Abstract
Yeast as unicellular organism, has shown multiple application due to exhibition of noble ability in its cells. And engineered yeast has found more suitability in bioprocesses application as well as adverse conditions adaptation. Different types of yeast strains showed their best capability to adapt the salt and sugar rich environment with their optimal growth capability. These strains, used as suitable and novel cell factories for production of value added bio-products (via utilization of fermentation processes) and also for different types of bioprocesses. Application of yeast species in biotechnology field, enhanced in current periods, due to conversion of its wild to engineer strain, suitable for bioprocesses utilization and also for different types of biochemical synthesis. Different yeast species identified due to known their genetic, regulatory mechanism and also competitive metabolic pathways. In this regards, different type of engineering approaches (for genetic or pathways modification), applied to construct the optimal and suitable cell factories for different types of bioprocesses as utilized in different sectors (foods with mineral or protein rich, bread, brewing, cosmetics, chemical, agriculture, pharmacy and distillation industries) via improving the quality of bio-products. Further, in silico designed based metabolic engineering technique showed the improvement in performance of yeast strain. System and synthetic biology with engineering approaches applied to further improve the yeast mediated bioprocesses as well as biochemical products formation for industrial or biotechnological application. Some bio-products such as functional bio-molecule, different types of alcoholic biofuels, organic acids and enzymes etc are good examples of yeast mediated biochemicals products, utilized more frequently in our life. Author will focus recent research and development on bio-product formation or bioprocesses with their regulatory control mechanism in different yeast strains.
Author Contributions
Academic Editor: JUN WAN, United States
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2019 Rajesh K. Srivastava, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
An author has written a cover letter to the editor that is no any conflict of interest to any author or any associations. There is no any conflict of interest in this manuscript. There is no current or pending relationship to consultant for the company supporting the research or manufacturing products being tested, a financial or managerial interest in such a company, or intellectual property rights.
Citation:
Introduction
Yeast strains (i.e. Saccharomyces cerevisiae), widely used as model microbial source for novelty possessing biochemical products synthesis and can be used for studying the bioprocesses optimization for enhanced desired products formation. Advanced disciplines (such as genetic and molecular biology) are emerged as platform to construct the engineered strain of yeast with enormous potential in research and development fields. A lot of works have performed to construct the genes, mRNA libraries, enzymes or proteins, and metabolites profile via study of genomics, transcriptomics, proteomics and metabolomics that help in efficient yeast mediated bioprocesses development.
These efforts can provide the complete knowledge of genetic components or its regulatory mechanism in yeast strains via helping in understanding of yeast biological behaviors. A lot of bio-products such as protein drugs (human insulin and vaccines against papillomavirus and hepatitis) or fine chemicals (lactic acid or n-butanol) are known to synthesize from engineered yeast (Saccharomyces cerevisiae) strains 1. Different yeast strains, isolated from different marine environments and these strains utilized to synthesis of various types of chemicals, enzymes, bioactive compounds, single cell protein and also nano-particles. Many yeast genes are known to involve in biosynthesis and regulation of bioactive or functional compounds or molecules (have more application of food, chemical, agriculture, biofuel, pharmacy, and cosmetics industries) and these had been cloned, expressed and characterized. Marine origin yeast now days frequently, used in industrial application due to have good tolerance capacity to adverse condition (high salts, sugars or alcohols conditions) 2.
Saccharomyces cerevisiae is reported as one of the most widely studied yeast strain and in employed as commercially significant whole cell systems for various types of products synthesis and bioprocesses application. It has shown more efficiency and stereo-selectivity for yeast mediated reduction of ketones in presence and the position of sulfur substituents. In benign reaction, reduction of 2-acetyl-3-methyl sulfolane 1 to corresponding alcohol 2, reported via mediation of baker’s yeast via exhibiting its excellent efficiency and enantioselectivity (> 98% excellent enantiopurity~ee) under mild environmentally conditions. In chemical reduction, it proceeds with poor yield (≤25%) and diastereocontrol. In instance biocatalysis, a higher degree of selectivity and efficiency are reported relative to its conventional chemical counterpart and enantiomerically pure alcohols 2a (cis-2-ethanol-3-methyl sulfolane) and 2b (trans-2-ethanol-3-methyl sulfolane) are shown as valuable chiral synthons in synthesis, as both the hydroxyl and sulfonyl moieties as synthetically versatile 3.
