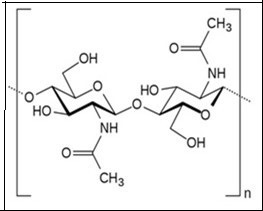
Figure 2.Schematic representation of three different polymeric configurations (α, β and γ) of chitin.
Traditionally chitin is extracted from the exoskeletons of crustaceans by chemical methods which include a combination of three basic steps viz. (i) deproteinisation, (ii) deminaralization and (iii) bleaching. Deproteinisation is performed by treating the crustacean shells in alkaline solutions such as NaOH and KOH37 . Demineralisation is generally performed by treating the shells in acidic solutions like HCl, HNO3, H2SO4, CH3COOH and HCOOH at a high temperature of 90-100°C38 . Finally bleaching is carried out to get colourless chitin. Alternatively chitin can also be extracted by using biological methods; in particular the deproteinisation is performed by using microbial extracellular proteases instead of alkaline solutions39 .
Biological demineralization of the crustacean shells is performed by enzymatic and microbiological methods by using natural probiotic organisms. Extraction of chitin by microbiological method is the most effective technique than the extraction of chitin by chemical methods40 . In biological process of chitin extraction, demineralization and deproteinisation occur simultaneously. Fermentation of shell wastes of shrimp (Penaeus monodon) were carried out with lactic acid bacteria where chitin was recovered by adding carbohydrates as a natural energy source41 . The chemical and biological (enzymatic) methods used for extraction of chitin is schematically represented in Figure 3.
Figure 3.A schematic representation of the chemical and biological (enzymatic) methods for chitin extraction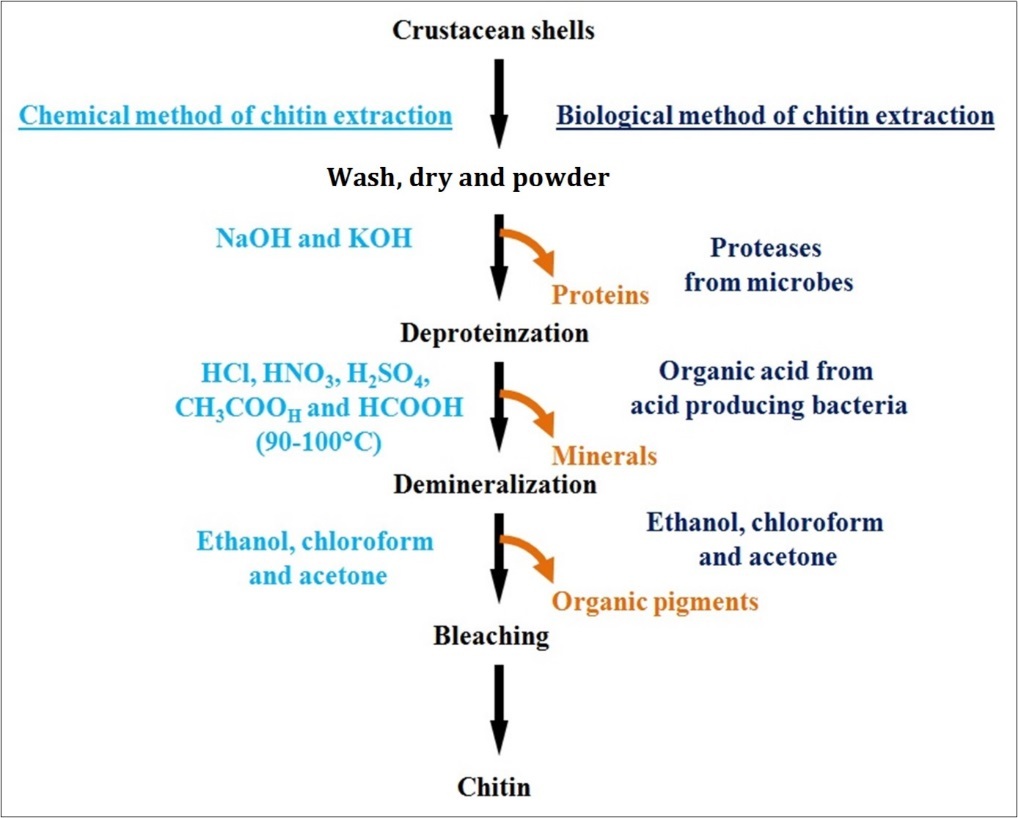
In the production of chitin derivatives, the roles of chitinase enzymes are also remarkable in recent decades. Chitinase enzymes have specific molecular structure and function besides and exhibit substrate specificity and catalytic mechanisms. Chitinases also promote degradation of chitin into novel products having industrial applications 41,42,43,44 .
Derivatives of Chitin
Chitosan
The utilization of chitin may be restricted due to its poor solubility, low porosity, and surface area45 . Hence to overcome the limitations and control the properties of chitin, various significant derivatives are produced. Chitosan is the one of the most important derivatives of chitin in terms of applicability46 . The chitin undergoes extensive deacetylation process to produce chitosan. The deacetylation is carried out using sodium hydroxide solution at 100 °C. The concentration of sodium hydroxide and variation in temperature influence the variation in DDA content during the process of production of chitosan. Depending upon the DDA content, the chitosan can be soluble in water or mild acidic solution47 .
Alkyl Chitin
Alkyl derivatives of chitin are known to significantly enhance the solubility and applicability of the chitin48 . For the production of N-alkyl-chitin, chitosan molecules are initially deacetylated completely and further treated with three kinds of aldehydes, namely formaldehyde, acetaldehyde, and pentanal to form Schiff bases of chitosan which in turn are reduced with sodium cyanoborohydride to form N-alkylated chitosans. The N-alkyl-chitosans are then transformed into the corresponding N-alkyl-chitins by acetylation with acetic anhydride followed by transesterification (process of exchanging the organic group R″ of an ester with the organic group R of an alcohol) to remove partly formed O-acetyl groups49. The amorphous alkyl chitin (N-methyl-, N-ethyl- and N-pentyl) produced at C2-carbon of the monomer show an enhanced affinity towards the organic solvents. Hence, the alkyl derivatives of chitin showed an excellent solubility and applicability.
N and O-Sulfated Chitin
Sulfated derivatives of chitin have attracted perennial research interests due to their functional similarity to heparin and hence the sulfated derivatives of chitin are used as an anticoagulant agent. Moreover attempts are made to prepare N- and/or O-sulfated-chitin using various reaction conditions and sulfating agents. Zou and Khor (2009), prepared sulfated-chitins of varying degree of sulfation (DS) by the reaction of chitin with sulfur trioxide–pyridine complex under homogeneous conditions in 5% LiCl/DMAc solvent system. Sulfation at 8°C or room temperature was regio-selective for the C6–OH position with the DS ranging from 0.53 to 1.00 depending on the reaction time. When the reaction temperature was elevated, sulfation at the C3–OH position also occurred. The degree of substitution and position of sulfation led to structure-activity relationship ambiguities50.
Dibutyryl Chitin
Dibutyryl chitin or ester derivative of chitin is known to be an easily soluble derivative of chitin which binds with butyryl groups at C-3 and C-6 positions51 . These chitin derivatives exhibited some desirable qualities like bioactivity, biocompatibility, biodegradability along with film and fiber-forming properties and also the derivatives have a huge potential for manufacturing a wide range of materials suitable for biomedical and industrial applications52 . Dibutyryl chitin was obtained by reaction of the chitin with butyric anhydride, and by using perchloric acid as catalyst and also from butyric anhydride and butyric acid using methanesulfonic acid as catalyst and solvent. Bhatt et al., (2011) reported the occurrence of synthesis of chitin butyrate by reaction chitin with butyric acid in the presence of TFAA/H3PO453 . Dibutyryl derivatives of chitin are known to provide potential biomedical and industrial applications and they could also be used as intermediates for further chemical modifications under mild conditions.
Carboxymethyl Chitin
Soluble carboxymethyl chitin (CMCH) is one of the most attractive derivatives of chitin for biomedical applications54 . Traditional method of synthesis of carboxymethyl chitin involve mixing of chitin slurry in the presence of concentrated NaOH (40-60% w/w) and isopropanol under the heterogeneous reaction conditions at 100°C. Huang et al., (2012) prepared CMCH by using a mixture of NaOH, 2-propanol and monochloroacetic acid55 . Recently, Liu et al., (2015) synthesized novel homogeneous carboxymethyl chitin with a broad range of degree of substitution (0.035 to 0.74), high DA and little de-polymerization in aqueous NaOH/urea solution. Homogenous carboxymethylation of natural chitin offers an advantage of a fair structural control56 . Based on the carboxymethylation percentage CMCH could be used as excipients (inert substances used as vehicles and diluents for drugs), especially for oral drug delivery.
Chitoligosaccharides
Chitoligosaccharides (COS) are partially hydrolyzed products of chitin, and have been recently focused for their solubility in acid-free aqueous media57 . The COS have been shown to posses more potential than chitin nutraceutical additive, since COS are easily absorbed through the intestine, quickly transported into the blood flow and are shown to exhibit systemic biological effects in the organism58 . Acid hydrolysis (hydrochloric, nitrous, phosphoric acid, hydrogen fluoride) and oxidative reductive depolymerization (mediated by peroxide, ozone, and persulfate) are important routes for synthesis of COS. Depolymerization under high energy impact (using ultrasound, microwave, etc.) and recombinant approaches (using enzymatic and microbial depolymerization) are also being tried for production of COS59 . Due to its low molecular weight chitoligosaccharides are thought to have several interesting bioactivities and applications.
Chitin Nanofibers
Chitin nanofibers (CNF) are biodegradable chitin derivatives, having typical width of 10-20 nm and large surface-to-mass ratio. The CNF are being prepared, and studied, more recently worldwide for various applications60 . When the CNF are blended with inorganic metals to prepare advanced hybrid organic-inorganic composites, they can have applications in electronics, electrical, optical devices and much needed solar energy production61 . CNF was prepared from the shrimp and crab shells by various chemical treatments. In brief minerals were removed by HCl treatment, removal of proteins was done by refluxing the suspension with NaOH, pigments and lipids were removed by ethanol. After completion of above treatments, suspension was filtered washed with distilled water and kept wet for mechanical grinding for fibrillation, this wet slurry was made to a concentration of 1% and called chitin slurry. Chemical treatment loosened the tightly bonded fibrils bundles to larger extent apart from removal of minerals, proteins, pigments, and lipids62 . CNFs have successfully been used in many applications, including tissue engineering, wound dressing, cosmetic and skin health, stem cell technology, anti-cancer therapy, drug delivery, anti-inflammatory treatment, and obesity management60.
Chitin Nanowhiskers
Chitin nano-whiskers (CNW) of slender parallelepiped rods have been successfully prepared from chitin, which has been recently explored in nanotechnology application. CNWs are currently being studied and used as reinforcing additives for high performance environmentally friendly and biodegradable nanocomposite materials, as biomedical composites for drug/gene delivery or nanoscaffolds in tissue engineering64 . Sriupayo et al., (2005) reported the chemical preparation of CNW from chitin. They treated the chitin with 3 N HCl at 100°C for 90 min under vigorous stirring. The ratio of 3N HCl to chitin was 100 mL/g. After treatment, the suspension was diluted with distilled water, followed by centrifugation at 10 000 rpm for 5 min. This process was repeated three times and the suspension was then transferred to a dialysis bag and dialyzed against deionized water up to neutral pH. The CNW suspension was sealed and preserved by storing in a refrigerator at 4°C65 . Qin et al., (2016) have used 3 M H2SO4 solution, for the hydrolysis of chitin in the preparation of CNW66 . CNWs have drawn attention in various applications due to their properties like nanosized dimensions, high surface area, high absorbability, biodegradability, nontoxicity, renewability, low density and easy modification63 . The schematic representation of the difference between CNF and CNW is represented in Figure 4.
