Abstract
Background
The aim of this study is to evaluate the outcomes of patients submitted to diaphragmatic pacing, and the impact on quality of life of patients who chronically depend on mechanical ventilation, as well as the effectiveness of phrenic stimulation to eliminate the need for mechanical ventilation.
Methods
From 2010- 2014, 10 patients completely dependent on mechanical ventilation were operated upon, with the implantation of phrenic pacing device. The diagnoses were quadriplegia and congenital central hypoventilation syndrome (CCHS). All patients underwent bilateral approach to the phrenic nerves, by video-assisted thoracic surgery or mini-thoracotomy.
Results
All patientsstarted pacing 30-40 days post-operatively. The mean age of patients was 12.1 years (2-27 years range) with a median of ten years. Six patients (54.5%) were as old as ten years, and three (27.2%) were older than 20 years. Younger patients had CCHS and older ones were quadriplegic. All patients with CCHS (n = 4) were nine years old or younger while only two quadriplegic patients were in this age group.
Conclusions
Diaphragmatic pacing can provide improvement in the quality of life of patients who depend on mechanical ventilation, allowing freedom to conduct daily activities, lower respiratory infections, and tracheostomy decannulation.
Author Contributions
Academic Editor: Sasho Stoleski, Institute of Occupational Health of R. Macedonia, WHO CC and Ga2len CC, Macedonia.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2019 Rodrigo A. S. Sardenberg, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Since the invention of the electric stimulators by Otto von Guericke in 1663, some doctors have tried using electricity in medical experiments that ranged from simple attempts to reverse paralysis to revive the patients. The first suggested stimulation of the phrenic nerve for cardiopulmonary resuscitation was proposed by Cavallo in 17771. However, only after the invention of electrical stimulation in animals by Galvani in 1787 and Ure in 1818, electricity was applied in a recently hanged patient and watched diaphragmatic contractions1. The science of phrenic stimulation was studied in more detail by Duchenne during the cholera epidemic in 1827, Israel in 1927, and by Sarnoff in 1950 in polio victims2. Such reports remained crucial to allow for better understanding of phrenic stimulation in patients with diaphragmatic paralysis. In 1972, Glenn et al. developed the modern phrenic stimulation systems, using diaphragmatic stimulation sending transcutaneous radio-frequency signals into surgically-implanted electrodes3.
Electrical stimulation of motor neurons has been successfully applied to diaphragmatic pacing, and long-term conditioning is possible by stimulating the low frequencies.4
The integrity of both the phrenic nerve and diaphragm is a basic required criteria for potential candidates. The DP was introduced about 40 years ago by Glenn et al4 to dependent quadriplegic patients who were on mechanical ventilation, through phrenic stimulation. Initial experience in five quadriplegic patients using DP between 11 and 33 months was subsequently reported in 19844. The DP can provide a significant improvement in the quality of life of patients who depend on mechanical ventilation. In addition to optimizing lung function and reducing the incidence of pulmonary infections, this procedure may allow the patient to have improved phonation. It is also possible due to the restoration of the negative pressure during ventilation with DP, that even the olfactory sensation improves 7. Furthermore, phrenic stimulation can eliminate the need for mechanical ventilation in some cases for up to 24 hours / day.
Methods
All patients from 2010 to 2014 (n=10), submitted to implantation were retrospectively analyzed (Table 1). The device used in all procedures was the AVERY system (MARCK IV-USA).
Table 1. Patients characteristics| Patient | Age | Diagnosis | Time on mechanical ventilation (years) | Date of implantation | Status (time/day/pacing) |
| 1 | 23 | SCI | 3 | 2010 | 24 hs |
| 2 | 10 | SCI | 8 | 2011 | 24 hs |
| 3 | 27 | SCI | 5 | 2011 | - |
| 4 | 15 | SCI | 3 | 2012 | 24 hs |
| 5 | 2 | CCHS | 2 | 2012 | 6 hs |
| 6 | 5 | CCHS | 5 | 2013 | 6 hs |
| 7 | 9 | CCHS | 9 | 2013 | 12 hs |
| 8 | 10 | CentralApnea | 10 | 2013 | 12 hs |
| 9 | 9 | SCI | 6 mo | 2013 | 12 hs |
| 10 | 2 | CCHS | 2 | 2013 | 12 hs |
| 11 | 22 | SCI | 2 | 2014 | 24 hs |
The median age of all patients was 10 years (2-27 years). Only three (27.2%) were older than 20 years. Youngest patient (two years old) had CCHS, and the oldest (27 years) was quadriplegic due spinal cord injury (SCI). All patients with CCHS (n = 4) were less than nine years old, while only two quadriplegic patients were in this age group.