Baker's‐yeast (Saccharomyces cerevisiae) ‐ mediated reduction of ketones, it has shown more efficiency and the stereoselectivity via strongly influencing the presence and the position of sulfur substituents or sulfur‐containing additives. And sulfur substituent oxidation level has shown powerful impact as outcome of the yeast reduction. It has shown that use of the sulfone moiety as a substituent can influence the efficiency and stereoselectivity in ketone reduction as more effective than the use of the analogous sulfide and sulfoxide moieties. Yeast mediated asymmetric reduction has been reported for wide variety of ketones and carbon‐carbon double bonds compounds. Alcohol dehydrogenases (ADHs) is reported as key enzymes, responsible for catalytic activity in the reduction of the carbonyl functional group via utilizing of nicotinamide cofactor, NAD(P)H and hydride is transferred to the substrate carbonyl carbon. Whole‐cell systems can show in situ regeneration of the coenzyme through the metabolic pathways of the microorganism via oxidizing co‐substrate (generally glucose or ethanol) 4. Biobutanol is a potential biofuel, exhibiting the similar energy to gasoline and complete designs and selection of industrial-scale biobutanol biosynthesis plant can be found from yeast-derived biofuels with decisive advantages in industrial processes. Genetically engineered yeast strains S. cerevisiae has been optimized for acetyl-CoA synthesis and NADH levels with overexpression of trans-2-enoyl-CoA reductase (ter) enzyme which has enhanced n-butanol production of titer. Saccharomyces cerevisiae is known to produce propanol and recently several synthetic propanol-producing pathways were developed via advanced metabolic engineering and fermentation strategies have facilitated significant progress in the biochemical production of propanol. Engineered propanol producer strains had efficiently utilized low-cost feedstocks with success of industrially relevant microbial propanol production processes 5.
In this chapter, author will elaborate more examples of yeast mediated products and bioprocesses development via focusing the key points of their regulation and optimization, utilized for biotechnological application. These are given below.
Yeast Strain Synthesized Bio-Products
HPLC analyses have applied to determine relative concentrations of anthocyanins, tannins and pigmented polymers in commercial-scale replicated fermentation. These compounds in red wines are involved during ageing process at small scale model ferments and also in chemical model reactions. Anthocyanin degradation and pigmented polymer formation rate can determine the anthocyanin stability in wine aging process. Analyte profiles and reaction kinetics can demonstrate the condensed tannins and anthocyanins for the formation of pigmented polymers. Maximal formation of pigmented polymers has been shown in presence of fermenting yeast via presence of soluble yeast metabolites in the condensation reaction of anthocyanins with tannins. Commercial-scale replicated fermentation can be confirmed by yeast-mediated biotransformation reactions via employing purified substrates, and by chemical model reactions 6. Saccharomyces cerevisiae is found to expose to baking-associated stresses (i.e. air-drying and freeze-thaw stress) Self-cloned diploid this baker's yeast strain with expression of Pro1-I150T and Mpr1-F65L in the presence of functional Put1 gene, is enhanced proline and nitric oxide (NO) synthesis via improving fermentation ability under multiple baking-associated stress conditions. Engineered yeast strain is reported to increase the intracellular NO level in response to air-drying stress via developing tolerant to oxidative stress as well as air-drying and freeze-thaw stresses due to maintain of reduced intracellular reactive oxygen species (ROS) level. Enhanced stress tolerance and fermentation ability has not occurred in the put1-deficient strain. NO synthesis in baker's yeast is reported from proline in response to oxidative stresses and it induces ROS generation. Importance of Put1- and Mpr1-mediated NO generation can be found from proline which is baking-associated stress tolerance in industrial baker's yeast 7. Some of examples yeast strain mediation bio-products are discussed below.
Biofuels from Yeast Strain
Fossil fuels produced huge quantity of carbon dioxide emission in our environment and also showed lack of sustainability. So, biofuel can motivate us as energy alternative and their development and production is needed to develop as energy option source for world requirement and currently about 80% of crude oil is used as liquid transportation fuels. We need to develop efficient and effective biotechnological processes for the biosynthesis of liquid transportation fuels. Recently dominating biofuel is bioethanol that produced via yeast fermentation. And due to development of efficient new and modified yeast strains, more quantity of ethanol can be produced as alternative biofuels with exhibition of properties close to diesel and jet fuels that can be achieved in optimized growth condition on feed stocks without competing with food production. Advanced biofuels, used as drop-in fuels in existing internal combustion engines in most of our transport means. Saccharomyces cerevisiae as yeast cell factory has shown its capability to easily turn into a high producer strain for ethanol or higher alcohols (1-butanol and isobutanol) and is shown in Figure 18.
