Abstract
Background
Male Infertility accounts for 30-40% of all cases of infertility and its evaluation requires a good history, thorough physical examination, and several investigations to include testicular biopsy which might be used to further categorize infertile males for proper management and prognostication. This study aims to determine the predominant histopathological patterns of testicular biopsies in infertile males and to compare the findings with previous studies.
Methods
A retrospective cross-sectional study of 225 selected cases of testicular biopsies reviewed for the evaluation of male infertility in the Pathology department, of a tertiary hospital, Southwest, Nigeria, between 1987 and 2012. Relevant clinical and histopathological information was extracted from the departmental records. All histologic cases were reviewed, and a classification based on histological patterns of spermatogenesis was utilized to group the cases into normal findings, hypo spermatogenesis, maturation arrest, Sertoli cell-only syndrome, peritubular hyalinization/ tubular fibrosis and mixed patterns. The data obtained were analysed using descriptive and inferential statistics at a 5% level of significance.
Results
Among the 225 cases reviewed with a mean age of 37.7 years (SD - 8.61), 82.7% had primary infertility of which 92.9% were azoospermic, while 7.1% had oligospermia. The histological patterns included 34.2% of Hypospermatogenesis, 32% of Peritubular hyalinization/ tubular fibrosis, 14.2% had maturation arrest and Sertoli cell-only syndrome was found in 6.7% of cases, only 0.9% had normal histologic pattern while the mixed histologic pattern was seen in 12% of cases.
Conclusion
The commonest morphological pattern was Hypospermatogenesis, which is similar to some of the previous local and international studies. A high percentage of peritubular fibrosis was noted with few tubules containing scanty late spermatids or spermatozoa when proper sampling and evaluation were made. Multiple patterns within a biopsy were seen with careful review, especially in non-obstructive azoospermic cases. This is significant in male infertility patient management in our environment because it suggests greater chances of successful sperm extraction for Assisted Reproduction Technique in such patients.
Author Contributions
Academic Editor: Amit Kant, Department of Physiology, U.P. University of Medical Sciences, House No. 168, Kaveri Kunj, Phase II, Kamla Nagar, Saifai, Etawah, Agra, 282005, Uttar Pradesh, India.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2021 Adesoji Adetona, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Background
Infertility is a medical and socio-cultural phenomenon that affects up to 15% of couples worldwide with male factors accounting for 30-40% of all cases of infertility.1 Its evaluation requires a comprehensive history and thorough physical examination along with investigations such as semen analysis, hormonal assay and testicular biopsy.2, 3
The causes of male infertility can be grouped into three categories: Pre-testicular, Testicular and Post-testicular causes.
The pre-testicular causes include extragonadal endocrine disorders originating in the hypothalamus, pituitary or adrenal glands; chronic illnesses, and certain medications such as some antihypertensive drugs like Nifedipine as well as antiviral agents like Ganciclovir and a few others.1, 4
The testicular causes are related to defects in the process of spermatogenesis such as maturation arrest, germ cell aplasia, etc.4
The post-testicular causes include obstruction of ducts draining the testes secondary to trauma or surgery, impaired sperm motility, morphological abnormalities of the spermatozoa and biochemical abnormality of the seminal fluid.1, 5
Biopsy Technique
The FNA procedure is performed on the testis by aspiration at three separate sites (upper, middle and lower pole). Four to five needle excursions were made at each site except when contraindicated6. Contraindications for bilateral testicular sampling included the presence of local skin infection, hydrocele, orchialgia or a previous biopsy6, 7. Another technique is by open biopsy at the same site of the needle biopsy. Both needle specimen and open tissue specimen are fixed in Bouin’s solution and sent for histologic examination. Routine haematoxylin and eosin (H&E) for the open tissue biopsy and Romanowsky stains is performed on the smears8.
An open testicular biopsy shows different morphological patterns, which can provide valuable information to the urologist to further categorize infertile males with azoospermia or oligospermia for prognostication and treatment. Whereas, obstructive and testicular causes usually show a narrow range of morphologic changes, which could be assessed with biopsy evaluation to determine the patient reproductive chances and treatment options.
This study aimed to determine the predominant histopathological patterns seen in the testicular biopsies of infertile males and to compare the findings with those seen in similar studies including a previous study in this centre.