As most of them was treated in a different State - through the country, the surgical access varied. However, bilateral thoracoscopy was the main surgery performed (70%).
The electrodes are implanted adjacent to the phrenic nerve, through thoracic access that includes: thoracotomy, thoracoscopy, or robotic surgery. After the inventory of the thoracic cavity, the phrenic nerve is located along its path in the pericardium. It is important to maintain viable perineural tissue adjacent to the nerve, to maintain its blood supply9. The mediastinal pleura is then incised above and below the nerve and the electrode is placed in contact with the phrenic nerve through the metal portion. Distortion or nerve traction can cause post-operative injury or nerve dysfunction.
All patients started pacing 4-6 weeks after the implantation, in order to accomplish complete healing on the internal components and phrenic nerves. Diaphragm conditioning requires a gradual increase, and the patient should be carefully monitored while performing muscle training10. Normally, we started pacing 15-20 minutes/ day for the SCI patients, and a weekly increase according to each patient’s response was stablished. Once there is a good outcome, it took four to six months to complete mechanical weaning. For the CCHS patients, we started one hour/ day, and after that, one hour in time increasing weekly. On this population, mechanical weaning is shorter, because the diaphragm muscle is normal and the patients needed pacing only during sleep. Fluoroscopy, whenever needed as an additional assessment test, can be used to determine the maximum muscle contraction 11,12.
Results
There is a predominant incidence of quadriplegic patients (n = 7), which represents 63.6% of the sample, the same as reported in the literature. Due to the increase of people suffering of sequelae following an accident, we see a growing increase in the rate of patients with SCI (Table 2).
Table 2. Quadrilegic patients| Patient | Lesion level | Etiology | Electromyography | Current Status on initial evaluation |
| 1 | C1-C2 | SCI | no | hospital |
| 2 | C1 | Meningitis sequelae | no | home care |
| 3 | C1-C4 | SCI | yes | hospital |
| 4 | C2 | hypoxia | no | hospital |
| 5 | bulbar | Brain injury | no | home care |
| 6 | bulbar | ICB | no | hospital |
Of these, one patient presented with a brain damage caused by a perforating object. The patient displayed intracranial bleeding and compression with bulbar ischemia. Central apnea followed and surgery was then indicated since the phrenic nerves were functionally intact.
The other three patients in this group had SCI, and two with paralysis below C3, the location where the phrenic roots emerge. The patient with injury C2 -C4 required bilateral intercostal nerve grafts, since there was no intra-operative response to the phrenic stimulation. However, four months after the procedure, he had a hemorrhagic stroke and died. There was a predominance of patients who were still in hospital, and of these, all were in the intensive care unit (ICU). Although, all patients presently on home care were at some point in the ICU. Some remained there for many years and others for shorter periods up to six months. One patient was hospitalized for eight years.
On the quadriplegic group, six patients achieved complete mechanical weaning overtime. But one patient presented excessive weight gain, and the pacing was discontinued. One patient remained 12 hs/ day on pacing, due to slow time progress on diaphragm conditioning by the family. Of these, four patients could leave their home or hospital only pacing, and visit their friends, go back to school, shopping mall, beaches or elsewhere as they wish.
The CCHS group, although needing shorter time on pacing, presented problems due to the patient’s excessive manipulation on the receiver. Two patients had damage done to their systems, one to the left, and the other to the right receiver. This complication occurred on the first year after implantation. Overall, three patients could completely pace during sleep, and one did not get fully accustomed to the diaphragm contraction.