Figure 1.Yeast mediated bioprocesses and biochemical products synthesis as biotechnological application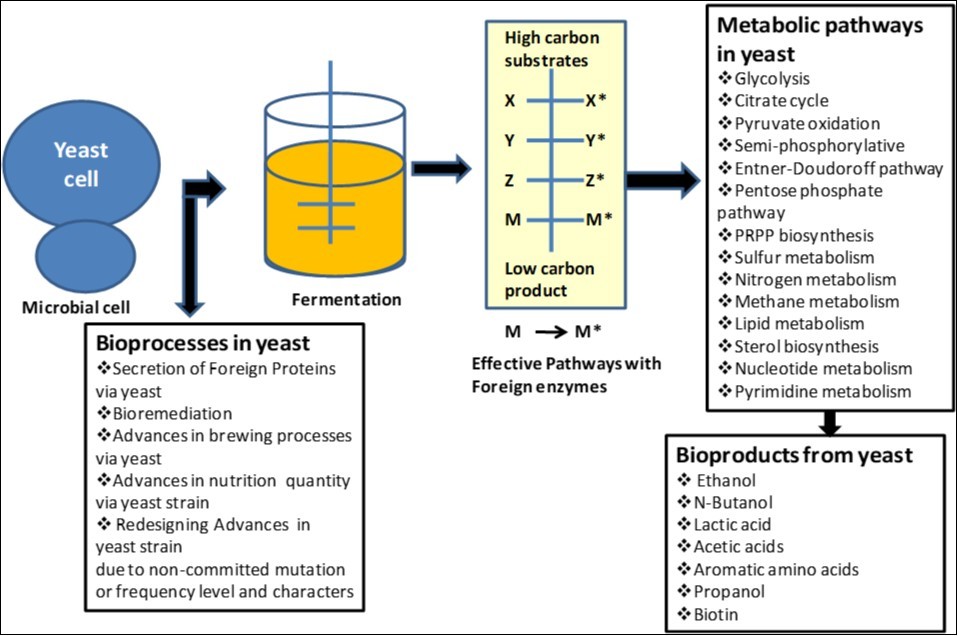
Some challenges (i.e. inhibition of ethanol production at high temperature or high ethanol concentration and also poor ability for pentose sugars fermentation) are reported for yeast mediated fermentation. And currently various types of yeast strains (i.e. hybrid, engineered, recombinant or wild-type) have been applied for ethanol production via expressing capability to directly ferment simple sugars into ethanol. Various types of feedstocks (i.e. non food source) have been converted to fermentable sugars that fermented to ethanol. The pretreatment, hydrolysis and fermentation are common processes involve in ethanol production. Temperature, sugar concentration, pH, fermentation time, agitation rate, and inoculum size has reported as critical factor effecting the bioethanol synthesis during fermentation and these can affect the efficiency and productivity of ethanol 9. The two genes (ADH, ADH1) are reported to express in modified yeast S. cerevisiae in constitutively manner for ethanol production. Reduction in the intracellular concentration of glucose has induced expression of ADH2 gene. The expression of ADH2 gene is regulated by transcription factors and genome sequencing and transcriptome analysis can help in confirmation of the structure and DNA binding elements of these regulatory proteins. ADH gene re-engineering has shown the greater substrate specificity and improvement of catalytic activity via understanding the yeast genome with protein coding genes 10, 11.
The immobilized yeast cells (under mild condition) can influence the productivity of ethanol by several factors (surface characteristics of the carrier, pore size, water content, hydrophilicity and magnetism) and these strain can maintain the activity of the cells for several cycles. Immobilized yeast cells have shown more effectiveness at higher cell density per volume of reactor. It has other benefits such as easier separation from the reaction medium, higher substrate conversion, less inhibition by products, shorter reaction time and control of cell replication 12.
Ethanol is synthesized from molasses, starch based substrate, sweet sorghum cane extract, lignocellulose, and other wastes. Lignocellulosic hydrolysates can contain more or less amount of inhibitors depending on pretreatment processes which can be reduced by utilization of repeated sequential fermentation, treatment with reducing agents and activated charcoal. Other detoxification methods are also reported that are overliming, anion exchanger, evaporation, enzymatic treatment with peroxidase and laccase, in situ detoxification by fermenting microbes and different extraction methods. Co-culturing S. cerevisiae with other yeasts or microbes has utilized for ethanol production optimizations via getting the benefits of shorten fermentation time, and reduced process cost. For ethanol production, immobilized yeast cells has arisen the potential alternative microbial agent via enhancing ethanol productivity, minimal risk of contamination easy cell mass separation from broth with stability of cell activities. It has helped in minimization production costs, biocatalyst recycling ability, reduced fermentation time, and also protection the cells from inhibitors. Production of ethanol is reported from wide range of substrates (i.e. molasses, starch based substrate, sweet sorghum cane extract, lignocellulose, and other wastes) 13.
Engineering in subunit of Rpb7 of RNAP II in yeast strain (Saccharomyces cerevisiae) has improved yeast ethanol tolerance and production. Improved ethanol resistance has enhanced ethanol production and comparison is reported among variant M1 strain with much improved resistance with 8 - 10% ethanol titer improvement. The 122 g.L-1 of ethanol titers had reported for M1 strain and this value is 96.58% of the theoretical yield and it was analyzed under laboratory at very high gravity (VHG) fermentation. This ethanol value is reported as 40% increase as compared to the control 14.
Mutant Saccharomyces cerevisiae strain Z5ΔGPD2 has better performed, widely used industrial strains because gene GPD2 (glycerol 3-phosphate dehydrogenase) was deleted and resulted a lower glycerol yield and poor ethanol productivity. Strain Z5ΔGPD2 was subjected to three rounds of genome shuffling to improve its VHG fermentation performance and it has developed best strain SZ3-1. This modified strain has produced less glycerol and increased the ethanol yield (more 8%) compared with the parent strain Z5. Strain SZ3-1 has shown the enhanced ethanol tolerance due to closely associated with the cell membrane fatty acid compositions and intracellular trehalose concentrations. Karyotype analysis has confirmed genome rearrangements in the optimized strain. Combined approaches of genome shuffling and metabolic engineering has recommended as efficient approaches for the rapid improvement of yeast strains for desirable industrial phenotypes 15.