This information might be useful in the management of cases of male infertility by guiding case selection of patients that can benefit from in vitro fertilization or surgical intervention in cases where especially there is duct obstruction with the normal histologic pattern.
Methods
This is a retrospective cross-sectional study carried out in the Department of Pathology of a large teaching hospital in South-Western Nigeria. We reviewed all testicular biopsies submitted for the evaluation of male infertility between 1987 and 2012 received from both the urology clinic of the hospital and peripheral hospitals, usually fixed either in Bouin’s fluid or 10% buffered formalin. The archival Haematoxylin & Eosin (H&E) stained histology slides of cases were retrieved. Cases whose slides and blocks were missing were excluded from the study. All the cases of fibrosis were easily diagnosed on H&E and there was no need for any special stains such as Periodic Acid Schiff or Verhoeff van Gieson. All histologic cases were reviewed and a morphological classification that is principally based on histological patterns of spermatogenesis was utilized to group the cases into normal findings, hypo spermatogenesis, maturation arrest, Sertoli cell-only syndrome, peritubular hyalinization/ tubular fibrosis and mixed patterns2, 9, 10. The data obtained were analysed using descriptive and inferential statistics at a 5% level of significance and Ethical approval was obtained from the appropriate Ethical Review Committee.
Results
A total of 225 cases were reviewed comprising of patients who presented with infertility and had either azoospermia or oligospermia diagnosed on routine seminal fluid analysis.
Their ages ranged from 20 years to 60 years with a mean age of 37.7 years (SD = 8.61). The highest proportion of the cases, 31.6%, was found among the age group 30-39 years. The majority, (82.7%), had primary infertility while 17.3% had secondary infertility.
Seminal fluid analysis reports had shown that most of the cases reviewed had azoospermia, (92.9%), while others had oligospermia. Only 9.8% had clinical evidence of obstruction. Azoospermia was seen in 93% of the primary and 92.5% of the secondary infertility cases.
All the cases were histologically classified into six categories (figure 1).
Hypospermatogenesis (Hypo) was the most common pattern seen comprising 34.2%, followed by peritubular hyalinization/ tubular fibrosis (PHTF) in 32%, maturation arrest (MA) in 14.2%, Sertoli cell-only syndrome (SCOS) in 6.7%, while the least pattern were cases of normal histologic pattern in 0.9%. The photomicrographs of each pattern are shown in figure 2 below. Twenty-seven cases (12%) had a mixed histological pattern where more than one morphologic picture was seen in the same biopsy (Figure 3).
Figure 1.Distributions of Different Histological Pattern of testicular biopsy
Figure 2.Different Histological Pattern of testicular biopsy
Figure 3.Photomicrographs of different mixed histologic patterns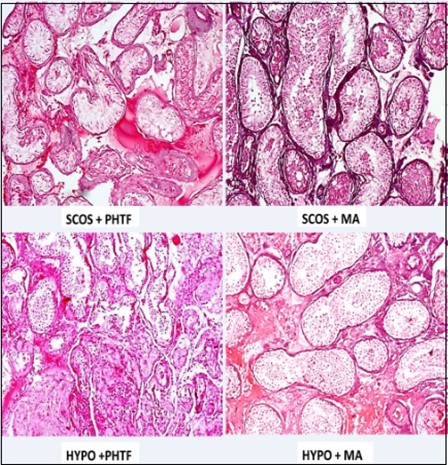
In relating the different histologic patterns with the types of infertility, more cases of primary infertility were seen in all the different histologic patterns compared with secondary infertility. Peritubular hyalinization/tubular fibrosis is the commonest pattern seen among cases of primary infertility (87.5%) while Hypospermatogenesis is the commonest in the secondary type (43.6%). The two histologically normal cases also had primary infertility (100%). Clinical evidence of obstruction was seen to be more associated with hypo spermatogenesis, (40.9%) and maturation arrest, (31.8%) (Table 1).