Discussion
All four types of devices available have the same components: receiver, antenna, and a radio transmitter electrode (Figure 1a and 1b). The receiver and internal components require a surgical implant while the radio transmitter and antenna are external devices.
Figure 1.(a-internal components/ b- external components)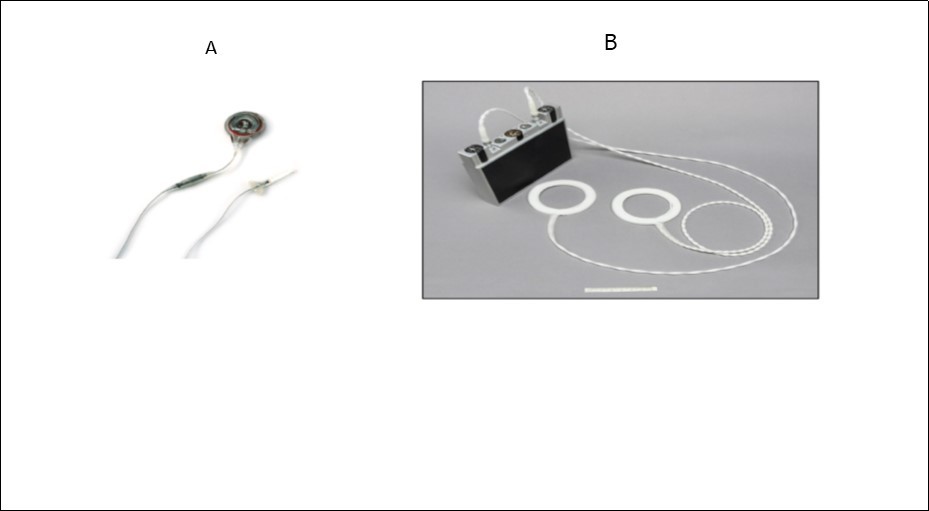
The receiver implanted subcutaneously transforms the radiofrequency signals from the radio transmitter into electrical impulses, which are transmitted to electrodes implanted along the phrenic nerve. The application of repetitive stimulus to the phrenic nerve causes smooth and rhythmic contraction of the diaphragm, causing air to enter the lungs.
The cervical access is considered by some as controversial, although easy to perform. Complications such as local infection associated with previous tracheostomy, conduction loss in the cervical branch, shoulder and upper limb pain due to stimulation of the brachial plexus, may occur8.
In our Patients, the Age Distribution can be Explained by the Following Reasons
The quadriplegic group is more heterogeneous in its incidence and prevalence;
Patients with CCHS have a respiratory condition from birth, a fact that leads to the search for a DP implant option earlier in life; patients with CCHS are usually in ICU since birth. Therefore, it is in the interest of both their family and physicians to seek a proper outcome to their medical condition.
Quadriplegic patients usually have significant atrophy of their diaphragm, although a progressive increase of pacing weekly is necessary for the prevention of muscle fatigue10. Patients with CCHS have a less atrophic diaphragm, therefore, a more aggressive training may be instituted.
When the patient’s family is involved in the patient’s care and when careful monitoring take place, the medical outcome is more successful13. Pulse oximetry is mandatory, as some patients with central hypoventilation may not have the drop in O2 and CO2 sensitivity.
Malfunction in the pacemaker system can occur inside or outside of the patient’s body. Internal issues account for 25% of cases per year according to a study conducted in children14,15.
Rarely the cause of DP failure is at the neuromuscular junction or level of the phrenic nerve. Such failure may occur in patients with diabetes, malnutrition, those who use anticholinesterase drugs, and patients who have hypermagnesemia and hypocalcemia. Infection at the surgical site of the receptor can also occur 16.
In a retrospective series of 12 quadriplegic patients who underwent implantation of DP, the authors suggested that diaphragmatic dysfunction was not associated with DP17.
Another study reviewed the case histories of 22 young patients, all with respiratory failure secondary to SCI, stroke, or CCHS, and showed that these patients had success in using the DP. Specifically, one patient did for 11 years, four patients 10 years, and 17 for five years18.