Improvements of n-butanol production are reported from glucose substrate consumption with yeast strain via utilization acetoacetyl-CoA-derived pathway. Improved n-butanol pathway was made by various isoenzymes of different pathway reactions and n-butanol titers (15 mg.L-1) in synthetic medium after 74 h was reported. In n-butanol pathway, acetyl-coenzyme A (acetyl-CoA) and most intermediates were bound to coenzyme A (CoA) for increased CoA synthesis by over-expression of the pantothenate kinase coA gene (i.e. from Escherichia coli). Supplementation with pantothenate was increased n-butanol production up to 34mg.L-1. Mutant adhEA267T/E568K strain has developed ATP independent acetylating acetaldehyde dehydrogenase, converts acetaldehyde into acetyl-CoA, and had enhanced butanol titer up to 95 mg.L-1. Genes of coA and adhE in A267T/E568K has stably integrated into the yeast genome with deletion of alcohol dehydrogenase gene of ADH6, and GPD2 (glycerol-3-phosphate dehydrogenase), leading to a further decrease in ethanol and glycerol as by-product formation with elevated redox power in the form of NADH. With the addition of pantothenate, this engineered strain has produced higher n-butanol up to a titer (130 m g.L-1) and a yield (0.012 g/g glucose) 16.
Major contribution of Acetone–butanol–ethanol (ABE) pathway has been reported for butanol production via mediation of specific mutants yeast (after gene deletion) and it shown significant impact on butanol levels. Threonine-based ketoacid (TBK) pathway is reported for endogenous mode of butanol synthesis in ADH1 mutants but endogenous butanol production is reported in our strains. Other endogenous butanol production is dependent on glycine metabolism via an α-ketovalerate intermediate. And this modified yeast cells is found to utilize α-ketovalerate as a supplement to synthesize high butanol titres (> 2 g.L-1). In future, characterization and optimization of the enzymatic activities in these pathway can be developed the exciting area in the generation of robust n-butanol production strategies. 17.
In anaerobic fermentation engineered S. cerevisiae strain YG5C4231 has shown sufficient activity of endogenous 2-keto acid decarboxylase and alcohol/aldehyde dehydrogenase for conversion of 2-ketobutyrate (2 KB) into 1-propanol (500 m g.L-1) in yeast. Enhanced production of 1-propanol was found to dependent on the construction of an artificial 2 KB biosynthetic pathway from pyruvate via citramalate (cimA) and also overexpression of threonine dehydratase (tdcB) gene. Further it has needed the enhancement of threonine biosynthesis from aspartate (thrA, thrB and thrC) with deletion of the GLY1 gene (regulates a competing pathway converting threonine to glycine). Under high-density condition, production of 1-propanol (180 m g.L-1) from glucose substrate is reported. Engineered citramalate-mediated pathway is reported as an effective production method for 1-propanol in S. cerevisiae 18.
Organic Acids from Yeast Strain
Two lactic-acid-producing strains of Saccharomyces cerevisiae, CEN.PK m850 and CEN.PK RWB876 are reported to express the heterologous l-lactate dehydrogenases and also good in tolerant of acidic environments (low pH, lactic acid exerts a high level of stress on the cells). Enhanced Lactic acid production was reported in the majority of the mutants compared to the parental strains. Intracellular pH (pHi) and viability by staining with cSNARF-4F and ethidium bromide was used for screening the mutant strain of yeast. Batch culture with 70 g.L-1 glucose was utilized for strains CEN.PK RWB876 and G33 up to 70 h periods, to produce 31 and 45 g.L-1 of lactic acid, respectively 19. Manipulation of the metabolic pathways of Saccharomyces cerevisiae is done to produce lactic acid and shown potential influence of acid transport across the plasma membrane in this process. L-LDH gene from Lactobacillus casei has been expressed in S. cerevisiae W303-1A and all strains (isogenic mutants jen1∆, ady2∆ and jen1∆ ady2∆) were able to produce lactic acid. In strains constitutively expressing both LDH and JEN1 or ADY2, a higher external lactic acid concentration was reported in presence of glucose rich medium at exhausted condition. Engineered S. cerevisiae W303-1A expressing LDH gene (Bacillus Taurus) has shown a lactic acid titer (20 mg L−1) after 30 h of growth periods in YNB glucose 2% medium. Expression of LDH gene (L. casei) in other engineered yeast has reported for a higher level of lactic acid (15-fold) in the same time frame. Further inactivation of Pdc and Adh, in engineered S. cerevisiae strains has shown the improve the yields of lactic acid 20. Two xylose-to-lactic acid converting strains of Saccharomyces cerevisiae (IBB14LA1 and IBB14LA1_5 (without pdc5 gene)) have found to express