Table 1. Correlation between Histologic Patterns, SFA, and Evidence of Obstruction| Histologic Patterns | Type of Infertility | Total | SFA | Total | Obstruction | Total | |||
| Primary | Secondary | Azoospermia | Oligo spermia | No | Yes | ||||
| PHTF | 64(28.4%) | 8(3.6%) | 72(32.0%) | 67(29.8%) | 5(2.2%) | 72(32.0%) | 69(30.7%) | 3(1.3%) | 72(32.0%) |
| HYP | 60(26.7%) | 17(7.6%) | 77(34.2%) | 72(32.0%) | 5(2.2%) | 77(34.2%) | 68(30.2%) | 9(4.0%) | 77(34.2%) |
| MA | 25(11.1%) | 7(3.1%) | 32(14.2%) | 28(12.4%) | 4(1.8%) | 32(14.2%) | 25(11.1%) | 7(3.1%) | 32(14.2%) |
| MIXED | 22(9.8%) | 5(2.2%) | 27(12.0%) | 25(11.1%) | 2(0.9%) | 27(12.0%) | 24(10.7%) | 3(1.3%) | 27(12.0%) |
| SCOS | 13(5.8%) | 2(0.9%) | 15(6.7%) | 15(6.7%) | 0(0.0%) | 15(6.7%) | 15(6.7%) | 0(0.0%) | 15(6.7%) |
| Normal | 2(0.9%) | 0(0.0%) | 2(0.9%) | 2(0.9%) | 0(0.0%) | 2(0.9%) | 2(0.9%) | 0(0.0%) | 2(0.9%) |
| Total | 186(82.7%) | 39(17.3%) | 225(100.0%) | 209(92.9%) | 16(7.1%) | 225(100.0%) | 203(90.2%) | 22(9.8%) | 225(100.0%) |
In this study, the mixed histologic pattern constituted 12% of the entire cases reviewed (Table 2) with various combinations which include peritubular hyalinization/tubular fibrosis with hypo spermatogenesis and peritubular hyalinization/tubular fibrosis with maturation arrest in 29.6% each, peritubular hyalinization/tubular fibrosis with Sertoli cell-only syndrome in 18.6%, both hypo spermatogenesis with Sertoli cell-only syndrome and maturation arrest with Sertoli cell-only syndrome in 11.1% of cases each.
Table 2. Frequency of Mixed Pattern| MIXED PATTERNS | FREQUENCY | PERCENTAGE |
| PHTF+HYPO | 8 | 29.6 |
| PHTF+MA | 8 | 29.6 |
| PHTF+SCOS | 5 | 18.6 |
| SCOS+HYPO | 3 | 11.1 |
| SCOS+MA | 3 | 11.1 |
| Total | 27 | 100.0 |
Most of the mixed histologic pattern cases, 77.8%, had primary infertility. Table 3 showed the association between the mixed histologic patterns, semen analysis report and evidence of obstruction. However, these relationships are not statistically significant (p ≥ 0.05)
Table 3. Correlation between Mixed Histologic Patterns, SFA, and Evidence of Obstruction| Histologic Patterns | Type of Infertility | Total | SFA | Total | Obstruction | Total | |||
| Primary | Secondary | Azoospermia | Oligospermia | No | Yes | ||||
| Single Pattern | 165 (72.9%) | 34 (15.1%) | 198 (88.0%) | 184 (81.8%) | 14 (6.2%) | 198 (88.0%) | 179 (79.6%) | 19 (8.4%) | 198 (88.0%) |
| HYP+SCOS | 6 (2.7%) | 2 (0.9%) | 8 (3.6%) | 3 (1.3%) | 0 (0.0%) | 3 (1.3%) | 3(1.3%) | 0(0.0%) | 3 (1.3%) |
| MA+SCOS | 6 (2.7%) | 2 (0.9%) | 8 (3.6%) | 2 (0.9%) | 0 (0.0%) | 2 (0.9%) | 2(0.9%) | 0(0.0%) | 2 (0.9%) |
| SCOS+PHTF | 5 (2.2%) | 0 (0.0%) | 5 (2.2%) | 4 (1.8%) | 1 (0.4%) | 5 (2.2%) | 4(1.8%) | 1(0.4%) | 5 (2.2%) |
| MA+ PHTF | 2 (0.9%) | 1 (0.4%) | 3 (1.3%) | 7 (3.1%) | 1 (0.4%) | 8 (3.6%) | 8(3.6%) | 0(0.0%) | 8 (3.6%) |
| HYP+ PHTF | 1 (0.4%) | 0 (0.0%) | 1 (0.4%) | 9 (4.0%) | 0 (0.0%) | 9 (4.0%) | 7(3.1%) | 2(0.9%) | 9 (4.0%) |
| Total | 186 (82.7%) | 39 (17.3%) | 225(100.0%) | 209 (92.9%) | 16 (7.1%) | 225 (100.0%) | 203 (90.2%) | 22 (9.8%) | 225 (100.0%) |
Discussion
We reviewed testicular biopsy specimens from 225 men diagnosed by seminal fluid analysis to be either azoospermic or oligospermic. The mean age of the cases was 37.7 years (SD 8.61, range 20-60 years11, 12
Primary infertility was commoner than secondary infertility in this study, as was the pattern seen in a few previous studies elsewhere in Nigeria.13, 14 reported 49.2% of patients with primary infertility and 22.2% with secondary infertility in Ilorin. Couples with primary infertility tend to present early for fertility evaluation in the health centre due to socio-cultural factors and family pressures than those with secondary infertility who had once had a pregnancy to term irrespective of the outcome or those with one or more children in this environment13.