Figure 4.Schematic illustration of morphological difference of a) CNF and b) CNW.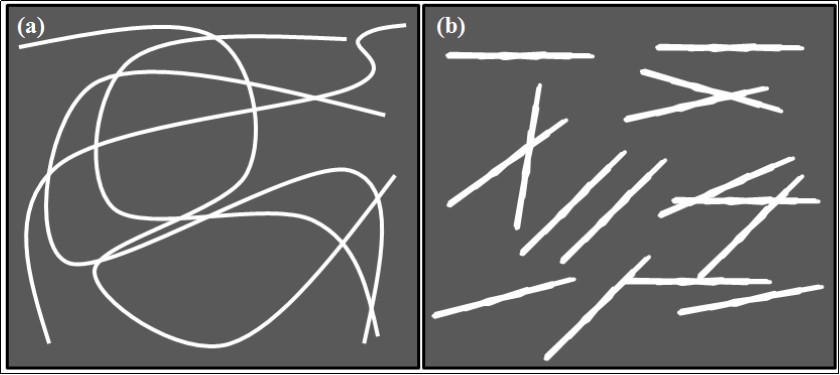
Chitin Nanoparticles
Chitin nanoparticles (CNP) with larger surface area are synthesized from powdered chitin and such CNP is known to have varied applications67 . CNP was isolated from the purified chitin by repeated acid hydrolysis. Chitin powder was soaked in 3 M HCl for 1.5 h at 90 °C in a water bath. The sample was centrifuged at 6000 rpm for 10 min and the pellets were collected. The acid hydrolysis step was repeated thrice and the pellets were suspended in distilled water to dilute the acid concentration. The suspension was dialyzed against distilled water until it reaches pH 6 and was homogenized using a tissue homogenizer. The homogenized sample was collected and lyophilized at −60 °C to get the powder form of CNP. Mechanical disruption and ultrasonication were carried out to cut down the size of nanoparticles17 . SEM and TEM micrographs of prepared CNP from the shells of Penaeus monodon was displayed in Figure 5. Smitha et al., (2013, 2015) have prepared chitin nanoparticle by cross linking the chitin using tripoly phosphate solution (TPP)68,69 . CNPs have been widely used in various applications due to its biocompatible, biodegradable and non-toxic nature.
Figure 5.a) SEM and b) show TEM micrograph of chitin nanoparticles synthesized from the shells of Penaeus monodon Fabricius (Reprinted from 17).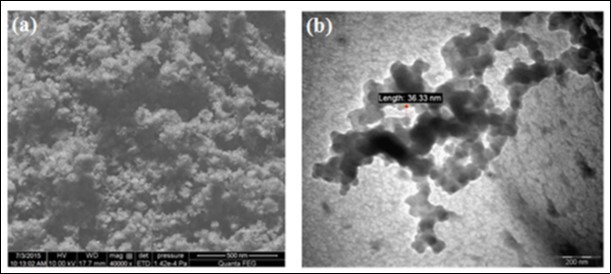
Chitin Nanocomposite
Chitin nanocomposites are multiphase materials consisting of a chitin matrix and nanosized fillers to alter the stability and the mechanical properties of the chitin70. Polymer nanocomposites can be produced by introducing a crosslinking agent into the polymer matrix. Chitin whisker and tannic acid cross link chitosan composite which was synthesized and the mechanical and physicochemical properties of such nanocomposites were studied by Rubentheren et al., (2015)71. Chitin nanocomposites can also be produced by introducing chitin nanofibers with high aspect ratio, high strength and high modulus into synthetic polymer matrices like polyacrylic acid (PAA). Bogdanova et al., (2016) has shown exfoliation of the squid β-chitin in aqueous acrylic acid (AA), after which a composite film of chitin microfibrils in polyacrylic acid (PAA) has been prepared by in situ polymerization of the AA72. Also chitin nanocomposites can be produced by incorporating metal nanoparticles into chitin matrix. In our recent study, we reported the synthesis of α-chitin/silver nanocomposite (CNP/AgNP) by incorporating α-chitin nanoparticles isolated from a mixture of the shrimp shells and silver nanoparticles (AgNP)73. The TEM micrograph of CNP/AgNP displayed in Figure 6 shows CNP nanocomposite which prevented the agglomeration of AgNP by stably encapsulating the AgNP.
Figure 6.TEM micrograph of CNP/AgNP; Blue arrow indicates the surface of CNP; Red arrow indicates AgNP (spherical shaped spots) embedded in the surface of CNP (Reprinted from 73).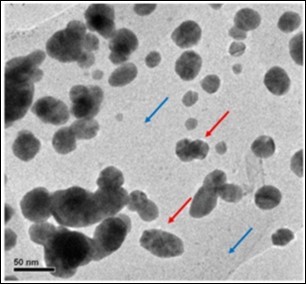
Chitin Hydrogels
Hydrogels are three-dimensional hydrophilic polymer-based networks with high water content resembling the native extracellular matrix74 . Kawata et al., (2016) prepared calcium phosphate cross linked chitin nanofiber hydrogel and it used for bone tissue regeneration applications75. Similarly, Liu et al., (2016) prepared CMCH hydrogel by simple NaOH treatment and it used for three-dimensional cell culture76 . Due to hydrogels shared resemblance with natural soft tissue (high water content, controllable porosity and generally acceptable biocompatibility) for the past several decades, hydrogels have been widely explored as promising biomaterial candidates for cell scaffolds and drug delivery vehicles77.
Biomedical Applications
Chitin and its derivatives are biodegradable and biocompatible natural polymers, safe and non-toxic, and bind to mammalian and microbial cells potentially. Here, we discussed some of the potential biomedical applications of chitin and its derivatives (Figure 7).
Figure 7.Schematic representation of the biomedical applications of chitin and its derivatives
Antimicrobial Activity
The increasing antibiotic resistance pattern exhibited in majority of the pathogenic microorganism is a major problem throughout the world78 . In recent years, there has been an increased interest in the development of antimicrobial substances from natural products.. Abdel-Rahman et al., (2015) studied the antibacterial activity of chitin and chitosan, isolated from shrimp shell by chemical treatments, these products were tested against E. coli strains and it was concluded to exhibit antibacterial activity. The chitosan had high DDA content as well as antibacterial activity than chitin79 . The antimicrobial activity of chitin and chitosan extracted from Para Penaeus Longirostris shrimp shell waste was studied against four different genera of bacteria viz. Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa and Klebsiella pneumonia and two fungi viz. Candida albicans and Candida parapsilosis. The results of the study further confirmed that generally the antimicrobial activity seem to be related with DDA80 . Jiang et al., (2016) investigated the antibacterial activity of lysozyme immobilized on CNW and the results of the study provided evidences so that the lysozyme immobilized CNW system exhibited greater antibacterial activity against Escherichia coli, Staphylococcus aureus, and Bacillus subtilis when compared with free lysozyme81 . Sahraee et al., prepared corn oil emulsified nanocomposite gelatin film with chitin nanofiber to study the antifungal activity and showed improved physical, mechanical, thermal and antifungal properties82 . In addition, the α-chitin nanofiber processed by dynamic high pressure homogenization exhibited a significant antifungal activity against Aspergillus niger83 . Similarly the enzymatically deproteinized chitin, chitosan and its by-products isolated from Norway lobster exhibited very good antimicrobial activity against bacterial and fungal strains84 . Likewise, CNF included carrageenan films exhibited strong antimicrobial activity against Listeria monocytogenes85 . Sun et al., (2017) synthesized a novel water-soluble sulfonated chitosan, as a kind of linear sulfated polysaccharide, by introducing 1,3-propane sulfone to the amino group of chitosan under mild acidic conditions. They also studied the antimicrobial activity of the sulfonated chitosan against bacterial and fungal strains and concluded that the microbial inhibition was dependent on the type of chitosan used and the type of microorganisms86. Zhang et al., (2016) reported that chitin enhances the biocontrol activity of Rhodotorula mucilaginosa against blue mold and Rhizopus decay of peaches87 . Gelatin nanocomposite film containing 0, 3, 5, and 10 % concentrations of chitin have been synthesized and the antifungal property was evaluated. The study confirmed that, incorporation of chitin with gelatin films not only improved physical properties of the film, but also can develop a functional nanocomposite biopolymer with potential antifungal activity88 . Solairaj & Rameshthangam (2016) have prepared CNP/AgNP and evaluated the antimicrobial activity against bacterial and fungal strains. They demonstrated that the prepared composite to exhibit potential antimicrobial activity, which is higher than the pure AgNP. The CNP/AgNP has also tested for mosquito larvicidal activity and reported that, the composite have potential larvicidal activity against Aedes aegypti73 . In a similar research, AgNPs-loaded chitin nanocrystal nanocomposites were produced and coated on a cellulose paper which showed potential antimicrobial activity against E. coli and S. aureus89 .
Anti-Inflammatory Effects
Synthetic anti-inflammatory agents possess some side effects such as gastric irritation, ulceration and decreased host resistance in the patients. In order to find some natural anti-inflammatory agents with biocompatibility and biodegradability property, extensive research works have been carried out in chitin and its derivatives. Khanal et al., (2000) studied the potential usefulness of phosphated chitin (P-chitin) as an anti-inflammatory agent in a mice model of acute respiratory distress syndrome. The research group reported that P-chitin with a molecular weight of 24000 D, 58% degree of substitution and 4% degree of deacetylation was found to be the most effective in blocking the lung injury when administered at 8 mg/kg level89 . Lee et al., (2009) prepared two kinds of COSs (90-COSs and 50-COSs) from 90% and 50% deacetylated chitosan and evaluated their anti-inflammatory activity. The results evidenced that; 90-COS has showed potential anti-inflammatory effect via down-regulation of (both transcriptional and translational expression) tumor necrosis factor (TNF)-a, interleukin (IL)-6, inducible nitric oxide synthase (iNOS) and cyclooxygenase (COX)-2 genes90 . Another study suggests that, COS possess anti-inflammatory activity, which is dependent on dose and molecular weight. A single dose of 500 mg/kg body weight may be suitable to treat acute inflammation cases91 . El-Badry and Fetih (2011) studied the anti-inflammatory activity of the celecoxib loaded chitosan formulations. The results suggested that chitosan concentration and molecular weight are very crucial factors on the release of celecoxib from gel formulations and also confirmed that the chitosan gel formulations have significant anti-inflammatory activity92 . Another similar research evidenced an increased anti-inflammatory activity of rutin encapsulated in chitosan microspheres. The researchers also suggested that, rutin loaded chitosan microspheres could be used in the treatment of mucosa inflammation, such as in the synovial, lung and bowel compartments93 . Wei et al., (2012) demonstrated that, COS could inhibit the inflammatory responses in N9 microglial cells through the suppression of nitric oxide (NO) production and down regulation of iNOS production at both transcription and translation levels94 . In various similar studies COS was proved for its anti inflammatory property in uveitis rats and asthmatic models95,96 . The recent reports state that, chitin and its derivatives may act as a promising candidate material for treating and preventing inflammation.
