Abstract
Distant metastases generally indicate disseminated disease and the standard treatment for these patients is palliative chemotherapy. Retrospective series showed that selected patients with metastatic lung cancer and a solitary extrathoracic disease could be effectively treated with curative intention by resection of both primary tumor and the single site of metastatic disease.
According to current data, adrenalectomy might be considered as an alternative option for patients with isolated adrenal metastases. Significant morbidity and mortality may be happened by these procedures, and a cautious analysis of pros and cons should be discussed with the patient.
We present a review of the literature and updated recommendations focusing lung cancer with solitary adrenal metastasis.
Author Contributions
Academic Editor: Sasho Stoleski, Institute of Occupational Health of R. Macedonia, WHO CC and Ga2len CC, Macedonia.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2019 Rodrigo A. S. Sardenberg, et al
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Isolated adrenal metastasis are reported in 1,6 -3,5% of patients with primary non-small cell lung cancer (NSCLC), presenting with resectable tumors, and it increases up to 40% as the disease progresses1,2.
The setting of distant metastasis generally indicates disseminated disease and the standard treatment for these patients is palliative chemotherapy. Retrospective series suggest that some stage IV non-small-cell-lung cancer (NSCLC) patients with a solitary synchronous/metachronous extrathoracic metastatic disease (“micrometastatic disease”) might be cured by resection of both primary tumor and isolated metastases2,3. Since 1982, when Twomey et al4 reported on prolonged survival after adrenalectomy, introducing the concept of curative management of patients with lung cancer and isolated adrenal metastases, many reports have addressed this topic and confirmed the feasibility of such approach.
Methods
We adopted a comprehensive assessment of the literature where studies published in English were included.
Studies on adrenal metastases and NSCLC were identified using Pubmed, (until December 2017) and the Cochrane Library (until December 2017).
The search words were: adrenal metastases, adrenal metastasectomy, adrenal metastases resection, surgery for adrenal metastases, non-small cell lung cancer adrenal metastases
To be considered to enter in this review, studies consisted of reviews and case series. Entry criteria for studies included: operative morbidity and mortality, type of resection, systemic therapy, primary tumor characteristics, disease free-interval, and adrenal nodules/ masses aspects. The surgical approach included unilateral or bilateral adrenalectomy.
The authors attempted to view adrenal metastasectomy as a surgical option for patients with adrenal nodules/masses resected – in patients with previous NSCLC diagnosed- also offering a critical review of this technique.
Results
The results of adrenal metastasectomy should be analyzed from the point of view according to the critical factors that can affect survival. Such analysis results should also be grounded in reviews of studies in primary tumor characteristics, or in patients with bilateral or unilateral metastases, and synchronous or metachronous metastases in a sufficient number of patients.
Prognostic factors were reviewed in several series in order to show its influence alone or in combination for survival analysis and the description that best fits the selection criteria. The results from the collected data confirm that adrenal metastasectomy is a form of potential therapy for healing that can be safe with low mortality 5,6.
According to the principles of oncological surgery, complete removal of all pulmonary foci is associated with increased survival. The data also suggest that the preoperative radiological work-up evaluation however, has higher accuracy with PET-CT 7,8.
Laparoscopic resection can not always provide efficiency for the complete nodule/ mass removal9,10.
A limitation of this study lies in the fact that no evaluation of variables related to the biological behavior of different histological types (adenocarcinoma vs epidermoid carcinoma), which may explain the evolution of clinical metastases. Currently, adrenalectomy may be indicated for the or resection of metastases in patients specially with single nodules and metachronous metastases, after PET-CT evaluation20,21,25.
The role of adrenalectomy is less evident in patients with bilateral and synchronous lesions, and in primary tumors with lymph node metastases25,28.
Discussion
Isolated adrenal gland metastases found during staging work-up in patients with resectable lung cancer are rare in the pre- PET-CT era. Although, adrenal metastases might be higher due to the low accuracy of image to detect small tumors1,5,12.
Direct lymphatic spread, via retroperitoneal channels, might be the main reason of adrenal metastasis5,which weakens the hematogenous spread hypothesis. This theory is based on the presumptions that ipsilateral adrenal metastases are lymphatic and thus, less advanced disease. The contralateral and/ or distant metastases might represent hematogenously spread, or more advanced disease. Chemokines detected by immunohistochemistry, like CCR6, may be involved in higher propensity to the development of such metastasis33. Identified as its receptor, high levels of CCL20 expression in adrenal metastasis is a molecular determinant for relapse at this particular site, supporting the "seed and soil" theory5.