the l-lactic acid dehydrogenase (from Plasmodium falciparum ~pfLDH) which were integrated at the pdc1 (pyruvate decarboxylase) locus. Principal engineering strategies such as pyruvate-to-lactic acid conversion with and without disruption of the competing pyruvate-to-ethanol pathway have shown high lactic acid yields (YLA) and productivities (QLA) on both sugar substrates (xylose and glucose). Conversion time courses together with results of activity measurements for pfLDH and PDC has shown in IBB14LA1 strain depending on distribution of fluxes at the pyruvate branching point for carbon source and oxygen. Genetically engineered S. cerevisiae are better suited for lactic acid biorefineries. The pfLDH/PDC5 ratio is found critical to shift the distribution of the carbon fluxes from the ethanol to the lactic acid producing pathway. Optimization of the gene expression level of the pfldh and pdc5 is reported successful approach which can be helped in high cell viability (PDC5 activity for cytosolic acetyl-coA production with good lactic acid yields and productivities (shift in distribution of the carbon fluxes towards the pfLDH-catalyzed reaction) 21. Whole genome sequencing of JHY5310 has helped in identification of four loss-of-function mutations in GSF2, SYN8, STM1, and SIF2 genes for LA tolerance. A nonsense mutation in GSF2 has contributed for improved lactic acid (LA) tolerance and LA production in JHY5310 strain via improved glucose uptake with derepressed glucose-repressed genes (involve in the respiratory pathway). Alleviation of glucose repression was done by deletion of MIG1 or HXK2 in JHY5210 has improved D-LA production. GSF2 deletion in yeast strain would be good for increased biomass yield or respiratory flux 22. Organic acid (malic, tartaric, citric, succinic, acetic and pyruvic) composition changes have been reported during fermentation by five industrial wine yeast strains in a synthetic grape must (MS300) under wine-like, fermentation conditions. Samples have been analyzed for three physiological stages during fermentation, (exponential growth phase at day, early stationary phase at day 5 and late stationary phase at day 14. These different stages have provided information on acid evolution throughout fermentation, as well as on the impact of nutritional and environmental conditions during aerobic and anaerobic fermentation. Some strains such as VIN13 and 285 were shown to higher producers of most acids in white and/ or red wine fermentation settings with some strains (such as DV10) with lower acid production. Different acid consumption and production patterns, has shown a first step towards enabling winemakers to appropriately select strains for acid management during fermentation or ageing processing 23 and Table 1 has shown few more information on various bio-products from yeast strain.
Table 1. Yeast strain mediated bioproducts formation via fermentation processes| Yeast strain | Metabolites (g.L-1) | Substrates | References |
| S.cerevisiae CHFY0321 (hybrid) | Ethanol~ 89.8 | Cassava starch with195.0 g.L-1 sugars | 24 |
| S. cerevisiae ZU-10(recombinant) | Ethanol~ 41.2 | Corn stover with 99.0 g.L-1 sugars | 25 |
| Saccharomyces cerevisiae M1 | Ethanol~ 122at very high gravity. | Glucose with 300 g.L-1 | 14 |
| Saccharomyces cerevisiae strain adhEA267T/E568K | n-butanol ~ 0.095-0.86 | Glucose withconcentration | 16 |
| Saccharomyces cerevisiae Δ+5g | n-butanol ~ 2.0 | Dextrose (2% w/v) media (YPD) | 17 |
| S. cerevisiae strain YG5C4231 | 1-propanol ~ 0.5 | 2-ketobutyrate (2 KB) via glucose | 18 |
| Saccharomyces cerevisiae, CEN.PK RWB876 | Lactic acid ~ 45 | 70 g.L-1 glucose | 19 |
| S. cerevisiae W303-1A | Lactic acid ~ 0.3 | YNB glucose 2% medium | 20 |
Yeast Mediated bio-Services/Processes
Synthetic Chromosome Rearrangement and Modification by LoxPsym-mediated Evolution (SCRaMbLE) is reported as a global recombination system, utilized for synthetic chromosomes during design to allow inducible genome evolution. Now L-SCRaMbLE is used as tool, utilized for light-controlled Cre-mediated recombination in yeast. It has boosted the potential for further customization with facile application in yeast genome re-engineering project Sc2.0 or in other recombination-based systems 31.
Cloning by homologous recombination (HR) is reported in most of yeast strains. Yeast-cloning cassette (YCC) has found to contain the 2-micron origin of replication (2 μm ori) and alos the ura3 gene for selection of multiple DNA fragments and these can be assembled into any DNA vector. Unlimited potential has been reported in yeast strain due to building of a variety of plasmids for different uses such as recombinant protein production, epitope tagging, site-directed mutagenesis, and expression of fluorescent fusion proteins 32.