Wound Healing Potential
The wound healing is a complex process, which includes hemostasis, inflammation, proliferation, and remodeling. A successful wound healing needs an appropriate treatment to modulate a series of complex interactions between different cells and cytokine mediators throughout the all phases of healing97 . There are numerous biomaterials used as wound dressings viz. alginates, polyurethane, hydrocolloids, collagen, pectin, hyaluronic acid and chitin derivatives for enhancing the wound healing process. Among them chitin derivatives have attractive wound healing properties. Minagawa et al., (2007) studied the effect of molecular weight and DDA of chitin/chitosan in wound healing. They found that, higher the DDA and low molecular weight of chitin/chitosan to shows the highest wound healing property98 . Abdel-Mohsen et al., (2016) reported the use of chitin/chitosan-glucan complex for the preparation of micro and non-wovenfiber/nonwoven sheets after dissolution in urea/sodium hydroxide aqueous solution at -15°C. They further reported that the prepared wound dressing sheets have shown excellent wound healing ability and promoted accelerated wound closure of the rat skin99 . Marei et al., (2017) compared the wound healing properties of chitosan isolated from locust (Schistocerca gregaria) and shrimp (Penaeus monodon). The research group found that chitosan isolated from locust has showed a better wound healing ability. The histopathological studies of the healed wounds showed earlier granulation as well as dermis active angiogenesis with a significantly higher count with early marked epithelization and formation of thicker epidermis with minimal inflammation100 . Likewise, Aragão-Neto et al., (2016) prepared a wound healing hydrogel combined with policaju (POLI) from cashew tree (Anacardium occidentale L.) gum and chitosan. They reported that the POLI-CS hydrogel contributed for a most effective wound healing and modulation of the inflammatory process. The research group also reported that the combined use of POLI-CS hydrogel with low level laser therapy showed a better wound contraction, larger collagen presence, minor focal necrosis and early epithelization101 . Chitosan hydrogel in combination with nerolidol, superficially deacetylated chitin nanofibrils are also reported for their wound healing properties102,103 . Moreover, the composite materials such as, sulfanilamide and silver nanoparticles-loaded polyvinyl alcohol-chitosan composite, chitosan-based copper nanocomposite and chitosan-Ag/ZnO composite have shown synergistic mechanism and an enhanced wound healing process104,105,106 . Freeze dried surface-deacetylated chitin nanofibers reinforced with sulfobutyl ether β-cyclodextrin are also reported as a new beneficial biomaterial for the treatment of wounds107 . Moura et al., (2014) reported that 5-methyl pyrrolidinone chitosan (MPC) wound dressings loaded with neurotensin could be used for the healing of diabetic foot ulcers. MPC foam combined with neurotensin can promote an anti-inflammatory response and stimulate re-epithelialization, which are important phases in the wound healing process108 . An ideal wound dressing should be able to absorb exudates and toxic components from the wound surface, maintain a high humidity at the wound/dressing interface, allow gaseous exchange, provide thermal insulation, and protect the wound from bacterial penetration and it must be non-toxic109 . The above studies proved that chitin based nanostructures, nanocomposites and hydrogels have all the beneficial characteristics and could be used as wound dressings and an effective wound healing material.
Anticancer Effects
The mortality rate due to cancer still remains very high. Chemotherapy still remains one of the popular treatment options for cancer treatment; however, a low level of drug accumulation in and around the cancerous cells and a high accumulation of anticancer drugs in the healthy tissues limits its potential clinical applications110 . The biocompatibility of chitin nanogels has been studied and reported by Rejinold et al., (2012) on an array of cell lines111. The anticancer property of chitooligosaccharides with the highest degree of DA and lowest molecular weight has been reported by Kim et al., (2012) in human myeloid leukemia HL-60 cells112 . Huang et al., (2006) reported that highly charged COS show cell specific anticancer activity against HeLa, Hep3B and SW480 cell lines. They reported that highly charged COS derivatives could significantly reduce cancer cell viability, regardless of the positive or negative charges113 . Salah et al., (2013) studied the active mechanism of chemically prepared low molecular weight chitin against human monocyte leukaemia cells (THP-1) and human monocytic cells (MRC-5). They speculate that low molecular weight chitin inhibited the action of YKL-40, a glycoprotein with anti-apoptotic effect. Consequently THP-1 cancer cells, which express YKL-40 undergoes mortality and noncancerous cell (MRC-5) which could not express YKL-40 can proliferate114 . In addition, Gibot et al., (2014) studied the cell line dependent anticancer property of chitosan in human melanoma cell lines. The research group reported that chitosan could trigger both mitochondrial and death receptor mediated apoptosis signaling pathways in melanoma cell line115 . The synergistic anticancer activity of chitosan combined with silver nanoparticles was also reported in human cervical cancer HeLa cells and human lung cancer A549 cells116,117 . The anticancer potential of β-chitosan nanoparticles was studied in human hepatocellular carcinoma cells and reported as promising anticancer agent118 . The anticancer responses displayed by the chitin and its derivatives can be attributed to the beginning of development of new anticancer agents.
Bio-Sensing
Electrochemical methods have shown the remarkable advantages in the analysis of components or ingredients in pharmaceutical preparations and other biological molecules in human body fluids. The advantages of electrochemical sensing are mainly due to the sensitivity, low cost and relatively short analysis time of the biological compounds as compared to the other routine analytical techniques including chromatography, ELISA and Western blot119 . The protonation of acetylamide group in chitin is effective for accumulating and separating some anions from a sample matrix, based on electrostatic interaction. This beneficial property makes chitin possible for immobilization of enzyme(s) and other materials that will provide effective sensing application. Sugawara et al., (2000) have demonstrated the glucose sensing ability of carbon-paste electrode which was modified with immobilization of glucose oxidase (GOD), demonstrated the electrostatic interactions of the chitin and GOD and they determined the amount of glucose present in the sports drinks120 . Chen et al., (2006) prepared chitosan membranes from the carapace of the soldier crab Mictyris brevidactylus and studied its application. The chitosan membrane was used to immobilizing enzymes for biosensor construction owing to their good electrochemical characteristics and excellent mechanical properties121 . Kumar et al., (2010) demonstrated that, polymer nano-omposites synthesized by the implementation of carbon nanotubes (CNT) in chitosan matrices that exhibited better mechanical property and electrical conductivity. The research groups also established that the composite material is very useful in designing electrochemical biosensor for the detection of organic vapours122 . Immobilisation of functionalised carbon nanotubes into chitosan matrices using crosslinkers was performed and applied to sense organic molecules such as hydroquinone, dipyrone, and glucose123 . Liu et al., (2012) electrodeposited chitosan and silver nanoparticles to form a positively charged surface on the glassy carbon electrode and used for the detection of the trichloroacetic acid (TCA). A sensitive amperometric sensor for trichloroacetic acid was constructed with low detection limit of 1.1 µM by using the fast diffusion and electron transfer process of the negatively charged TCAA in the positively charged silver nanoparticles doped chitosan hydrogel film124 . In a similar study, Liu et al., (2012) electrodeposited and prepared molecularly imprinted polymers (MIP) using combination of chitosan and graphene. These MIPs have been recognized as optimal elements to construct sensor with specific binding sites to target molecule. The researchers developed a sensor for dopamine detection based on the CS dispersed with graphene mixture as the functional matrix. Dopamine is a naturally occurring catecholamine which is an important neurotransmitter of mammals and it becomes a key marker for schizophrenia and Parkinson’s disease125 . Similarly, Palanisamy et al., (2017) prepared a novel hybrid hydrogel composite of chitin stabilized graphite for selective and simultaneous electrochemical detection of dihydroxybenzene isomers in water126 . A electrochemical biosensor was fabricated using copper immobilized chitin nanostructures which exhibited rapid and sensitive detection of 0.776 µM glucose127 . All these demonstrate that chitin and its derivatives could be used for the development of sensors for sensing various chemicals, biomolecules and drugs.
Drug Delivery Potential
Nowadays, polymer based materials are known to act as a very promising platform for delivery of bioactive macromolecular drugs. A number of interesting properties of drug carriers include muco- and bioadhesiveness, a high capacity to associate and release therapeutic macromolecules, as well as their ability to enhance the transport of bioactive compounds across the epithelial barriers, such as the ocular, nasal and intestinal routes. Among the polymers used in drug delivery platform, chitosan (CS) is one of the well known molecules because of their biocompatibility, low toxicity, biodegradability, and muco- and bioadhesiveness128 . Cover et al., (2012) studied the effect of transcervical administration of doxycycline-loaded chitosan nanoparticles (DCNPs) for the treatment of uterine infections. The DCNPs showed improved and sustained delivery of doxycycline, thereby minimizing the adverse effects and improved the drug efficacy129 . In various studies, CS scaffolds were fabricated and used for the delivery of therapeutic agents such as docetaxel, curcumin, 5-fluorouracil, pentoxifylline, ampicillin, dexamethasone, tetracyclinehydrochloride, amikacin, vancomycin and ketoprofen109,130,131. Apart from drug molecules, chitin derived nanoparticles was used for the delivery of RNA, proteins and peptides. Nascimento et al., (2014) formulated epidermal growth factor receptor targeted CS nanoparticles loaded with small interfering RNAs (siRNAs) against mitotic arrest deficient 2 (Mad2) gene. Mad2 is an essential mitotic checkpoint component required for accurate chromosome segregation during mitosis and its complete abolition leads to cell death. The study confirmed that EGFR targeted CS loaded with Mad2 siRNAs was a potent delivery system for selective killing of cancer cells132 . In another study, CS nanoparticles modified with T cell-specific antibodies were used for the delivery of siRNA to T cells. CD7-specific single-chain antibody was chemically conjugated to CS by carbodiimide chemistry, and nanoparticles were prepared by a complex coacervation method in the presence of siRNA. The results showed that the expression levels of CD4 receptors on T cells were greatly reduced by the delivery of CD4 siRNA using antibody-conjugated chitosan nanoparticles133 . Development of therapeutic peptides and its clinical use has been restricted to non-central nervous system diseases due to the poor permeation of peptides across the gastrointestinal mucosa and the blood−brain barrier. To overcome such restrictions, Lalatsa et al., (2012) fabricated the quaternary ammonium palmitoyl glycol chitosan nanoparticles (GCPQ) that facilitated delivery of orally administered peptides such as leucine-enkephalin (neurotransmitter) into the brain. The research concluded that GCPQ particles facilitated absorption of the oral mucus adhering drug peptide by protecting the peptide from gastrointestinal degradation, and by increasing the drug gut residence time and transporting GCPQ associated peptide across the enterocytes and to the systemic circulation, enabling the GCPQ stabilized peptide to be transported to the brain134 . With these recent research findings, this section briefly revisited the application potential of chitin and its derivatives in drug, RNA and peptide delivery.