Usually, the imaging screening used for diagnosis of adrenal metastasis are: CT scans (Figure 1), magnetic resonance imaging (MRI) and PET-CT. The sensitivity of PET-CT has conflicting results, being of 96% and with false-positive detection rate even for smaller masses (without swelling and not evident on CT or MRI) of only 4% as reported in some series8,9,18. Another study confirms these findings, showing sensitivity and specificity of PET/CT for distant metastasis of 94% and 85%, respectively31.
Figure 1. CT scan showing isolated right adrenal NSLC metastasis (Arrow)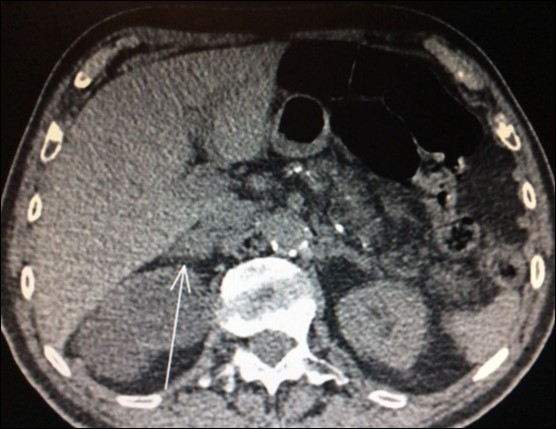
Nevertheless, in another study, only 28 out of 39 (71.8%) patients who underwent adrenalectomy for suspect adrenal tumor by FDG-PET/CT confirmed metastasis. Ten of those were benign adenoma and one was a non-functional pheochromocytoma29. Thus, FDG-PET/CT produced an unexpected high rate (28.2%) of false-positive results in this trial. Despite benign adenoma may mimicking metastasis and produce a positive preoperative FDG-PET/CT, history of primary lung malignancy and SUV max >2.65 were more commonly associated with metastasis in this paper (Figure 2).
Figure 2. PET-CT with high uptake on right adrenal 
PET-CT results for adrenal glands increases the likelihood of malignancy, but may only be considered indicative, not certainty of malignancy.
Adrenal biopsies (Figure 3) before adrenalectomy is useful in order to identify the nature of the lesion10,11,12. Even with fine-needle aspirate is possible a diagnosis and also a successful molecular testing by next-generation sequencing in order to the identification of pathogenic alterations linked to available or developing targeted therapies30.
Figure 3. Right adrenal needle biopsy (arrow)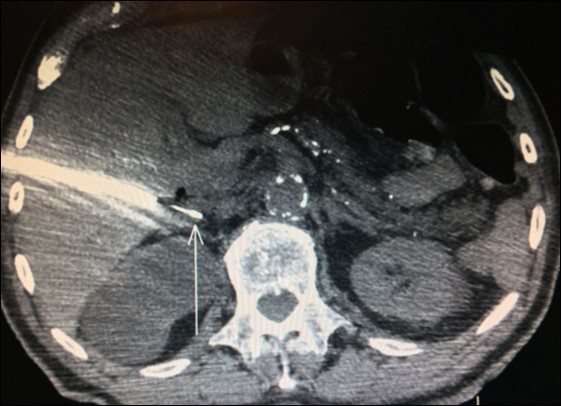
There is limited understanding of the efficacy of surgical resection because of low incidence of isolated adrenal metastasis in patients with stage IV NSCLC. Some series showed that the time from diagnosis of primary lung cancer diagnosis of adrenal metastasis - disease-free interval (DFI) – is a prognostic factor6. Synchronous metastases (Figure 4), defined as a DFI of 6 months or less, has been associated with a poor prognosis.