As shown, the majority of cases were in the age range 30-39 years corresponding to the peak of male reproductive activity and the age of psychological maturity when infertile men are most concerned about their condition and seek medical attention15.
In this study, 209 (92.9%) patients were azoospermic by the SFA report while others were oligospermic. Also, the clinical history of obstruction and clinical evidence of obstruction was noted in 10% of cases with azoospermia while none of the oligospermic patients had any associated clinical obstruction. This finding is similar to what was reported in some of the previous local and international studies.16, 17 The evaluation of the possible aetiopathogenesis of testicular damage in non-obstructive azoospermia, which has a high incidence in this environment may help in the prevention and management of male infertility.
Normal spermatogenesis has been seen in a variable proportion of testicular biopsies done in the investigation of primary infertility. These usually represent post-testicular problems in such patients (including obstruction). In many studies, these were reported in up to between 33% and 50% of cases. 18, 19, 20 The frequency of normal spermatogenesis in the present study is much lower at 0.9%.
Hypospermatogenesis (Hypo) was the most common pattern seen in this study constituting 34.2% of the cases. A similar pattern but with a higher proportion was seen in the study by Obafunwa et al. from Lagos 5, Ahmed et al from Zaria11 and Nagpal et al. from India21 while a lower prevalence was seen in other previous studies done in this environment including the previous study from this centre by Thomas18 who reported 19% of cases. Ikuerowo et al22 from Lagos, Ojo et al14 from Ilorin and Oranusi et al23 from Nnewi reported 3.9%, 4.8% and 10% of cases with Hypospermatogenesis respectively. Various reasons were adduced for the sharp differences in the percentages by some of these authors, which are also similar to the reasons identified among the few that are specified in this study too, which include exposure to noxious chemicals (lead, various toxic industrial fumes especially gasoline vapour), drugs e.g. Nifedipine, Moduretic and physical agents (especially heat, tightly fitted underwear which apposes the testes closely to the body).3, 5, 13
Maturation arrest (MA) was observed in 32 (14.2%) cases with an arrest at different levels of maturation, none reaching up to the level of spermatid. Those reviewed in this study were at the level of spermatogonia and primary spermatocytes. The incidence of maturation arrest was similarly lower in the previous study from Ibadan where 5.3% was reported. Philip et al12 reported 5.9% and a very low incidence of 0.4% was reported by Ali from Saudi.24 However, a higher relative proportion of 27.5% and 25.4% were reported by Ikuerowo et al22and Ojo et al. from Lagos and Ilorin respectively.14.
Sertoli cell-only syndrome (SCOS) was found in 6.7% of cases. This pattern usually has a very low incidence and was so documented by Thomas18 in Ibadan who reported 9.2% and by Obafunwa et al5 and Phillip et al12 who documented 1.1% and 1.5% respectively. In contrast, some of the studies reported higher incidences. Ahmed et al11 from Zaria reported 28.2% of cases, Rasheed et al.4 from Egypt reported 34% of cases. In India, Mehrotra et al19 and Nagpal et al21 reported 29.3% and 17% respectively.