Addition of sugar is found to favourable for biotransformation process, as medium and high concentration of glucose can facilitate the regeneration of enzyme cofactors. Small amounts of organic solvents addition to the medium has also found to succeed in biotransformation process. And ethanol, glycerol, hexane and isopropanol are used in various proportions. For carbonyl reductase, isopropanol can be used as a co-substrate for the regeneration of NAD+ or NADP+ and it increased substrate solubility 33. Triggering of yeast apoptosis is reported to link with mitochondrial dysfunction due to the production of ROS and caused to oxidative damage in mitochondria with changes in mitochondria compartment morphology, a phenotype as thread-grain transition state. Exposing to yeast cell with bostrycin can produce such a phenotype, cells expressing a mitochondria-targeted protein fused green fluorescent protein (GFP), induce apoptosis 34. An anthracenedione (bostrycin) belongs to the large family of quinones and it has exhibited the phytotoxic and antibacterial activity. Isolation of bostrycin (as secondary metabolites) has reported in a mangrove endophytic fungus, no. 1403 (collected from South China Sea). Saccharomyces cerevisiae as a model, bostrycin has inhibited cell proliferation via blocking the cell cycle at G1 phase. It caused cell death in a time- and dose-dependent manner. Bostrycin-induced lethal cytotoxicity is reported due to increased levels of intracellular reactive oxygen species (ROS). It has shown the hallmarks of apoptosis (i.e. chromatin condensation, DNA fragmentation and externalization of phosphatidylserine). Bostrycin worked via decreasing mitochondrial membrane electric potential, inducing mitochondrial destruction with progression of cell death. Bostrycin-induced cell death has found in YCA1 null yeast strain, partially rescued (in AIF1 null mutant) in fermentative and respiratory media. Bostrycin has induced apoptosis in yeast cells through a mitochondria-mediated via caspase-independent pathway 35. Advantage of viticutural techniques have utilized for optimization of anthocyanin levels in grapes via identifying essential factors, involved in the stabilization of anthocyanins in wine. It has also helped to characterize the reactions, involved in the transformation of grape-derived anthocyanins into stable red wine pigments 36. And Table 2 has shown few more information on various bio-processes or services from yeast strain. (Table 3).
Table 2. Yeast mediated bioprocesses or services for industrial application| Bioprocesses | Products | References |
| Modular pathway rewiring (MPR) strategy | Production of l-ornithine with high-level (5.1 g.L−1) in fed-batch cultivations | 26 |
| Biotransformation of geraniol into citronellol through continuous-closed-gas-loop bioreactor (CCGLB) via helps in situ product removal | The gas loop led to a maximum citronellol concentration of 2.38 g.L−1 | 27 |
| Potential stereospecificreduction tool for biotransformation of mono and sesquiterpenoids | Conversion of parthenin (a sesquiterpene lactone) to dihydrocoronopilin with 20% yield.6,7-epoxygeraniol (a mono-terpene epoxide with a rosylike smell) to 2-methyl-2-(2-hydroxy ethyl)-5-(2-hydroxy prop 2-yl) tetrahydrofuran (a furanoid derivative), 6,7-epoxycitronellol and 6,7-epoxynerol | 28 |
| Hydration of oleic acid | Transformation of oleic acid into (R)- 10-hydroxystearic acid (10-HSA). It will help in preparation of the high-value flavour γ-dodecalactone. | 29 |
| Stereoselective reduction ofα-acetylbutyrolactone into α’-1’-hydroxyethyl-γ-butyrolactone | Transformation α-acetyl butyrolactone 1 preferentially to(3S,1’S)-α’-1’-hydroxyethyl-γ-butyrolactone(3R,1’R)-α’-1’-hydroxyethyl-γ-butyrolactone (2b) | 30 |
| Yeast strain | Industry Types | References |
| Saccharomyces,Candida,Kluyveromyces,PichiaandTorulopsis | Food grade | 37 |
| S. cerevisiae. | Bakery industry | 38 |
| Rhodotorula mucilaginosa (NCYC 65) | Ethyl acetate, arabinitol, glycerol and acetate production | 39 |
| Spent brewer's yeast from a lager fermentation | Protein, mineral, and vitamin B complex constituents production | 40 |
| Engineered the baker's yeast Saccharomyces cerevisiae | Antibiotic nonribosomal peptide penicillin | 41 |
| Candida speciesTrichosporon asahii | Antimicrobial substances for preserving food | 42 |
Conclusions
High capital costs and low reaction rates via yeast mediated fermentation are big challenges in the current bio-economy system. High cell density cultures of yeast can help us in efficient way to increase the volumetric productivity via enabling faster and more robust processes in smaller reactors. High local cell density can offer significant advantages for sustainable second generation bioethanol production. Advances in the knowledge of fundamental and applied bioprocessing systems at cellular, molecular and production levels can help in better utilization of various carbohydrates (like dextrin, maltose or lactose) and flocculation characters of yeast. Yeasts are heterogeneous group of fungi with unicellular morphology in at least one phase of their vegetative life cycles. Successful product development in yeast is limited by current manufacturing processes which are difficult to scale up, high in cost. Yeast strains have the potential to affect the structure-activity properties of food protein-derived bioactive peptides (BPs). Yeast apoptosis is triggered due to the production of ROS via exposing to bostrycin and caused to oxidative damage in mitochondria with changes in mitochondria compartment morphology. Yeast is found to involve in many biotransformation processes for conversion of one compound to another one. It has contributed in synthesis of various biochemical products, utilized in our daily life and industrial needs.
Affiliations
As I have mentioned that all the mental and research support is done fromDepartment of Biotechnology, GIT, Gitam Institute of Technology and Management (GITAM) (Deemed to be University), Visakhapatnam. I am very much thankful to our university to support it.