Figure 4. PET CT showing simultaneous uptake on right lung mass and 2 ipsilateral adrenal nodules.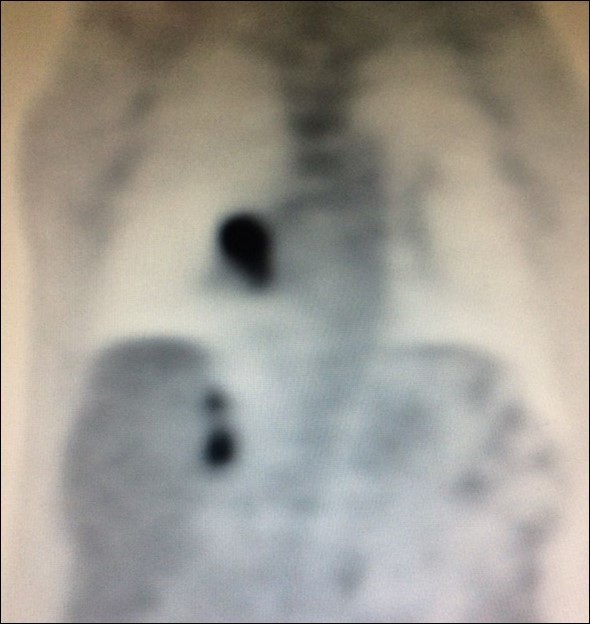
It is not known why patients with metachronous metastases performedbetter. Certainly, patients in the synchronous group experience early morbidity/mortality due to lung tumor resection, and those with a metachronous metastases had already recovered from thoracic surgery. Also, theoretically, the biology of the tumors is different in those presenting as synchronous lesions tend to be more aggressive.
In one study, none of the 10 patients with synchronous metastasis survived beyond two years 7. A recent systematic review and pooled analysis performed in 13 retrospective studies (98 patients with adenocarcimoma and squamous cell carcinoma) from PubMed, Embase and Cochrane Library databases confirmed that patients with metachronous had a significantly better prognosis than synchronous metastasis (p < 0.05) 27.
The results associated with surgical treatment between metachronous and synchronous metastasis is clinically relevant. For patients with a synchronous metastasis, the decision for an adrenalectomy depends on the definitive management of the primary lung cancer. As opposed to palliative chemotherapy alone, these procedures may result in a significant morbidity and mortality, and the decision should be discussed with the patient in a multidisciplinary setting. Node involvement decreased significantly the prognosis when identified (p < 0.05) and any further invasiveness must be discouraged. 27
Unfortunately, to date, there have been few reviews on this subject. Most studies analyze and report data combining synchronous and metachronous metastases. Others include patients with only synchronous or metachronous metastases alone (Table 1). Some publications with a high number of patients did not establish a statistically significant difference between these groups of patients 9,12,16,17.
Table 1. Characteristics of the studies comparing synchronous and metachronous adrenal metastases survival.| Author | Year | No. patients | Survival (mo)Synchronous | Survival (mo)Metachronous |
| Higashiyama | 1994 | 9 | 9 | 17-40 |
| Wade | 1998 | 47 | - | 0.7-61 |
| Bretcha-Boix | 2000 | 14 | - | 8-16 |
| Lucchi | 2005 | 15 | 9-12 | 14-80 |
| Mercier | 2005 | 23 | 0.3-16 | 2-110 |
| Pfannschmidt | 2005 | 11 | 9-72 | 6-40 |
| Porte | 2001 | 43 | - | - |
| Sebag | 2006 | 16 | 1-68 | 24 |
| Strong | 2007 | 89 | 2-127 | 3-97 |
| Moreno | 2013 | 148 | 23 | 30 |
| Romero | 2014 | 29 | - | - |
| Kawai | 2014 | 10 | 9 | 25 |
In one of this series, there was no difference in median disease- free survival (DFS amongst 72 patients who underwent synchronous adrenalectomy (9 months) versus metachronous (11 months) diseases (p=0.79)17. A systematic review by Tanvetyanon and cols 16 including 114 patients, 42% underwent resection for synchronous metastases, and overall survival was shorter in this group; however, for both groups, long-term survival was similar, with 25% of the patients estimated to be alive 5 years after surgery. Conversely to those data, a large retrospective series – including others histologies beyond NSCLC - by Moreno et al 21, the differences in overall survival between synchronous and metachronous were significant (P = .038). The same findings favoring metachronous versus synchronous adrenalectomy was seen in another study (p < 0.01). 20
Tamura e cols reported recently the rare, but potentially fatal, occurrence of bilateral adrenal hemorrhage due to adrenal metastasis of lung cancer. This is an additional argument reinforcing the appeal for surgical approaches for oligometastatic diseases32.There have been only retrospective reports and reviews of patients undergoing resection of a primary NSCLC and solitary adrenal metastasis and the data are strikingly conflicting. In a review by Beitler et al. 2, there were synchronous adrenal metastases in 59% of patients; the lung primary tumor stage was I in 22%, II in 16%, and III in 43% of the patients. Surgical treatment of all lesions resulted in an average survival time of 24 months; with one third of the patients surviving five years. These results are superior to historic data regarding overall survival of metastatic lung cancer patients without driver oncogenes and who did not receive targeted therapies 34.