Peritubular hyalinization/tubular fibrosis (PHTF) was found in 32% of the cases in this study. This was less frequent than in the previous study done by Thomas with 23% cases.18. Two recent studies from Lagos by Ikuerowo et al22 and Ojo et al14 similarly reported high incidences of 58.8% and 55.6% while some other studies reported low incidences such as those of Ahmed et al11 from Zaria (6.1%) and Rasheed et al4 from Egypt (6.0%). Al-Rayess et al16 and Ali et al24 from Saudi reported 12% and 19% respectively.
A mixed morphological pattern was diagnosed in this study when over 30% of either pattern was present and this was the case in 28 (12%) patients. Of these, there were 9 cases of peritubular hyalinization/tubular fibrosis admixed with hypo spermatogenesis; 8 cases of peritubular hyalinization/tubular fibrosis mixed with Maturation arrest; 5 cases of peritubular hyalinization/tubular fibrosis mixed with Sertoli cell-only syndrome; 3 cases of Sertoli cell-only syndrome mixed with hypo spermatogenesis and 3 cases of Sertoli cell-only syndrome mixed with maturation arrest.
The limitation of this study includes the smaller number of samples over a long period, absence of fertility hormone profile and lack of molecular or genetic testing capability of the institution to further screen the participants for genetic anomalies for a better correlation with morphologic patterns seen.
Some studies identified different discordant patterns in right and left testes, supporting the use of bilateral testicular biopsies for a more comprehensive evaluation of male infertility. Although the absence of spermatozoa in one testis in such cases does not rule out their presence in the other2 a unilateral biopsy has been seen to suffice in revealing the pathology in most instances2, 25. A bilateral biopsy seems needful only when there is a significant difference in the size of the testes and especially when considering sperm retrieval for an intracytoplasmic sperm injection (ICSI) in the management of infertile couples with male infertility26, 27, 28 .
From the literature, there is significant geographical variation in the histological patterns in male infertility as shown in Table 4
Table 4. Geographical Variation of Histologic Patterns in male infertility| Normal | Hypo | MA | SCOS | PHTF | Mixed | Klinefel-ter synd. | Inflam- matory | Varico-coele | |
| PresentStudy(n=225) | 0.9 | 34.2 | 14.2 | 6.7 | 32 | 12 | - | - | - |
| Thomas Ibadan(n=152) | 38.2 | 19.1 | 5.3 | 9.2 | 23 | - | 5.3 | - | - |
| Obafunwa et al. Lagos (n=177) | 20 | 49 | - | 1.1 | - | - | 1.1 | 24.3 | 3 |
| Ahmed et al. Zaria (n=472) | 15.9 | 35.2 | 14.6 | 28.2 | 6.1 | - | - | - | - |
| Ojo et al. Ilorin (n=63) | 14.1 | 4.9 | 25.4 | - | 55.6 | - | - | - | - |
| Ali et al. Egypt (n=272) | 5 | 39 | 1.0 | 26 | 19 | - | - | - | - |
| Al-Rayess et al. Saudi(n=230) | 31.4 | 13 | 11 | 39.3 | 5.3 | - | - | - | - |
| Nagpal et al. India | 16 | 42 | 18 | 17 | 1 | - | 3 | 3 | - |
The high prevalence of normal spermatogenesis in a previous study was attributed to the high incidence of chronic inflammation resulting in fibrosis of the excurrent ducts. This seems to occur with much lower frequency in recent studies due to the advent of indiscriminate antibiotic use in our environment which has reduced the incidence of chronic inflammatory patterns seen in these patients.5, 21 Besides, Klinefelter syndrome is reported less frequently in recent studies due to improved methods for early diagnosis including in-utero diagnosis negating the need for testicular biopsy in the evaluation of such patients for infertility.5, 18, 21. In another study, 2.7% had chromosomal abnormalities, of which less than half had sex chromosome abnormalities (mainly 47, XXY) while Y-chromosome microdeletions were detected in 5.4%.29
The variations observed between this study and some other previous studies are not well understood. However, previous local and international studies have suggested increasingly, the importance of occupation, environmental, sociocultural habits, consanguineous marriages, and in particular, genetic factors in the cause of male infertility30, 31, 32.