Acknowledgements
Author is main source for writing this manuscript. He has done only all the respective literature paper to writing this manuscript. There is no contribution of any fund or any honorarium money for this paper. But I am thankful to our university to encourage for paper writing.
Abbreviations
References
- 1.Gerngross T. (2005) Production of complex human glycoproteins in yeast. , Adv Exp Med Biol 564, 139.
- 2.Zhe C, Guang-Lei L, Yi L, Hong J, Zhen-Ming C. (2016) Bio-products produced by marine yeasts and their potential applications. , Bioresour Technol 202, 244-252.
- 3.Deasy R E, O'Riordan N, Maguire A R. (2014) Baker’s Yeast Mediated Reduction of 2-Acetyl-3-methyl Sulfolane. , Catalysts 4(2), 186-195.
- 4.Deasyand R E, Maguire A R. (2014) Baker’s-Yeast-Mediated Reduction of Sulfur-Containing Compounds. , Eur J Org Chem 2014(18), 3737-3756.
- 5.Swidah R, Wang H, Reid P J, Ahmed H Z, Pisanelli A M et al. (2015) Butanol production inS. cerevisiaevia a synthetic ABE pathway is enhanced by specific metabolic engineering and butanol resistance. , Biotechnology for Biofuels 8, 97.
- 6.Eglinton J, Griesser M, Henschke P, Kwiatkowski M, Parker M et al. (2004) Yeast-Mediated Formation of Pigmented Polymers in Red Wine. , Waterhouse AL, Kennedy JA, (Eds):, Red Wine Color, Chapter 2, ACS Symposium Series, American Chemical Society 886, 7-21.
- 7.Sasano Y, Haitani Y, Hashida K, Ohtsu I, Shima J et al. (2012) Enhancement of the proline and nitric oxide synthetic pathway improves fermentation ability under multiple baking-associated stress conditions in industrial baker's yeast. , Microb Cell Fact 1(11), 40.
- 8.Buijs N A, Siewers V, Nielsen J. (2013) Advanced biofuel production by the yeastSaccharomyces cerevisiae.Curr. , Opin Chem Biol 17(3), 480-488.
- 9.SHM Azhar, Abdulla R, Jambo S A, Marbawi H, Gansau J A et al. (2017) Yeasts in sustainable bioethanol production: A review. , Biochem Biophys Rep 10, 52-61.
- 10.Raj S B, Ramaswamy S, Plapp B V. (2014) Yeast alcohol dehydrogenase structure and catalysis. , Biochemistry 53, 5791-5803.
- 11.Alper H, Moxley J, Nevoigt E, Fink G R, Stephanopoulos G. (2006) Engineering yeast transcription machinery for improved ethanol tolerance and production. , Sci 314, 1565-1568.
- 12.Vucurovic V, Razmovski R, Popov S. (2009) Ethanol production usingSaccharomyces cerevisiaecells immobilized on corn stem ground tissue. , Zb. Matic-. Srp Prir Nauk 116, 315-322.
- 13.Tesfaw A, Assefa F. (2014) Current Trends in Bioethanol Production bySaccharomyces cerevisiae: Substrate, Inhibitor Reduction, Growth Variables, Coculture, and Immobilization. Int Sch Res Notices. 532852.
- 14.Qiu Z, Jiang R. (2017) ImprovingSaccharomyces cerevisiaeethanol production and tolerance via RNA polymerase II subunit Rpb7. , Biotechnol Biofuels 10, 125.
- 15.Tao X, Zheng D, Liu T, Wang P, Zhao W et al. (2012) A novel strategy to construct yeastSaccharomyces cerevisiaestrains for very high gravity fermentation. PLoS One,7(2):. 31235.
- 16.Schadeweg V, Boles E. (2016) n-Butanol production inSaccharomyces cerevisiaeis limited by the availability of coenzyme A and cytosolic acetyl-CoA. , Biotechnol Biofuels 9, 44.
- 17.Swidah R, Ogunlabi O, Grant C M, Ashe M. (2018) n-Butanol production inS. cerevisiae: co-ordinate use of endogenous and exogenous pathways. , Appl Microbiol Biotechnol 102(22), 9857-9866.
- 18.Nishimura Y, Matsui T, Ishii J, Kondo A. (2018) Metabolic engineering of the 2-ketobutyrate biosynthetic pathway for 1-propanol production inSaccharomyces cerevisiae.Microb Cell Fact. 17, 38.
- 19.Valli M, Sauer M, Branduardi P, Borth N, Porro D et al. (2006) Improvement of Lactic Acid Production inSaccharomyces cerevisiaeby Cell Sorting for High Intracellular pH. , Appl Environ Microbiol 72(8), 5492-5499.
- 20.Pacheco A, Talaia G, Sá-Pessoa J, Bessa D, Gonçalves M J et al. (2012) Lactic acid production inSaccharomyces cerevisiaeis modulated by expression of the monocarboxylate transporters. Jen1 and Ady2. FEMS Yeast Res 12(3), 375-381.