The retrospective review of the Memorial Sloan-Kettering Cancer Center 3, suggests that the median survival time of patients treated with chemotherapy alone is approximately 8.5 months, but the overall survival of those treated with chemotherapy and surgical resection was 31 months. Barone et al. performed 18 adrenalectomies according to different therapeutic strategies, and benefit in survival was found in patients who underwent adrenalectomy. A statistically significant difference was found between adrenalectomy (p < 0.01), metachronous metastasis (p < 0.01), ipsilateral disease (p < 0.05) and overall survival 20.
Aranda e cols advocate that adrenalectomy not only is feasible in the synchronous setting, but also is safe and must be done first, followed further by lung resection. This approach resulted in a disease-free survival at 2 years of 60%, 5-year overall survival of 30% and a median survival of 41.5 months in 108 laparoscopic adrenalectomies performed by this group 23. However, synchronous bilateral adrenal metastases seem to have poorer survival (8 months survival) when compared to metachronous bilateral adrenal metastases (44 months survival) according to the outcomes based on two patients 16.
Luketich and Burt 3 indicated that excision significantly extended the survival period (31 months) compared with no excision (8.5 months) in metastatic cases of primary NSCLC. New techniques, like laparoscopic adrenalectomy, was also evaluated in such cases and pros and cons were associated with its use.
Laparoscopic adrenalectomy was not inferior to open adrenalectomy regarding safety and the anticancer effect, and it has proven itself as a minimally invasive treatment in another study 18.
Adrenal metastases in both sides do not appear to be a contraindication for a more intensive treatment13. A case report was described in a patient with bilateral adrenal metastases who remained nine years free of diseaseafter bilateral adrenalectomy 14. Nevertheless, few cases of surgical treatment of bilateral adrenal metastases were reported, perhaps due to primary adrenal insufficiency - after bilateral adrenalectomy - and/or the coexistence of multiple metastasis at other organs 19.
Conversely to the data showing benefit in outcomes of patients submitted to adrenalectomy, there are reports opposing that approach. A study performed between 2001 and 2015 in 22 out 1,302 patients with NSCLC and solitary adrenal metastasis who underwent adrenalectomy did not show any gain of survival compared to those undergoing nonsurgical treatment. With a median overall survival of 11 months and 1-year survival rate of 51.4% (p=0.209), these data do not support metastasectomy and primary radical resection since this approach did not improved the outcomes in this population 22.
A multicenter study reported by Porte et al. 12, reviewed 43 patients with solitary resectable adrenal metastasis who were treated for primary NSCLC during a 10-year period; the metastatic disease was synchronous in 21 patients and metachronous in 21 patients. All patients had the metastatic disease resected. With a mean follow-up of 23 months, 74% had died, 11% were alive with disease, and 14% had no evidence of disease. They found no association between survival and TNM stage, tumor histology, adjuvant or neoadjuvant therapy, synchronous vs metachronous disease, and ipsilateral vs contralateral disease. A conclusion that long-term survival could be reached by surgical resection in few patients was the main thought of the authors. Gao e cols addressed the same points and histology and laterality did not impacted on prognosis as well. 27
We need to point out that none of the studies included were prospectivelyrandomized between adrenalectomy or no adrenalectomy; maybe, the poor outcomes associated to palliative chemotherapy is difficult to conduct, since no long-term survival is expected in patients with advanced NSCLC.
The following criteria were proposed for the indication of adrenalectomy for the treatment NSCLC with isolated adrenal metastases: (1) primary lung cancer could be resected or cured by radical treatment, (2) metastasis restricted to the adrenal gland, (3) adrenal metastases had not invaded the surrounding organs, and (4) the size of the adrenal tumor did not exceed 10 cm 18.