Tissue Engineering
Tissue engineering is one of the basic approaches to recuperate/replace the tissues and organs that are damaged or diseased. However, limited availability of grafts, risk of disease transmission, pain at the graft site, lack of enough fusion, morbidity at the donor site and cost, are some of restraining factors of tissue engineering. Biomaterials with appropriate physio and biochemical properties thereby used to achieve successful survival rates over tissue engineering109 . Recently, chitin and its derivatives have shown remarkable promise in tissue engineering. Kumar et al., (2013) have developed a nanocomposite scaffold for use in tissue engineering, using a mixture of pectin, chitin and nano CaCO3 by lyophilization, The research group evaluated the cytocompatibility of the scaffold on mouse fibroblast cell lines (NIH3T3 and L929) and human dermal fibroblast (HDF) cells. The results confirmed that the scaffold showed negligible toxicity towards cells. Cell attachment and proliferation studies were also conducted using these cells, which showed that cells attached onto the scaffolds and started to proliferate after 48 h of incubation135 . In another study, graphene oxide (GO)–chitosan (CS)-hyaluronic acid (HA) based bioactive composite scaffold containing an osteogenesis-inducing drug simvastatin was fabricated for bone tissue engineering application. The in vitro results showed that the scaffold material offered a significant influence on osteogenesis and biomineralization and it possess an excellent biocompatibility and to be used as a bone tissue engineering scaffold136 . Liu et al., (2016) prepared CS/chitin nanocrystals (CNC) composite scaffolds by a dispersion-based freeze dry approach which exhibited significant enhancement in compressive mechanical strength of the composite scaffolds which were successfully applied as scaffolds for MC3T3-E1osteoblast cells, which in turn showed excellent biocompatibility and low cytotoxicity. The results of the study also revealed that CNCs can markedly promote the cell adhesion and proliferation of the osteoblast on CS and it can have potential application in bone tissue engineering137 . In a similar research, novel porous composite scaffolds consisting of chitin, chitosan and nano diopside powder were prepared by using the freeze-drying method. Cytocompatibility of the scaffolds and cell attachment were studied by using human gingival fibroblast cells. The scaffolds demonstrated no sign of cellular toxicity and the cells were found to be attached to the pore walls within the scaffolds and the results suggested that the developed composite scaffolds could be a potential candidate for tissue engineering138. Pangon et al., (2016) have used chitin whisker (CNW) to enhance the mechanical properties of chitosan/poly (vinyl alcohol) (CS/PVA) nanofibers and to offer osteoblast cells to grow with hydroxyapatite mineralization. The CNW combined with hydroxyapatite in bionanocomposite was shown to act as a key to promote osteoblast cell adhesion and proliferation139. Although these research findings supported the use of chitin and its derivatives for tissue engineering, further studies on toxicity, degradation and in vivo effects of these chitin scaffolds are required before using them for clinical trials/human use.
Environmental Applications
Soil and water pollution by organic and inorganic contaminants is of a growing concern because of their potential detrimental effects on human health and the environment. As environmental protection is becoming an important global problem and industries pay attention to the development of technology which limits the environmental problems. Recently, the commercial value of employing chitin and its derivatives for environmental applications gathered considerable interests. CT and its derivatives have been used for several environmental applications, including remediation of both organic and inorganic contaminants from water and soil. Also biocompatible nature of CT and its derivatives making them suitable, for immobilizing sensing elements such as, enzymes and nanoparticles for the sensing of environmental hazardous chemicals. Especially, Chitinases are known to play different roles in various organisms, their induction in the sensor elements is not yet beneficially unified. In chitin-utilizing organisms, chitinases require the presence of an inducer in the medium. Expression of induced hydrolases, in general, is controlled by hydrolysis products which are synthesized in very low concentration in the absence of an inducer and this allows for appropriate changes to be made in the composition of the medium for the generation of a signal to increase in the production of target enzymes140. Herein, we have summarized the applications of CT and its derivatives in the removal of dyes, organic and inorganic pollutants, and remediation of metal pollution.
Removal of Dyes
Wastewater effluents in some industries, such as dyestuff, textiles, leather, paper, and plastics, contain several kinds of synthetic dyestuffs. A very small dye amount in water is highly visible and can be toxic to life in water and harmful to human beings. Hence, the removal of dyes from process or waste effluents becomes of fundamental importance to the environment. Chitin and its derivates have excellent adsorption capacities and low cost when compared to activated carbon and therefore they received considerable interests for decontaminating the environment or removal of dyes and toxins141. Prado et al., 2004 compared the adsorption behavior of indigo carmine dye on chitin and chitosan. They reported that due to the presence of more basic nitrogen centers in chitosan, indigo carmine dye adsorbed more spontaneously in chitosan than chitin141. In a similar study, Dolphen et al., (2007) compared the adsorption behavior of chitin and chitin modified with sodium hypochlorite solution in Reactive Red 141 from wastewater. The hydroxyl group of the modified chitin was transformed into CH2OCl that cannot react with the dye solution. Therefore, dye adsorption by modified chitin involves mainly physical adsorption and adsorption capacity was higher than that of chitin142 . In another research, chitin was modified into pure chitin hydrogel (CG3), which showed excellent mechanical properties and biocompatibility, for wastewater treatment. CG3 exhibited microporous structure, large surface area and affinity on malachite green, leading to the high uptake capacity of dye143. To extend the applicability of chitin as dye adsorbent, Dotto et al., (2015) used ultrasound–assisted technology to modify the chitin surface and investigated the adsorption of methylene blue. Ultrasonic surface modified chitin (USM–chitin) presented more adequate characteristics, such as higher surface area, higher porosity, lower crystallinity and a more rugged surface for adsorption purposes, than raw chitin. Also USM–chitin can be reused for seven times maintaining the same adsorption capacity144, 144. The follow up of the same work was carried out as fixed bed adsorption of methylene blue by USM-chitin supported on sand. The optimal bed performance was attained with flow rate of 10 ml min−1 with initial MB concentration of 50 mg L−1 and also the bed performance was maintained after five adsorption–elution cycles145. Wang et al., (2015) fabricated a sunlight photocatalyst by in situ synthesis of Cu2O in the regenerated chitin (RC)/grapheneoxide (GO) composite film, where the porous chitin film was used as the microreactor for the formation of nano Cu2O. The Cu2O/RC photocatalyst exhibited good photodegradation of dyes146. In another study, chitin/graphene oxide (Chi:nGO) hybrid gels were prepared and investigated the biosorption property. Remazol Black (RB) and Neutral Red (NR) were used as an acid and basic dye model for adsorption study. The results revealed that the adsorption was dependent on both the solution pH and the Chi:nGO proportion147. Chitin nano whiskers (ChNW) are obtained from native chitin by acid hydrolysis, and considered as a very attractive class of nanomaterial with high surface to volume ratio and with hydroxyl and acetamide functional groups. Gopi et al., (2016) reported enhanced adsorption of crystal violet achieved using ChNW isolated from shrimp shells148. In a similar study, Solairaj et al., (2016) prepared chitin nanoparticles from shrimp shells and studied the adsorption property of methylene blue, bromophenol blue, and coomassie brilliant blue. The results evidenced that the chitin nanoparticle showed significant increase in mechanical, thermal stability and dye adsorption property17. The findings confirmed that CT and its derivatives are simple, fast reacting, low cost biodegradable materials that can be used for effective dye removal process.
Remediation of Inorganic Contaminants
Metals are the major inorganic contaminant worldwide and the removal of toxic metals from water is a matter of great interest in the field of water pollution control. Numerous metals such as chromium, mercury, lead, copper, etc., are known to be toxic which are a serious cause for water pollution. Chitin and its derivatives have been evaluated for remediating heavy metals, such as Cu(II), Pb(II), Hg(II), Cd(II) and Zn(II) in recent years140. Gandhi et al., (2010) prepared a composite material by combining nano-hydroxyapatite (n-HAp) with chitin and chitosan for the removal of copper(II) from aqueous solution. The adsorption capacity of n-HAp/chitin (n-HApC) and n-HAp/chitosan (n-HApCs) composite were found to be 5.4 and 6.2 mg g-1 respectively with a minimum contact time of 30 min. The research group also confirmed that due to the presence of more numerous number of chelating reactive amino groups in chitosan than the acetamide groups present in chitin, the n-HApCs composite experienced a higher efficiency than the n-HApC composite149. Kousalya et al., (2010) suitably modified the chitin for enhancing the metal sorption capacity and as an alternate for chitosan. The research group prepared protonated chitin (PC), carboxylated chitin (CC) and grafted chitin (GC) to study the metal sorption property using Cu(II) and Fe(III) ions. Among the modified forms of chitin, GC showed higher SC towards Cu(II) and Fe(III) than CC, PC and CT150. Saravanan et al., (2013) have prepared chitin/bentonite composite for better metal adsorption capacity and resistance to acidic environment. They evaluated the chitin/bentonite as the adsorbent for the sorption process of chromium from aqueous solution. The results confirmed that the composite material can act as a biosorbent at the optimum pH of 4.0151. Similarly chitin nanofibrils (CNF) was evaluated for the removal of Cd(II), Ni(II), Cu(II), Zn(II), Pb(II), Cr(III) in aqueous solution. The CNF showed much higher adsorption behavior than chitin micro-particles152. Likewise various modifications such as functionalization of chitin using polypyrrole, irradiated grafting of acrylonitrile on to chitin, acetophenone derivative of nano-chitosan, crosslinking chitosan into poly(alginic acid) nanohydrogel and thiol-functionalization chitin nanofibers were used for the removal of Cr(VI), As (III), Cu(II), Cd(II), Hg(II) and Pb(II)153,154,155,156,157.The above findings summarize the use of chitin and modified chitin for the removal of metals from the aqueous environment.
Remediation of Organic Contaminants
Wastewater that was contaminated with organic contaminants can be remediated with chitin and its derivatives dependent on the characteristics of the contaminants. Yoshizuka et al., (2000) prepared chitosan micro particles (CMs) and silver-complexed CMs (SCMs) by using different cross linking agents, i.e. glutaraldehyde and epichlorohydrin. The research group investigated the adsorption and release behaviors of CMs and SCMs towards a typical pesticide, methyl parathion (MP). The results of the study concluded that SCM cross linked with glutaraldehyde could be used for the removal of methyl parathion158. Dolphen & Thiravetyan (2011) synthesized chitin nanofibers from shrimp shells and studied the adsorption of melanoidin, a food additive which cause some mutagenic, carcinogenic and cytotoxic effects. They exhibited a maximum adsorption capacities of melanoidins by chitin nanofibers and they were 131, 331 and 353 mg/g at 20 °C, 40 °C, and 60 °C, respectively. They also found that temperature could play a major role in the adsorption behavior of chitin nanofibers159 . Similarly, Lu et al., (2011) prepared the chitosan beads and porous crab shell powder from shrimp shells and studied the removal of 17 organochlorine pesticides (OCPs) from the polluted water solution. The study confirmed that the surface morphology of chitosan beads having a rough surface and pores, can serve as the adsorption site for pesticides160 . Chitosan-carbon based biocomposite are used for the efficient removal of phenols from aqueous solutions161. Recently Elanchezhiyan & Meenakshi (2016) studied the recovery of oil from oil-in-water emulsion by metal incorporated chitin using adsorptive method162 .
Along with chitin and its derivatives, the environmental applications of chitinase enzymes are studied and reported. Chitinases can be used to convert chitinous waste of marine organisms into simpler useful depolymerized components, and thus promoting reduction of water pollution. Chitinases are also used in conversion of chitinous waste into biofertilizers163. Chitinases can be used in the production of single cell protein by utilize the chitinous waste effectively 164. All together chitinases, CT and its derivatives can be used for the remediation of various organic contaminants from the environment.
Conclusion and Future Perspective
At the outset, this review focused on the recent developments related to biomedical and environmental applications of chitin, chitinases, and chitin derivatives. In the first part of the review, various methods that have been employed to improve the functionality of chitin have been discussed. Chitin and its enzymes can be readily derivatized into various forms which can find applications in diversified fields. Chitin's biomedical applications are not only from its easy availability, but also from its inherent material and chemical properties such as degradability, mechanical strength and biological activity. The activities of chitin in specific applications greatly depend on its degree of acetylation, molecular weight and functionalization. CT and its derivatives provide highly valuable components with health benefits such as anti-microbial, anti-cancer, wound healing and anti-inflammatory effects. As chitin is an eco-friendly biodegradable material, the environmental remediation process using chitin and its derivatives, may lead to the development of futuristic methods and materials to reduce the environmental toxins. Though enzyme chitinase, chitin and its chitin derivatives showed potential applications, these biocompatible materials are underutilized and their use in all of the above applications need further research and validation to exploit their potential medical and environmental applications. With the recent advances in the applications of chitinases, chitin and its derivatives, it is hoped that this review will encourage aspiring researchers to use chitin and chitinases in various approaches for the development of valuable innovative biomaterials, technologies and methodologies for the benefit of mankind.