The evaluation of 50 patients with SCI treated with phrenic stimulation showed a 96% success rate. It also showed that these patients were able to support the phrenic stimulation with DP for at least 4 hours per day; in another set of 38 patients with amyotrophic lateral sclerosis, the implantation and use of DP delayed by two years the need for mechanical ventilation19. Yet, the area of greatest enthusiasm for diaphragmatic training is the resuscitation of the phrenic nerve using microsurgery techniques with intercostal nerve transfer. Such embodiment allows an increase in the spectrum of indications for implantation of the DP in patients that theoretically would not have been initially referred for such a procedure20. We emphasize that for such a clinical situation, the pacemaker should not be started. After grafting, pacing should start 9- 12 months after the procedure, giving the phrenic nerve enough time to regenerate.
According to studies included in the Ministry of Health’s guidelines, assistive technology is now considered a useful tool that allows patients with SCI at the cervical level to enjoy more independent and functional lives 21,22. While some options are rather expensive and therefore only accessible to a few, there exist others viable and low-cost options that also give substantial gains to patients. Each year in the world, 1.2 million people are killed in traffic accidents while another 15 million are significantly injured 1. In Brazil, the official number of deaths per year is 30.000, while the official number of wounded individuals that require hospitalization is 350.000. However, in reality, these numbers are much higher. If we consider the deaths that happen within the first 24 hours following the injury, there is a 50% increase in mortality. As a result, these accident victims occupy 55% of hospital beds and cost USD 3 billion annually. Of the injured, 100.000 patients have irreversible lesions 23,24,25.
High quadriplegia, or above C3, affects the respiratory medullary center but preserves the phrenic nerve integrity - a situation in which the diaphragmatic stimulation is possible. There is a correlation between the level of injury and the corresponding conditions as well as their effects on respiration.
CCHS is a rare disease with a worldwide estimated incidence of 1: 200,000 births 31,32. There are many patients with CCHS that need support 24 hours / day and who have not reached full weaning for reasons not yet well understood32,33. In São Paulo, we had a child who underwent surgery in August 2012 and remained 24 hrs. / day with only the DP. After a few months, the child used the device only during the sleep period. There are no criteria on the best age for surgery. In the United States, two CCHS neonates as young as 50 and 54 days underwent the procedure.
CCHS is classically characterized by normal breathing during hypoxia day and at night. Patients with the most severe form of the disease (20-30%) exhibit hypoxia both during the day and at night. Children with CCHS often have anatomical and physiological manifestations of a widespread regulatory autonomic dysfunction. A subset of patients develop abnormal neural crest disorders (ex. Hirschsprung Disease) and tumors such as neuroblastoma, ganglioneuroma, and ganglioneuroblastoma. Recently, some individuals with the nocturnal form of the disease, which have mutation in PHOX2B gene - characteristic of CCHS- , presented in adolescence and adulthood. Another form of this syndrome has been identified after clinical deterioration in neonates with normal Apgar. It is necessary to exclude cardiac and respiratory abnormalities, after thorough research and continuous monitoring26.
The term "Ondine´s Curse" was first used by Severinghaus and Mitchell in 1962 when they described three patients with central apnea acquired after brain stem surgery. Such patients could breathe when awake, but required mechanical ventilation to treat severe central sleep apnea. In 1970, Mellins et al. described the first case of a child who presented with signs and symptoms of congenital central hypoventilation syndrome, now called CCHS27.
The CCHS is a type of sleep apnea without neuromuscular specific primary disorder or respiratory disease. The brain fails to control the ventilation process properly. This disease comes from malfunction to the level of the respiratory center of the spinal cord. The etiology of the disease is variable. It can originate from an infection (e.g. encephalitis), cerebrovascular accident (especially trunk), cancer, and trauma28. In some cases, however, failure to control ventilation may be idiopathic, and probably represents a dysfunction that normally detects chemoreceptors hypoxia and hypercapnia27,28.