Finally, a safe and feasible alternative for adrenalectomy are noninvasive methods for disease control. In a study, 31 patients were submitted to computed tomography (CT)-guided percutaneous microwave ablation (MWA) of unilateral adrenal metastasis from lung cancer (1.5 - 5.4 cm in diameter) 25. The median local tumor progression-free survival time was 9 months. Only 5.6% of major complications (hypertensive crisis) were reported. Other trial evaluating the role of stereotactic ablative radiotherapy (SABR) in 43 individuals with adrenal metastasis showed a median time to local failure not reached and the 1-year freedom from local failure rate of 74% (9 failures were in field and 1 was marginal)26.
The indication of resection or non-surgical local therapy (SBRT or microwave) is feasible, but the biggest question imposed is the real validity of adrenal local control in the metastatic setting, while the most frequent cause of death is systemic progression. However, it is important to emphasize that there is a compelling new evidence from a randomized, multicenter phase 2 trial 35 that shows a real gain in progression free survival for patients who had histological confirmation of stage IV NSCLC with three or fewer metastatic disease lesions that receive local consolidative therapy ((chemo) radiotherapy or resection of all lesions) after first-line systemic therapy versus maintenance treatment alone, which could be observation only. In this article, 27,6% of the patients have the adrenal glands as the only site of disease. The median progression-free survival in the local consolidative therapy group was 11·9 months versus 3·9 months in the maintenance treatment group (hazard ratio 0·35 (90% CI0·18–0·66), log-rank p=0·0054); they also found that the addition of local consolidative therapy could delay the appearance of new lesions. Concerning the type of consolidative therapy done for adrenal metastasis were: surgery in 54,2%, stereotatic radiosurgery in 20,8% and external radiotherapy in 25%. The overall survival observed in the adrenal oligometastatic group were in line with other classical single site metastatic disease in witch local therapy are usually indicated (37,1 months -adrenal - ; 38,2 months – brain -; 37,4 months - contralateral lung metastase). Suggesting that the benefit of consolidation could extend beyond known sites of disease; these data corroborate the evidence of offering aggressive treatment in this setting and reinforces the idea of testing the biology of cancer first with chemotherapy, before doing an aggressive procedure. In the era of targeted therapy, when disease control and overall survival reached unprecedented rates in metastatic patients, it is worthy the discussion of methods for metastasis local control, particularly for patients in the oligometastatic setting, metachronous metastasis and negative node involvement.
Comparing this analysis along with molecular data, until now is not known if the prognosis of patients particularly with adrenal metastasis has any correlation with molecular alteration or any difference from those without involvement of this specific site as happens in case of bone metastasis and Kras mutation. In a study with 500 caucasian metastatic lung cancer patients (28.6% were Kras-mutated), a significantly higher tendency for intrapulmonary metastasis was identified in the Kras mutated group (35% versus 26.5%, p = 0.0125) compared to those with extrapulmonary ones 28. In contrast, those with liver (17% versus 33% (p < 0.001)) and pleural dissemination (16% versus 33% (p = 0.02)) had lower Kras mutation incidence. In this analysis, 17.4% had adrenal gland metastasis, but no relationship with Kras status and propensity to adrenal implants was observed in this study.
Adrenal metastasis control does not differ from the others, being necessary a cautious analysis case-by-case in order to improve quality of life and delay symptoms and the necessity to change therapy. The NCCN recommendation for localized treatment of isolated adrenal metastasis is grade 2B – that is, based on “lower-level” evidence; there is consensus that the intervention is appropriate36.
Conclusion
Adrenalectomy should be considered as a therapeutic option for patients with isolated adrenal metastases, especially with metachoronous presentation.
Although the median survival in the adrenal metastases synchronous group is comparable to palliative chemotherapy in patients with advanced disease, there was an expected five-year overall survival of 25%.
Review of the literature suggests different outcomes in patients with ipsilateral vs. contralateral adrenal metastasis. Adrenalectomy should be considered as a therapeutic option for patients with isolated adrenal metastases, especially with metacoronary presentation. Although the median survival in the synchronous group of adrenal metastases is comparable to palliative chemotherapy in patients with advanced disease, there was an overall expected five-year survival of 25%. The review of the literature suggests a difference in the results of patients with ipsilateral adrenal metastasis vs. contralateral. However, the current guidelines of the National Comprehensive Cancer Network do not make a distinction in the treatment of adrenal metastasis based on laterality.