- 21.Novy V, Brunner B, Nidetzky B. (2018) l-Lactic acid production from glucose and xylose with engineered strains ofSaccharomyces cerevisiae: aeration and carbon source influence yields and productivities. , Microb Cell Fact 17, 59.
- 22.Baek S H, Kwon E Y, Kim S Y, Hahn J S. (2016) GSF2 deletion increases lactic acid production by alleviating glucose repression inSaccharomyces cerevisiae. , Scient Rep 6, 34812.
- 23.Chidi B S, Rossouw D, Buica A S, Bauer F F. (2015) Determining the impact of industrial wine yeast strains on organic acid production under white and red wine-like fermentation conditions. , S. Afr J Enol Vitic Stellenbosch 36(3), 316-327.
- 24.Choi G W, Um H J, Kang H W, Kim Y, Kim M et al. (2010) Bioethanol production by a flocculent hybrid, CHFY0321 obtained by protoplast fusion betweenSaccharomyces cerevisiae and Saccharomycesbayanus. , Biomass- Bioenergy 34, 1232-1242.
- 25.Zhao J, Xia L. (2010) Bioconversion of corn stover hydrolysate to ethanol by a recombinant yeast strain. , Fuel Process Technol 91, 1807-1811.
- 26.Qin J, Zhou Y J, Krivoruchko A, Huang M, Liu L et al. (2015) Modular pathway rewiring ofSaccharomyces cerevisiaeenables high-level production of l-ornithine. , Nat Comm 6, 8224.
- 27.Arifin A A, Don M M, Uzir M H. (2011) Baker's yeast mediated biotransformation of geraniol into citronellol using a continuous-closed-gas-loop bioreactor (CCGLB) system. , Biochem Eng J 56(3), 219-224.
- 28.Khor G K, Uzir M H. (2011) cerevisiae: a potential stereospecific reduction tool for biotransformation of mono and sesquiterpenoids. Yeast,28 93-107.
- 29.Serra S, D. (2018) New insights on the baker's yeast-mediated hydration of oleic acid: the bacterial contaminants of yeast are responsible for the stereoselective formation of (R)-10-hydroxystearic acid. , J Appl Microbiol 124(3), 719-729.
- 30.Maczka W, Winska K, Grabarczyk M, Zarowska B. (2018) Yeast-Mediated Stereoselective Reduction of α-Acetylbutyrolactone. , Appl. Sci 8, 1334.
- 31.Hochrein L, Mitchell L A, Schulz K, Messerschmidt K.Mueller-Roeber B,(2018) L-SCRaMbLE as a tool for light-controlled Cre-mediated recombination in yeast. , Nature Comm 9-1931.
- 32.Joska T M, Mashruwal A, Boyd J M, Belden W J. (2014) A universal cloning method based on yeast homologous recombination that is simple, efficient, and versatile. , J Microbiolog Method 100, 46-51.
- 33.Xu Q, Xu X, Huang H, Li S. (2015) Efficient synthesis of (R)-2-chloro-1-phenylethol using a yeast carbonyl reductase with broad substrate spectrum and 2-propanol as cosubstrate. , Biochem Eng J 103, 277-285.
- 34.Du L, Su Y, Sun D, Zhu W, Wang J et al. (2008) Formic acid inducesYca1p-independent apoptosis-like cell death in the yeastSaccharomyces cerevisiae. , FEMS Yeast Res 8, 531-539.
- 35.Xu C, Wang J, Gao Y, Lin H, Du L et al. (2010) The anthracenedione compound bostrycin induces mitochondria-mediated apoptosis in the yeastSaccharomyces cerevisiae. , FEMS Yeast Res 10(3), 297-308.
- 36.Li Z, Pan Q, Cui X, Duan C. (2010) Optimization on anthocyanins extraction from wine grape skins using orthogonal test design. , Food Sci Biotechnol 19(4), 1047-1053.
- 38.Goldammer T. (2008) The Brewers’ Handbook. The Complete Book to Brewing Beer. , Apex Publishers, Clifton, Virginia, USA 496.
- 39.Wu J, Elliston A, Gall G L, Colquhoun I J, SRA Collins et al. (2017) Yeast diversity in relation to the production of fuels and chemicals. , Scientific Reports 7, 14259.
- 40.Djuragic O, Levic J, Serdanovic S. (2010) Use of new feed from brewery by-products for breeding layers. , Romanian Biotechnol. Lett 15, 5559-5565.
Cited by (2)
- 1.de Campos Fraga-Silva Thais Fernanda, Mimura Luiza Ayumi Nishiyama, de Oliveira Larissa Ragozo Cardoso, dos Santos Toledo Juliana Helena, Borim Patrícia Aparecida, et al, 2020, Selenization of S. cerevisiae increases its protective potential in experimental autoimmune encephalomyelitis by triggering an intestinal immunomodulatory loop, Scientific Reports, 10(1), 10.1038/s41598-020-79102-7
- 2.Granata Tim, Rattenbacher Bernd, Kehl Florian, Egli Marcel, 2024, Microbial Factories and Exploiting Synergies of Bioreactor Technologies to Produce Bioproducts, Fermentation, 10(3), 135, 10.3390/fermentation10030135