Abbreviations
AA – Acrylic acid
AgNP – Silver nanoparticles
Chi:nGO – Chitin/graphene oxide
CMCH – Carboxymethyl chitin
CMs – Chitosan microparticles
CNF – Chitin nanofibers
CNP – Chitin nanoparticles
CNP/AgNP – α–chitin/silver nanocomposite
CNW – Chitin nano-whiskers
COS – Chitoligosaccharides
COX – Cyclooxygenase
CS – Chitosan
DA – Degree of acetylation
DDA – Degree of deacetylation
DMAc – N,Ndimethylacetamide
DS – degree of sulfation
EGFR – Epidermal growth factor receptor
EIS – Electrochemical impedance spectroscopy
GCPQ – Quaternary ammonium palmitoyl glycol chitosan
GO – Graphene oxide
GOD – Glucose oxidase
H3PO4 – Phosphoric acid
HA – Hyaluronic acid
HAp – Hydroxyapatite
IL – Interleukin
iNOS – Inducible nitric oxide synthase
LiCl – Lithium chloride
Mad2 – Mitotic arrest deficient 2
MIP – Molecularly imprinted polymers
MP – Methyl parathion
MPC – Methyl pyrrolidinone chitosan
Mw – Molecular weight
NaOH – Sodium hydroxide
OCPs – Organochlorine pesticides
PAA – Polyacrylic acid
P-chitin – Phosphated chitin
POLI – policaju
scFvCD7 – CD7-specific single-chain antibody
siRNA – Small interfering RNA
TCAA – Trichloroacetic acid
TFAA – Trifluoroacetic anhydride
TNF – Tumor necrosis factor
USM chitin – Ultrasonic surface modified chitin
References
- 1.Gooday G W. (1994) Physiology of microbial degradation of chitin and chitosan. In Biochemistry of microbial degradation,SpringerNetherlands 279-312.
- 3.Józef S, Nadia A A. (2003) Production, Properties, and Some New Applications of Chitin and Its Derivatives. , Crit Rev Food Sci Nutrition 43, 145-171.
- 4.Revathi M, Saravanan R.Shanmugam A (2012), Production and characterization of chitinase from Vibrio species, a head waste of shrimp. Metapenaeus dobsonii (Miers, 1878) and chitin of Sepiella inermis Orbigny, 1848. Adv Biosci Tech 4, 392-397.
- 5.Kuddus M, Ahmad I Z. (2013) Isolation of novel chitinolytic bacteria and production optimization of extracellular chitinase. , J Genet Eng Biotechnol 11, 39-46.
- 6.Xu Y, Bajaj M, Schneider R, Grage S L, Ulrich A S et al. (2013) Transformation of the matrix structure of shrimp shells during bacterial deproteination and demineralization. , Microb Cell Factories 12, 90-102.
- 8.Zhang M, Haga A, Sekiguchi H, Hirano S. (2000) Structure of insect chitin isolated from beetle larva cuticle and silkworm (Bombyx mori) pupa exuvia. , Int J Biol Macromol 27, 99-105.
- 9.Liu S, Sun J, Yu L, Zhang C, Bi J et al. (2012) Extraction and characterization of chitin from the beetle Holotrichia parallela motschulsky. , Molecules 17, 4604-4611.
- 10.Nemtsev S V, Zueva O Y, Khismatullin M R, Albulov A I, Varlamov V P. (2004) Isolation of chitin and chitosan from honeybees. , Appl Biochem Microbiol 40, 39-43.
- 11.Farnesi L C, RFS Menna-Barreto, Martins A J, Valle D, Rezende G L. (2015) Physical features and chitin content of eggs from the mosquito vectors Aedes aegypti, Anopheles aquasalis and Culex quinquefasciatus: Connection with distinct levels of resistance to desiccation. , J Insect Physiol 83, 43-52.
- 12.Harkin C, Brück W M, Lynch C. (2015) Isolation & identification of bacteria for the treatment of brown crab (Cancer pagurus) waste to produce chitinous material. , J Appl Microbiol 118, 954-965.
- 13.Shanmugam K A. (2016) Modified Process for deprotinization of green grab shells (Carcinus maenas) extraction of chitin/chitosan. , J Chem Pharm Sci 9, 163-165.
- 14.Sorokoumov I, Zagorskiy I, Zagorskaya D, Uryash V, Kokurina N et al. (2014) Physicochemical properties of chitin isolated from shell of industrial crabs of various species. Progress on Chemistry and Application of Chitin and its Derivatives 15, 5-10.
- 15.Percot A, Viton C, DomardA. (2003) Optimization of chitin extraction from shrimp shells. , Biomacromol 4, 12-18.
- 16.Puvvada Y S, Vankayalapati S, Sukhavasi S. (2012) Extraction of chitin from chitosan from exoskeleton of shrimp for application in the pharmaceutical industry. , Intl Curr Pharm J 1, 258-263.
- 17.Solairaj D, Rameshthangam P, Srinivasan P. (2016) Adsorption of methylene blue, bromophenol blue, and coomassie brilliant blue by α-chitin nanoparticles. , J Adv Res 7, 113-124.
- 18.Cahú T B, Santos S D, Mendes A, Córdula C R, Chavante S F et al. (2012) Recovery of protein, chitin, carotenoids and glycosaminoglycans from Pacific white shrimp (Litopenaeus vannamei) processing waste. , Process Biochem 47, 570-577.
- 19.Auerswald L, Gäde G. (2008) Simultaneous extraction of chitin and astaxanthin from waste of lobsters Jasus lalandii, and use of astaxanthin as an aquacultural feed additive. , Afr J Mar Sci 30, 35-44.
- 20.Raabe D, Al-Sawalmih A, Yi S B, Fabritius H. (2007) Preferred crystallographic texture of α-chitin as a microscopic and macroscopic design principle of the exoskeleton of the lobster Homarus americanus. , Acta Biomaterialia 3, 882-895.
- 21.Rhazi M, Desbrieres J, Tolaimate A, Alagui A, Vottero P. (2000) Investigation of different natural sources of chitin: influence of the source and deacetylation process on the physicochemical characteristics of chitosan. , Polym Intl 49, 337-344.
- 22.Hartmann M H, Kaplan D L. (1998) Biopolymers from renewable resources. , Kaplan, DL, Ed 367, 629-638.
- 23.Muzzarelli R A, Ilari P, Tarsi R, Dubini B, Xia W. (1994) Chitosan from Absidia coerulea. , Carbohydr Polym 25, 45-50.
- 24.Kumaresapillai N, Basha R A, Sathish R. (2011) Production and evaluation of chitosan from Aspergillus niger MTCC strains. , Iranian J Pharm Res 10, 553-558.
- 25.Synowiecki J, Al-KhateebNAAQ. (1997) Mycelia of Mucor rouxii as a source of chitin and chitosan. , Food Chem 60, 605-610.
- 26.Ruiz-Flores E, Lopez-Romero E, Gutierrez-Corona F. (1990) Chitin synthetase activity in a developmental mutant of Phycomyces Blakesleeanus. , Antonie van Leeuwenhoek 58, 67-72.
- 27.Maw T, Tan T K, Khor E, Wong S M. (2002) Selection of Gongronella butleri strains for enhanced chitosan yield with UV mutagenesis. , J Biotechnol 95, 189-193.
- 28.Vaingankar P N, Juvekar A R. (2014) Fermentative Production of Mycelial Chitosan from Zygomycetes: media optimization and physico-chemical characterization. , Adv Biosci Biotechnol 5, 940-956.
- 29.Ramanathan A, Kittusamy R. (2011) Antihepatotoxic effect of isolated chitin from Rhizopus oryzae against paracetamol-induced hepatotoxicity. , Bangladesh J Pharm 6, 64-67.
- 30.Messner R, Kubicek C P. (1990) Synthesis of cell wall glucan, chitin, and protein by regenerating protoplasts and mycelia of Trichoderma reesei. , Can J Microbiol 36, 211-217.
- 31.Vetter J. (2007) Chitin content of cultivated mushrooms Agaricus bisporus, Pleurotus ostreatus and Lentinula edodes. , Food Chem 102, 6-9.
- 32.Khor E, Lim L Y. (2003) Implantable applications of chitin and chitosan. , Biomater 24, 2339-2349.
- 33.Aranaz I, Mengíbar M, Harris R, Paños I, Miralles B et al. (2009) Functional characterization of chitin and chitosan. , Curr Chem Biol 3, 203-230.
- 34.Mark H. (2004) . Encyclopedia of Polymer Science and Technology, 12 Volume Set. Wiley-Interscience , New York .
- 35.Zhang Y, Xue C, Xue Y, Gao R, Zhang X. (2005) Determination of the degree of deacetylation of chitin and chitosan by X-ray powder diffraction. , Carbohydr Res 340, 1914-1917.
- 36.Kasaai M R. (2009) Various methods for determination of the degree of N-acetylation of chitin and chitosan: a review. , J Agric Food Chem 57, 1667-1676.
- 37.Dash M, Chiellini F, Ottenbrite R M, Chiellini E. (2011) Chitosan—A versatile semi-synthetic polymer in biomedical applications. , Prog Polym Sci 36, 981-1014.
- 38.Percot A, Viton C, DomardA. (2003) Optimization of chitin extraction from shrimp shells. , Biomacromol 4, 12-18.
- 39.No H K, Hur E Y. (1998) Control of foam formation by antifoam during demineralization of crustacean shell in preparation of chitin. , J Agric Food Chem 46, 3844-3846.
- 40.Prameela K, Mohan C M, Smitha P V, KPJ Hemalatha. (2010) Bioremediation of shrimp biowaste by using natural probiotic for chitin and carotenoid production an alternative method to hazardous chemical method. , Int J Appl Biol Pharm Technol 1, 903-910.
- 41.Khanafari A, REZA Marandi, Sanatei S. (2008) Recovery of chitin and chitosan from shrimp waste by chemical and microbial methods. , J Environ Health Sci Eng 5, 1-24.
- 42.Jung W J, Jo G H, Kuk J H, Kim Y J, Oh K T et al. (2007) Production of chitin from red crab shell waste by successive fermentation with Lactobacillus paracasei KCTC-3074 and Serratia marcescens FS-3. , Carbohydr Polym 68, 746-750.
- 43.Synowiecki J.Al-Khateeb NAAQ (2000), The recovery of protein hydrolysate during enzymatic isolation of chitin from shrimp Crangon crangon processing discards. , Food Chem 68, 147-152.
- 44.Bautista J, Jover M, Gutierrez J F, Corpas R, Cremades O et al. (2001) Preparation of crayfish chitin by in situ lactic acid production. , Process Biochem 37, 229-234.
- 45.Adour L, Arbia W, Amrane A, Mameri N. (2008) Combined use of waste materials—recovery of chitin from shrimp shells by lactic acid fermentation supplemented with date juice waste or glucose. , J Chem Technol Biotechnol 83, 1664-1669.
- 46.Sudha P N. (2010) Chitin/chitosan and derivatives for wastewater treatment. In SK Kim (Ed.), Chitin, Chitosan, Oligosaccharides and Their Derivatives: Biological Activities and Applications,CRCPress,561-585 .
- 47.Mohammed M H, Williams P A, Tverezovskaya O. (2013) Extraction of chitin from prawn shells and conversion to low molecular mass chitosan. , Food Hydrocoll 31, 166-171.