Proper evaluation of the testicular biopsy of infertile males is very necessary for the management of such patients and pathologists are required to report any form of germ cells seen in the specimen, especially if late spermatids or spermatozoa are seen. Quantitative assessment of human spermatogenesis has been shown by recent studies to be possible and can be beneficial.33
This is very important in offering the chance of assisted fertilization that will require testicular sperm extraction and intracytoplasmic sperm injection, and also for some patients with smaller testicles and slightly elevated levels of FSH.34
Conclusion
The findings of this study are similar to some of the previous local and international studies.
Most infertile males had primary infertility and azoospermia, and the commonest identified histologic pattern of testicular biopsy in cases of male infertility identified in this study was hypo spermatogenesis, followed by peritubular hyalinization/tubular fibrosis.
This study has shed some light on the possibility of multiple patterns within a biopsy if properly sampled and reviewed carefully especially in non-obstructive azoospermic cases with greater chances of successful sperm extraction for Assisted Reproduction Technique.
Bilateral testicular biopsies, as well as meticulous pathological examination of all seminiferous tubules in view, should be carried out to identify mixed and discordant patterns and this is highly recommended.
This study was self-funded with no support from any quarters.
List of Abbreviations
H&E - Haematoxylin & Eosin
FSH – Follicle Stimulating Hormone
SFA – Seminal Fluid Analysis
HYP–Hypospermatogenesis
PHTF–Peritubular Hyalinization/ Tubular fibrosis
MA–MaturationArrest
Mixed–MixedPattern
SCOS–Sertoli Cell Only Syndrome
Normal – Normal Pattern
ICSI - Intracytoplasmic Sperm Injection.
References
- 1.Cerilli L A, Kuang W RD, Cerilli L A, Kuang W, Rogers D.A practical approach to testicular biopsy interpretation for male infertility.ArchPatholLab Med. 134(8), 1197-204.
- 2.Abdullah L, Bondagji N. (2011) Histopathological patterns of testicular biopsy in male infertility: A retrospective study from a tertiary care center in the western part of Saudi Arabia.UrolAnn. 3.
- 3.Naz M, Kamal M. (2017) Classification, causes, diagnosis and treatment of male infertility: a review. Oriental Pharmacy and Experimental Medicine.
- 4.Rashed M, Ragab N. (2008) Shalaby A RW. , Patterns Of Testicular Histopathology In Men With Primary Infertility.Internet J Urol 5(2), 1-5.
- 5.Obafunwa J O, Elesha S O. (1993) Morphological changes found in the testes of 177 Nigerian males investigated for infertility.AfrJ MedMedSci. 22(4), 35-40.
- 6.Mehrotra R, Chaurasia D. (2005) Fine needle aspiration cytology of the testis as the first-line diagnostic modality in azoospermia a comparative study of cytology and histology. 363-8.
- 7.Cohen M S, Frye S, Warner R S, Leiter E. (1984) Testicular needle biopsy in diagnosis of infertility.Urology. 24, 439-42.
- 8.Craft I, Tsirigotis M, Farrer-brown G. (1997) Testicular needle aspiration as an alternative to biopsy for the assessment of spermatogenesis. 12(7), 1483-7.
- 9.Ticko S K, Amin M B, Cramer H M, Harik L R, Ulbright T M. (2006) The testis, paaratesticular structures, and male external genitalia. In: Silverberg SG, DeLellis RA, Frable WJ, LiVolsi VA WM, editor. Principles and Practice of Surgical Pathology and Cytopathology. fourth. Philadelphia: Elsevier Churchill Livingstone. . 1731-89.
- 10.Mushtaq H, Alam S, Khan M A. (2013) Histopathological Patterns of Testicular Biopsies in Male Infertility.J Islam Med Dent Coll.
- 11.Ahmed S A, Mohammed A, Shehu S M, MOA Samaila.Mbibu NH,et al.(2007). , Morphological Pattern of Testicular Biopsies in Zaria, Nigeria.Niger Med J 48(3), 69-70.