- 48.Barikani M, Oliaei E, Seddiqi H, Honarkar H. (2014) Preparation and application of chitin and its derivatives: A review. , Iranian Polym J 23, 307-326.
- 49.Kurita K, Mori S, Nishiyama Y, Harata M. (2002) N-Alkylation of chitin and some characteristics of the novel derivatives. , Polym Bull 48, 159-166.
- 50.Zou Y, Khor E. (2009) Preparation of sulfated-chitins under homogeneous conditions. , Carbohydr Polym 77, 516-525.
- 51.Blasinska A, Drobnik J. (2008) Effects of nonwoven mats of Di-O-butyrylchitin and related polymers on the process of wound healing. , Biomacromol 9, 776-782.
- 52.Castagnino E, Ottaviani M F, Cangiotti M, Morelli M, Casettari L et al. (2008) Radical scavenging activity of 5-methylpyrrolidinone chitosan and dibutyryl chitin. , Carbohydr Polym 74, 640-647.
- 53.Bhatt L R, Kim B M, Hyun K, Kang K H, Lu C et al. (2011) Preparation of chitin butyrate by using phosphoryl mixed anhydride system. , Carbohydr Res 346, 691-694.
- 54.CKS Pillai, Paul W, Sharma C P. (2009) Chitin and chitosan polymers: Chemistry, solubility and fiber formation. , Prog Polym Sci 34, 641-678.
- 55.Huang X, Wu Y, Wei S, Chen Q, Liu C. (2012) The effect of carboxymethyl chitin on sustained drug release of aspirin tablet. , Mater Lett 66, 206-208.
- 56.Liu H, Yang Q, Zhang L, Zhuo R, Jiang X. (2016) Synthesis of carboxymethyl chitin in aqueous solution and its thermo-and pH-sensitive behaviors. Carbohydr Polym. 137, 600-607.
- 57.Lin C W, Chen L J, Lee P L, Lee C I, Lin J C et al. (2007) The inhibition of TNF-α-induced E-selectin expression in endothelial cells via the JNK/NF-κB pathways by highly N-acetylated chitooligosaccharides. , Biomater 28, 1355-1366.
- 58.Chae S Y, Jang M K, Nah J W. (2005) Influence of molecular weight on oral absorption of water soluble chitosans. , J Controlled Release 102, 383-394.
- 59.Mourya V K, Inamdar N N, Choudhari Y M. (2011) Chitooligosaccharides: Synthesis, characterization and applications. , Polym Sci Ser A 53, 583-612.
- 60.Azuma K, Osaki T, Wakuda T, Ifuku S, Saimoto H et al. (2012) Beneficial and preventive effect of chitin nanofibrils in a dextran sulfate sodium-induced acute ulcerative colitis model. , Carbohydr Polym 87, 1399-1403.
- 61.Ifuku S, Saimoto H. (2012) Chitin nanofibers: preparations, modifications, and applications. , Nanoscale 4, 3308-3318.
- 62.Ifuku S, Nogi M, Abe K, Yoshioka M, Morimoto M et al. (2009) Preparation of chitin nanofibers with a uniform width as α-chitin from crab shells. , Biomacromol 10, 1584-1588.
- 63.Villanueva M E, Salinas A, Díaz L E, Copello G J. (2015) Chitin nanowhiskers as alternative antimicrobial controlled release carriers. , New J Chem 39, 614-620.
- 64.Mincea M, Negrulescu A, Ostafe V. (2012) Preparation, modification, and applications of chitin nanowhiskers: a review. , Rev Adv Mater Sci 30, 225-242.
- 65.Sriupayo J, Supaphol P, Blackwell J, Rujiravanit R. (2005) Preparation and characterization of α-chitin whisker-reinforced chitosan nanocomposite films with or without heat treatment. , Carbohydr Polym 62, 130-136.
- 66.Qin Y, Zhang S, Yu J, Yang J, Xiong L et al. (2016) Effects of chitin nano-whiskers on the antibacterial and physicochemical properties of maize starch films. , Carbohydr Polym 147, 372-378.
- 67.Goodrich J D, Winter W T. (2007) α-Chitin nanocrystals prepared from shrimp shells and their specific surface area measurement. , Biomacromol 8, 252-257.
- 68.Smitha K T, Anitha A, Furuike T, Tamura H, Nair S V et al. (2013) In vitro evaluation of paclitaxel loaded amorphous chitin nanoparticles for colon cancer drug delivery. , Colloids Surf B 104, 245-253.
- 69.Smitha K T, Nisha N, Maya S, Biswas R, Jayakumar R. (2015) Delivery of rifampicin-chitin nanoparticles into the intracellular compartment of polymorphonuclear leukocytes. , Int J Biol Macromol 74, 36-43.
- 70.Shams M I, Ifuku S, Nogi M, Oku T, Yano H. (2011) Fabrication of optically transparent chitin nanocomposites. , Appl Phys A 102, 325-331.
- 71.Rubentheren V, Ward T A, Chee C Y, Tang C K. (2015) Processing and analysis of chitosan nanocomposites reinforced with chitin whiskers and tannic acid as a crosslinker. , Carbohydr Polym 115, 379-387.
- 72.Bogdanova O I, Polyakov D K, Streltsov D R, Kulebyakina A I, Orekhov A S et al. (2017) Fabrication and mechanical properties of composite based on β-chitin and polyacrylic acid. , Carbohydr Polym 157, 1496-1502.
- 73.Solairaj D, Rameshthangam P. (2016) Silver nanoparticle embedded α-chitin nanocomposite for enhanced antimicrobial and mosquito larvicidal activity. , J Polym Environ 1-18.
- 74.Annabi N, Tamayol A, Uquillas J A, Akbari M, Bertassoni L E et al.Khademhosseini A (2014), 25th anniversary article: rational design and applications of hydrogels in regenerative medicine. , Adv Mater 26, 85-124.
- 75.Kawata M, Azuma K, Izawa H, Morimoto M, Saimoto H et al. (2016) Biomineralization of calcium phosphate crystals on chitin nanofiber hydrogel for bone regeneration material. , Carbohydr Polym 136, 964-969.
- 76.Liu H, Liu J, Qi C, Fang Y, Zhang L et al. (2016) Thermosensitive injectable in-situ forming carboxymethyl chitin hydrogel for three-dimensional cell culture. , Acta Biomaterialia 35, 228-237.
- 77.Wang Q, Chen S, Chen D. (2017) Preparation and characterization of chitosan based injectable hydrogels enhanced by chitin nano-whiskers. , J Mech Behav Biomed Mater 65, 466-477.
- 78.Kaplan S L, Mason E O. (1998) Management of infections due to antibiotic-resistant Streptococcus pneumonia. , Clin Microbiol Rev 11, 628-644.
- 79.Abdel-Rahman R M, Hrdina R, Abdel-Mohsen A M, Fouda M M, Soliman A Y et al. (2015) Chitin and chitosan from Brazilian Atlantic Coast: Isolation, characterization and antibacterial activity. , Pinto TD, Int J Biol Macromol 80, 107-120.
- 80.Hafsa J, Smach M A, Charfeddine B, Limem K, Majdoub H et al. (2016) Antioxidant and antimicrobial proprieties of chitin and chitosan extracted from Parapenaeus Longirostris shrimp shell waste. , Annales Pharmaceutiques Françaises 74, 27-33.
- 81.Jiang S, Qin Y, Yang J, Li M, Xiong L et al. (2017) Enhanced antibacterial activity of lysozyme immobilized on chitin nanowhiskers. , Food Chem 221, 1507-1513.
- 82.Sahraee S, Milani J M, Ghanbarzadeh B, Hamishehkar H. (2017) Effect of corn oil on physical, thermal, and antifungal properties of gelatin-based nanocomposite films containing nano chitin. LWT-Food. , Sci Technol 76, 33-39.
- 83.Salaberria A M, Fernandes S C, Diaz R H, Labidi J. (2015) Processing of α-chitin nanofibers by dynamic high pressure homogenization: characterization and antifungal activity against A. niger. , Carbohydr Polym 116, 286-291.
- 84.Sayari N, Sila A, Abdelmalek B E, Abdallah R B, Ellouz-Chaabouni S et al. (2016) Chitin and chitosan from the Norway lobster by-products: Antimicrobial and anti-proliferative activities. , Int J Biol Macromol 87, 163-171.
- 85.Shankar S, Reddy J P, Rhim J W, Kim H Y. (2015) Preparation, characterization, and antimicrobial activity of chitin nanofibrils reinforced carrageenan nanocomposite films. , Carbohydr Polym 117, 468-475.
- 86.Sun Z, Shi C, Wang X, Fang Q, Huang J. (2017) Synthesis, characterization, and antimicrobial activities of sulfonated chitosan. , Carbohydr Polym 155, 321-328.
- 87.Zhang H, Yang Q, Ge L, Zhang G, Zhang X et al. (2016) Chitin enhances biocontrol of Rhodotorula mucilaginosa to postharvest decay of peaches. , Int J Biol Macromol 88, 465-475.
- 88.Sahraee S, Milani J M, Ghanbarzadeh B, Hamishehkar H, Kafil H S. (2016) Physicochemical and antifungal properties of bio-nanocomposite film based on gelatin-chitin nanoparticles. , Int J Biol Macromol 97, 373-381.
- 89.Khanal D R, Okamoto Y, Miyatake K, Shinobu T, Shigemasa Y et al. (2001) Protective effects of phosphated chitin (P-chitin) in a mice model of acute respiratory distress syndrome (ARDS). , Carbohydr Polym 44, 99-106.
- 90.Lee S H, Senevirathne M, Ahn C B, Kim S K, Je J Y. (2009) Factors affecting anti-inflammatory effect of chitooligosaccharides in lipopolysaccharides-induced RAW264. 7 macrophage cells. , Bioorg Med Chem Lett 19, 6655-6658.
- 91.Fernandes J C, Spindola H, V De Sousa, Santos-Silva A, Pintado M E et al. (2010) Anti-inflammatory activity of chitooligosaccharides in vivo. , Mar Drugs 8, 1763-1768.
- 92.El-Badry M, Fetih G. (2011) Preparation, charactarization and anti-inflammatory activity of celecoxib chitosan gel formulations. , J Drug Deliv Sci Technol 21, 201-206.
- 93.Cosco D, Failla P, Costa N, Pullano S, Fiorillo A et al. (2016) Rutin-loaded chitosan microspheres: characterization and evaluation of the anti-inflammatory activity. , Carbohydr Polym 152, 583-591.
- 94.Wei P, Ma P, Xu Q S, Bai Q H, Gu J G et al. (2012) Chitosan oligosaccharides suppress production of nitric oxide in lipopolysaccharide-induced N9 murine microglial cells in vitro. , Glycoconjugate J 29, 285-295.
- 95.Fang I M, Yang C H, Yang C M. (2014) Chitosan oligosaccharides attenuate ocular inflammation in rats with experimental autoimmune anterior uveitis. , Mediators of Inflammation 1-15.
- 96.Chung M J, Park J K, Park Y I. (2012) Anti-inflammatory effects of low-molecular weight chitosan oligosaccharides in IgE–antigen complex-stimulated RBL-2H3 cells and asthma model mice. , Intl Immunopharmacol 12, 453-459.
- 97.Diegelmann R F, Evans M C. (2004) Wound healing: an overview of acute, fibrotic and delayed healing. , Front Biosci 9, 283-289.
- 98.Minagawa T, Okamura Y, Shigemasa Y, Minami S, Okamoto Y. (2007) Effects of molecular weight and deacetylation degree of chitin/chitosan on wound healing. , Carbohydr Polym 67, 640-644.
- 99.Abdel-Mohsen A M, Jancar J, Massoud D, Fohlerova Z, Elhadidy H et al. (2016) Novel chitin/chitosan-glucan wound dressing: Isolation, characterization, antibacterial activity and wound healing properties. , Int J Pharm 510, 86-99.
- 100.Marei N H, El-Mazny W, El-Shaer A, Zaki K D, Hussein Z S et al. (2017) Enhanced wound healing activity of desert locust (Schistocerca gregaria) vs. shrimp (Penaeus monodon) chitosan based scaffolds. , Int J Biol Macromol 97, 23-33.
- 101.Aragão-Neto A C, Soares P A, Lima-Ribeiro M H, Carvalho E J, Correia M T et al. (2017) Combined therapy using low level laser and chitosan-policaju hydrogel for wound healing. , Int J Biol Macromol 95, 268-272.
- 102.MOG Ferreira, LLR Leite, de Lima IS, Barreto H M, LCC Nunes et al.da Silva FilhoEC (2016)Chitosan Hydrogel in combination with Nerolidol for healing wounds. , Carbohydr Polym 152, 409-418.
- 103.Izumi R, Komada S, Ochi K, Karasawa L, Osaki T et al. (2015) Favorable effects of superficially deacetylated chitin nanofibrils on the wound healing process. , Carbohydr Polym 123, 461-467.
- 104.Ganesh M, Aziz A S, Ubaidulla U, Hemalatha P, Saravanakumar A et al. (2016) Sulfanilamide and silver nanoparticles-loaded polyvinyl alcohol-chitosan composite electrospun nanofibers: Synthesis and evaluation on synergism in wound healing. , J Industr Eng Chem 39, 127-135.
- 105.Gopal A, Kant V, Gopalakrishnan A, Tandan S K, Kumar D. (2014) Chitosan-based copper nanocomposite accelerates healing in excision wound model in rats. , Eur J Pharm 731, 8-19.
- 106.Lu Z, Gao J, He Q, Wu J, Liang D et al. (2017) Enhanced antibacterial and wound healing activities of microporous chitosan-Ag/ZnO composite dressing. , Carbohydr Polym 156, 460-469.
- 107.Tabuchi R, Azuma K, Izumi R, Tanou T, Okamoto Y et al. (2016) Biomaterials based on freeze dried surface-deacetylated chitin nanofibers reinforced with sulfobutyl ether β-cyclodextrin gel in wound dressing applications. , Int J Pharm 511, 1080-1087.
- 108.Moura L I, Dias A M, Leal E C, Carvalho L, de Sousa HC et al. (2014) Chitosan-based dressings loaded with neurotensin—an efficient strategy to improve early diabetic wound healing. , Acta biomaterialia 10, 843-857.
- 109.Anitha A, Sowmya S, Kumar P S, Deepthi S, Chennazhi K P et al. (2014) Chitin and chitosan in selected biomedical applications. , Prog Polym Sci 39, 1644-1667.
- 110.Liang T J, Zhou Z M, Cao Y Q, Ma M Z, Wang X J et al. (2016) Gemcitabine-based polymer-drug conjugate for enhanced anticancer effect in colon cancer. , Int J Pharm 513, 564-571.
- 111.Rejinold N S, Nair A, Sabitha M, Chennazhi K P, Tamura H et al. (2012) Synthesis, characterization and in vitro cytocompatibility studies of chitin nanogels for biomedical applications. , Carbohydr Polym 87, 943-949.
- 112.Kim E K, Je J Y, Lee S J, Kim Y S, Hwang J W et al. (2012) Chitooligosaccharides induce apoptosis in human myeloid leukemia HL-60 cells. , Bioorg Med Chem Lett 22, 6136-6138.
- 113.Huang R, Mendis E, Rajapakse N, Kim S K. (2012) Strong electronic charge as an important factor for anticancer activity of chitooligosaccharides (COS). , Life Sci 78, 2399-2408.
- 114.Salah R, Michaud P, Mati F, Harrat Z, Lounici H et al. (2013) Anticancer activity of chemically prepared shrimp low molecular weight chitin evaluation with the human monocyte leukaemia cell line, THP-1. , Int J Biol Macromol 52, 333-339.
- 115.Gibot L, Chabaud S, Bouhout S, Bolduc S, Auger F A et al. (2015) Anticancer properties of chitosan on human melanoma are cell line dependent. , Int J Biol Macromol 72, 370-379.
- 116.Banerjee S L, Khamrai M, Sarkar K, Singha N K, Kundu P P. (2016) Modified chitosan encapsulated core-shell Ag Nps for superior antimicrobial and anticancer activity. , Int J Biol Macromol 85, 157-167.
- 117.Arjunan N, HLJ Kumari, Singaravelu C M, Kandasamy R, Kandasamy J. (2016) Physicochemical investigations of biogenic chitosan-silver nanocomposite as antimicrobial and anticancer agent. , Int J Biol Macromol 92, 77-87.
- 118.Subhapradha N.Shanmugam A (2017), Fabrication of β-chitosan nanoparticles and its anticancer potential against human hepatoma cells. , Int J Biol Macromol 94, 194-201.
- 119.Shahrokhian S, Ghalkhani M. (2010) Glassy carbon electrodes modified with a film of nanodiamond–graphite/chitosan: Application to the highly sensitive electrochemical determination of Azathioprine. , Electrochimica Acta 55, 3621-3627.
- 120.Sugawara K, Takano T, Fukushi H, Hoshi S, Akatsuka K et al. (2000) Glucose sensing by a carbon-paste electrode containing chitin modified with glucose oxidase. , J Electroanal Chem 482, 81-86.
- 121.Chen P C, Hsieh B C, Chen R L, Wang T Y, Hsiao H Y et al. (2006) Characterization of natural chitosan membranes from the carapace of the soldier crab Mictyris brevidactylus and its application to immobilize glucose oxidase in amperometric flow-injection biosensing system. , Bioelectrochem 68, 72-80.
- 122.Kumar B, Feller J F, Castro M, Lu J. (2010) Conductive bio-Polymer nano-Composites (CPC): Chitosan-carbon nanotube transducers assembled via spray layer-by-layer for volatile organic compound sensing. , Talanta 81, 908-915.
- 123.Pauliukaite R, Ghica M E, Fatibello-Filho O, Brett C M. (2010) Electrochemical impedance studies of chitosan-modified electrodes for application in electrochemical sensors and biosensors. , Electrochimica Acta 55, 6239-6247.
- 124.Liu B, Deng Y, Hu X, Gao Z, Sun C. (2012) Electrochemical sensing of trichloroacetic acid based on silver nanoparticles doped chitosan hydrogel film prepared with controllable electrodeposition. , Electrochimica Acta 76, 410-415.
- 125.Liu B, Lian H T, Yin J F, Sun X Y. (2012) Dopamine molecularly imprinted electrochemical sensor based on graphene–chitosan composite. , Electrochimica Acta 75, 108-114.
- 126.Palanisamy S, Thangavelu K, Chen S M, Velusamy V, Chen T W et al. (2017) Preparation and characterization of a novel hybrid hydrogel composite of chitin stabilized graphite: Application for selective and simultaneous electrochemical detection of dihydroxybenzene isomers in water. , J Electroanal Chem 785, 40-47.
- 127.Solairaj D, Rameshthangam P, Muthukumaran P, Wilson J. (2017) Studies on electrochemical glucose sensing, antimicrobial activity and cytotoxicity of fabricated copper nanoparticle immobilized chitin nanostructure. , Int J Biol Macromol 101, 668-679.
- 128.Goycoolea F M, Lollo G, Remunán-López C, Quaglia F, Alonso M J. (2009) Chitosan-alginate blended nanoparticles as carriers for the transmucosal delivery of macromolecules. , Biomacromol 10, 1736-1743.
- 129.Cover N F, Lai-Yuen S, Parsons A K.Kumar A (2012), Synergetic effects of doxycycline-loaded chitosan nanoparticles for improving drug delivery and efficacy. , Int J Nanomed 7, 2411-2419.
- 130.Jain A, Thakur K, Sharma G, Kush P, Jain U K. (2016) Fabrication, characterization and cytotoxicity studies of ionically cross-linked docetaxel loaded chitosan nanoparticles. , Carbohydr Polym 137, 65-74.
- 131.Rameshthangam P, Solairaj D. (2016) In-vitro and in-silico studies on curcumin loaded chitin and chitosan nanoparticles from shrimp shells. , DOI: 10.4172/2157-7439.C1.037 J Nanomed. Nanotechnol
- 132.Nascimento A V, Singh A, Bousbaa H, Ferreira D, Sarmento B et al. (2014) Mad2 checkpoint gene silencing using epidermal growth factor receptor-targeted chitosan nanoparticles in non-small cell lung cancer model. , Molecular Pharm 11, 3515-3527.
- 133.Lee J, Yun K S, Choi C S, Shin S H, Ban H S et al. (2012) T cell-specific siRNA delivery using antibody-conjugated chitosan nanoparticles. , Bioconjugate Chem 23, 1174-1180.
- 134.Lalatsa A, Garrett N L, Ferrarelli T, Moger J, Schatzlein A G et al. (2012) Delivery of peptides to the blood and brain after oral uptake of quaternary ammonium palmitoyl glycol chitosan nanoparticles. , Molecular Pharm 9, 1764-1774.
- 135.Kumar P S, Ramya C, Jayakumar R, Lakshmanan V K. (2013) Drug delivery and tissue engineering applications of biocompatible pectin–chitin/nano CaCO3 composite scaffolds. , Colloids Surf B 106, 109-116.
- 136.Unnithan A R, ARK Sasikala, Park C H, Kim C S. (2017) A unique scaffold for bone tissue engineering: An osteogenic combination of graphene oxide–hyaluronic acid–chitosan with simvastatin. , J Ind Eng Chem 46, 182-191.
- 137.Liu M, Zheng H, Chen J, Li S, Huang J et al. (2016) Chitosan-chitin nanocrystal composite scaffolds for tissue engineering. , Carbohydr Polym 152, 832-840.
- 138.Moatary A, Teimouri A, Bagherzadeh M, Chermahini A N, Razavizadeh R. (2017) Design and fabrication of novel chitin hydrogel/chitosan/nano diopside composite scaffolds for tissue engineering. , Ceramics Int 43, 1657-1668.
- 139.Pangon A, Saesoo S, Saengkrit N, Ruktanonchai U, Intasanta V. (2016) Hydroxyapatite-hybridized chitosan/chitin whisker bionanocomposite fibers for bone tissue engineering applications. , Carbohydr Polym 144, 419-427.
- 140.Yong S K, Shrivastava M, Srivastava P, Kunhikrishnan A, Bolan N. (2015) Environmental applications of chitosan and its derivatives. In Reviews of Environmental Contamination and Toxicology,SpringerInternationalPublishing 233, 1-43.
- 141.Prado A G, Torres J D, Faria E A, Dias S C. (2004) Comparative adsorption studies of indigo carmine dye on chitin and chitosan. , J Colloid Interf Sci 277, 43-47.
- 142.Dolphen R, Sakkayawong N, Thiravetyan P, Nakbanpote W. (2007) Adsorption of Reactive Red 141 from wastewater onto modified chitin. , J Hazard Mater 145, 250-255.
- 143.Tang H, Zhou W, Zhang L. (2012) Adsorption isotherms and kinetics studies of malachite green on chitin hydrogels. , J Hazard Mater 209, 218-225.