- 12.Philip C, Bilodi AK PS. (2014) . Histological Evaluation of Testicular Biopsies and Spermatogenesis Distubance in Infertile Men with Varicocoele.World J Pharm Sci 3(3), 1789-803.
- 13.Vincent Deye, Michel F, Ehrmann P, Da Silva S, Piagnerelli D. (2013) M.,et al.,(2016). Changes in cardiac arrest patients TM temperature management after the. , Journal of Medicine 52(1), 61-66.
- 14.Ojo B A, Rahman G A. (2005) The Value of Testicular Biopsy in Male Infertility:. , Experience with 63 Nigerians.African J Urol 11(2), 105-10.
- 15.Articles on Psychology, Psychiatry, M.Psychological Maturity [Internet]. New Horizons. Available from: http://sabryfattah.com/psychology/psychological-maturity/.
- 16.Molham M Al-Rayess, Al-Rikabi A C. (2000) Morphologic patterns of male infertility in Saudi patients:. , A University Hospital experience.Saudi Med J 21, 625-8.
- 17.Colgan T J, Bedard Y C, Strawbridge H T, Buckspan M B, Klotz P H. (1980) Reappraisal of the value of testicular biopsy in the investigation of infertility.FertilSteril. 33, 56-60.
- 18.Thomas J O. (1990) Histological pattern of testicular biopsies in infertile males in Ibadan. , Nigeria.EastAfrMed J 67(8), 578-84.
- 19.Mehrotra R Chaurasia D. (2008) Fine needle aspiration cytology of the testis as the first-line diagnostic modality in azoospermia: a comparative study of cytology and histology.Cytopathology. 19(6), 363-368.
- 20.Caucci M, Barbatelli G CS. (1997) The retractile testis can be a cause of adult infertility.FertilitySteril. 68(6), 1051-8.
- 21.Nagpal B L, Manjari M, Kapoor K, Dhaliwal U S. (1993) Testicular biopsy in cases of male infertility: a retrospective study.J Indian Med Assoc. 91, 171-4.
- 22.Ikuerowo S O, Izegbu M C, Benebo A S, Fadeyibi I O, Omodele F O. (2010) Testicular Biopsies of Azoospermic Men at The Lagos State University Teaching Hospital.African. , J Urol 16(3), 69-72.
- 23.Oranusi C K, Onyiaorah Ukah CO. (2014) Pattern of Testicular Biopies as Seen in a Tertiary Institution in Nnewi. , Southeast Nigeria.Niger J Surg 20(2), 55-58.
- 24.Ali M A, Akhtar M, Woodhouse N, Burgess A, Faulkner C. (1991) Role of testicular fine-needle aspiration biopsy in the evaluation of male infertility: cytologic and histologic correlation.DiagnCytopathol. 7(2), 128-31.
- 25.Brannen G E, Roth R R. (1979) Testicular abnormalities of the subfertile male.J Urol. 122, 757-62.
- 26.McLachlan R I, E Rajpert-De Meyts, Hoei-Hansen C E, de Kretser DM. (2007) Histological evaluation of the human testis—approaches to optimizing the clinical value of the assessment.HumReprod. 22(1), 2-16.
- 27.Levin H S. (1979) Testicular biopsy in the study of male infertility: its current usefulness, histologic techniques, and prospects for the future.HumPathol. 10, 569-84.
- 28.Venkatachala S, Malur P R, Nerli R B, Desai B R, Dhorigol V. (2007) Testicular biopsies--histomorphologic patterns in male infertility.Indian JPatholMicrobiol. 50, 726-9.
- 29.Olesen I A, Andersson A M, Aksglaede L, Skakkebaek N E, E Rajpert-de Meyts et al. (2017) Clinical, genetic, biochemical, and testicular biopsy findings among 1,213 men evaluated for infertility.FertilSteril.
- 30.D Stewart Irvine. (1998) Epidemiology and aetiology of male infertility. Human Reproduction. 33-44.
- 31.Dada R, Kumar M, Jesudasan R, Fernández J L.Gosálvez J,et al.,(2012). Epigenetics and its role in male infertility. , Journal of Assisted Reproduction and Genetics 213-23.
- 32.Krausz C, Escamilla A R, Chianese C. (2015) Genetics of male infertility: From research to clinic. Reproduction