- 144.Dotto G L, JMN Santos, Rodrigues I L, Rosa R, Pavan F A. (2015) Adsorption of Methylene Blue by ultrasonic surface modified chitin. , Lima EC, J Colloid Interf Sci 446, 133-140.
- 145.Dotto G L, dos Santos JN, Rosa R, LAA Pinto, Pavan F A. (2015) Fixed bed adsorption of Methylene Blue by ultrasonic surface modified chitin supported on sand. , Lima EC, Chem Eng Res Des 100, 302-310.
- 146.Wang Y, Pei Y, Xiong W, Liu T, Li J et al. (2015) New photocatalyst based on graphene oxide/chitin for degradation of dyes under sunlight. , Int J Biol Macromol 81, 477-482.
- 147.González J A, Villanueva M E, Piehl L L, Copello G J. (2015) Development of a chitin/graphene oxide hybrid composite for the removal of pollutant dyes: adsorption and desorption study. , Chem Eng J 280, 41-48.
- 148.Gopi S, Pius A, Thomas S. (2016) Enhanced adsorption of crystal violet by synthesized and characterized chitin nano whiskers from shrimp shell. , J Water Process Eng 14, 1-8.
- 149.Gandhi M R, Kousalya G N, Meenakshi S. (2010) Removal of copper (II) using chitin/chitosan nano-hydroxyapatite composite. , Int J Biol Macromol 48, 119-124.
- 150.Kousalya G N, Gandhi M R, Viswanathan N, Meenakshi S. (2011) Preparation and metal uptake studies of modified forms of chitin. , Int J Biol Macromol 47, 583-589.
- 151.Saravanan D, Gomathi T, Sudha P N. (2013) Sorption studies on heavy metal removal using chitin/bentonite biocomposite. , Int J Biol Macromol 53, 67-71.
- 152.Liu D, Zhu Y, Li Z, Tian D, Chen L et al. (2013) Chitin nanofibrils for rapid and efficient removal of metal ions from water system. , Carbohydr Polym 98, 483-489.
- 153.Karthik R, Meenakshi S. (2014) Synthesis, characterization and Cr (VI) uptake studies of polypyrrole functionalized chitin. , Synth Met 198, 181-187.
- 154.Hanh T T, Huy H T, Hien N Q. (2015) Pre-irradiation grafting of acrylonitrile onto chitin for adsorption of arsenic in water. , Radiat Phys Chem 106, 235-241.
- 155.Mahmoud M E, MTA Kana, Hendy A A. (2015) Synthesis and implementation of nano-chitosan and its acetophenone derivative for enhanced removal of metals. , Int J Biol Macromol 81, 672-680.
- 156.Sharma G, Naushad M, Ala’a H, Kumar A, Khan M R et al.Sharma A (2017), Fabrication and characterization of chitosan-crosslinked-poly (alginic acid) nanohydrogel for adsorptive removal of Cr (VI) metal ion from aqueous medium. , Int J Biol Macromol 95, 484-493.
- 157.Yang R, Su Y, Aubrecht K B, Wang X, Ma H et al. (2015) Thiol-functionalized chitin nanofibers for As (III) adsorption. , Polym 60, 9-17.
- 158.Yoshizuka K, Lou Z, Inoue K. (2000) Silver-complexed chitosan microparticles for pesticide removal. , React Funct Polym 44, 47-54.
- 159.Dolphen R, Thiravetyan P. (2011) Adsorption of melanoidins by chitin nanofibers. , Chem Eng J 166, 890-895.
- 160.Lu L C, Wang C I.Sye WF (2011), Applications of chitosan beads and porous crab shell powder for the removal of 17 organochlorine pesticides (OCPs) in water solution. , Carbohydr Polym 83, 1984-1989.
- 161.Soni U, Bajpai J, Singh S K, Bajpai A K. (2017) Evaluation of chitosan-carbon based biocomposite for efficient removal of phenols from aqueous solutions. , J Water Process Eng 16, 56-63.
- 162.Elanchezhiyan S S, Meenakshi S. (2016) Facile synthesis of metal incorporated chitin for the recovery of oil from oil-in-water emulsion using adsorptive method. , J Cleaner Prod 139, 1339-1350.
Cited by (27)
- 1.Iroegbu Austine Ofondu Chinomso, Ray Suprakas Sinha, 2023, Chitin Nanomaterials as Multifunctional Systems in Advanced Applications – Progress and Challenges toward Sustainability, Macromolecular Materials and Engineering, 308(9), 10.1002/mame.202300053
- 2.Sujka Witold, Draczynski Zbigniew, Kolesinska Beata, Latanska Ilona, Jastrzebski Zenon, et al, 2019, Influence of Porous Dressings Based on Butyric-Acetic Chitin Co-Polymer on Biological Processes In Vitro and In Vivo, Materials, 12(6), 970, 10.3390/ma12060970
- 3.Jabeen F., Younis T., Sidra S., Muneer B., Nasreen Z., et al, 2023, Extraction of chitin from edible crab shells of Callinectes sapidus and comparison with market purchased chitin, Brazilian Journal of Biology, 83(), 10.1590/1519-6984.246520
- 4.Mukarram Mohammad, Naeem M., Aftab Tariq, Khan M. Masroor A., 2022, , , (), 339, 10.1016/B978-0-323-85672-0.00012-X
- 5.Rahimi Mahdi, Noruzi Ehsan Bahojb, Sheykhsaran Elham, Ebadi Baharin, Kariminezhad Zahra, et al, 2020, Carbohydrate polymer-based silver nanocomposites: Recent progress in the antimicrobial wound dressings, Carbohydrate Polymers, 231(), 115696, 10.1016/j.carbpol.2019.115696
- 6.Dave Uday, Somanader Esther, Baharlouei Parnian, Pham Linh, Rahman M. Azizur, 2021, Applications of Chitin in Medical, Environmental, and Agricultural Industries, Journal of Marine Science and Engineering, 9(11), 1173, 10.3390/jmse9111173
- 7.Su Xiaojuan, Tan Mengtian, Duan Bo, Cai Jie, Jiang Wei, et al, 2019, Hierarchical microspheres with macropores fabricated from chitin as 3D cell culture, Journal of Materials Chemistry B, 7(34), 5190, 10.1039/C9TB01046G
- 8.González-Camejo J., Andreola C., Maceratesi V., Toscano G., Eusebi A.L., et al, 2023, , , (), 127, 10.1016/B978-0-323-88510-2.00002-6
- 9.Amobonye Ayodeji, Bhagwat Prashant, Pandey Ashok, Singh Suren, Pillai Santhosh, 2020, Biotechnological potential of Beauveria bassiana as a source of novel biocatalysts and metabolites, Critical Reviews in Biotechnology, 40(7), 1019, 10.1080/07388551.2020.1805403
- 10.Kertmen Ahmet, Ehrlich Hermann, 2022, Patentology of chitinous biomaterials. Part I: Chitin, Carbohydrate Polymers, 282(), 119102, 10.1016/j.carbpol.2022.119102
- 11., 2022, , , (), 589, 10.1039/9781788013024-00589
- 12.Han Dong Jin, Park In Woo, Zhang Chengmin, Heo Kwang, Lee Byung Yang, 2024, Chitin‐Based Functional Films for the Colorimetric Detection of Ethylene Glycol, Advanced Engineering Materials, 26(7), 10.1002/adem.202301796
- 13.Akram Fatima, Haq Ikram ul, Roohi Ayesha, Akram Rabia, 2022, Acinetobacter indicus CCS-12: A New Bacterial Source for the Production and Biochemical Characterization of Thermostable Chitinase with Promising Antifungal Activity, Waste and Biomass Valorization, 13(7), 3371, 10.1007/s12649-022-01753-6
- 14.Poerio Aurelia, Petit Chloé, Jehl Jean-Philippe, Arab-Tehrany Elmira, Mano João F., et al, 2020, Extraction and Physicochemical Characterization of Chitin from Cicada orni Sloughs of the South-Eastern French Mediterranean Basin, Molecules, 25(11), 2543, 10.3390/molecules25112543
- 15.Samanta Dipayan, Govil Tanvi, Saxena Priya, Thakur Payal, Narayanan Adhithya, et al, 2022, , , (), 1, 10.1016/B978-0-323-90274-8.00005-8
- 16.Mallakpour Shadpour, Naghdi Mina, 2021, , , (), 193, 10.1007/978-3-030-61837-7_12
- 17.Song Jio, Lee Eung Take, Lee Ji Hyun, Kim Gyu Hyun, Lee Yong Hyun, et al, 2023, Highly efficient production of N,N'-diacetylchitobiose according to substrate modification and changes in enzyme kinetics, Process Biochemistry, 133(), 179, 10.1016/j.procbio.2023.08.011
- 18.Raja Sebastian, Mattoso Luiz H. C., 2020, , , 34(), 167, 10.1007/978-3-030-15608-4_7
- 19.Souza Maurício Palmeira Chaves de, Sábio Rafael Miguel, Ribeiro Tais de Cassia, Santos Aline Martins dos, Meneguin Andréia Bagliotti, et al, 2020, Highlighting the impact of chitosan on the development of gastroretentive drug delivery systems, International Journal of Biological Macromolecules, 159(), 804, 10.1016/j.ijbiomac.2020.05.104
- 20.Machałowski Tomasz, Amemiya Chris, Jesionowski Teofil, 2020, Chitin of Araneae origin: structural features and biomimetic applications: a review, Applied Physics A, 126(9), 10.1007/s00339-020-03867-x
- 21.Akram Fatima, Jabbar Zuriat, Aqeel Amna, Haq Ikram ul, Tariq Shahbaz, et al, 2022, A Contemporary Appraisal on Impending Industrial and Agricultural Applications of Thermophilic-Recombinant Chitinolytic Enzymes from Microbial Sources, Molecular Biotechnology, 64(10), 1055, 10.1007/s12033-022-00486-0
- 22.Liu Cong, Shen Naikun, Wu Jiafa, Jiang Mingguo, Shi Songbiao, et al, 2020, Cloning, expression and characterization of a chitinase from Paenibacillus chitinolyticus strain UMBR 0002, PeerJ, 8(), e8964, 10.7717/peerj.8964
- 23.Otuechere Chiagoziem A., Adewuyi Adewale, Oluwabayo Tanitoluwa, Afolayan Folashade, Avwioroko Oghenetega, et al, 2020, Salubrious effects of a vermiculite–cellulose‐based bionanocomposite on oxidative stress indices and histomorphology of male Wistar rats, Andrologia, 52(1), 10.1111/and.13426
- 24.De Lise Federica, Iacono Roberta, Moracci Marco, Strazzulli Andrea, Cobucci-Ponzano Beatrice, 2023, Archaea as a Model System for Molecular Biology and Biotechnology, Biomolecules, 13(1), 114, 10.3390/biom13010114
- 25.Hefft Daniel Ingo, 2023, , , (), 15, 10.1016/B978-0-323-85593-8.00019-9
- 26.Zhang Zuying, Ma Zhenmin, Song Lili, Farag Mohamed A., 2024, Maximizing crustaceans (shrimp, crab, and lobster) by-products value for optimum valorization practices: A comparative review of their active ingredients, extraction, bioprocesses and applications, Journal of Advanced Research, 57(), 59, 10.1016/j.jare.2023.11.002
- 27.Saravanan A., Kumar P. Senthil, Yuvaraj D., Jeevanantham S., Aishwaria P., et al, 2023, A review on extraction of polysaccharides from crustacean wastes and their environmental applications, Environmental Research, 221(), 115306, 10.1016/j.envres.2023.115306