Over time, pulmonary vasoconstriction secondary to chronic hypoxia can lead to pulmonary hypertension and cor pulmonale 29. The diagnosis is confirmed by clinical and molecular genetic testing. All individuals with the phenotype of CCHS are heterozygous for the mutation in the gene PHOX2B that is present in 92% of cases 30.
The use of DP at night allows a safer sleep, dismissing concerns such as accidental disconnection of the tracheostomy, risk of electrical failure, and sudden ventilator stopage 34. There is also a significant decrease in noise since the unit stimulates the patient's own breathing35.
Conclusion
We conclude that DP can provide significant improvement in the quality of life of patients who are dependent on mechanical ventilation. In addition to optimizing lung function and reducing the incidence of respiratory infections, this procedure may allow the patient a proper phonation. It is also possible as the negative pressure during ventilation with DP is restored that the olfactory function will improve. Furthermore, the phrenic stimulation can eliminate the need for mechanical ventilation in some cases for 24 hours per day.
References
- 1.Sarnoff S J. (1972) Electrophrenic respiration in acute bulbar poliomyelitis, JAMA 1950,143: 1383,Glenn WWL, Holcomb WG, McLaughlin AJ, Total ventilatory support in a quadriplegic patient with radiofrequency electrophrenic respiration. , N Engl J Med; 286, 513.
- 2.Talonem P P, Baer G A, Hakkinen V, Ojala J K. (1990) Neurophysiological and technical considerations for the design of an implantable phrenic nerve stimulator. , Med Biol Eng Comput 28, 31-7.
- 3.Lieberman D A, Faulker J A, Craig A B, Maxwell A C. (1973) Perfusion and histochemical composition of guinea pig and human diaphragm. , J Appl Physiol 34, 233.
- 4.Glenn WW,Hogan JF,Loke JS,Ciesielski TE,Phelps ML,Rowedder R.(1984). Ventilatory Support by Pacing of the Conditioned Diaphragm in. , Quadriplegia,N Engl J Med,May3 310(18), 1150-5.
- 5.Adler D, Gonzalez-Bermejo J, DuguetA DemouleA, LePimpec-Barthes F, HurbaultA. (2009) Diaphragm pacing restores olfaction in tetraplegia. Eur Respir J,Aug, Epub 2009,Feb 27 34(2), 365-70.
- 6.Strippanayam S. (2002) Mother-daughter transmission of congenital central hypoventilation syndrome. , Am J Resp Crit Care Med 166, 367.
- 7.Miller J I, Farmer J A, Stuart W, Apple D. (1990) Phrenic nerve pacing of the quadriplegic patient. , J Thorac Cardiovasc Surg, Jan, discussion 39-40 99(1), 35-9.
- 8.Chervin R D, Guilleminault C. (1997) Diaphragm pacing for respiratory insufficiency. , J Clin Neurophysiol Sep 14(5), 369-77.
- 9.Adler D, Gonzalez-Bermejo J, Duguet A, Demoule A, F Le Pimpec-Barthes et al. (2009) Diaphragm pacing restores olfaction in tetraplegia. , Eur Respir 34(2), 365-70.
- 11.Weese-Mayer D E, Hunt C E, Brouillette R T, Silvestri J M. (1992) Diaphragm pacing in infants and children. , J Pediatr 120, 1-8.
- 12.Flageole H, Adolph V R, Davis G M. (1995) Diaphragmatic pacing in children with central alveolar alveolar hypoventilation syndrome. , Surgery 118, 25-8.
- 13.Glenn WWL, Phelps M L, Elefteriades J A. (1986) Twenty years of experience in phrenic nerve stimulation to pace the diaphragm. , PACE 9, 780.
- 14.Chervin R D, Guilleminault C. (1997) Diaphragm pacing in respiratory insufficiency. , J Clin Neurophysiol; 14, 369-77.
- 15.Elefteriades J A, Quin J A, Hogan J F. (2002) Long-term follow-up of pacing of the conditioned diaphragm in quadriplegia. , Pacin Clin Electrophysiol 25, 897.
- 16.Garrido-Garcia H, Alvarez Mazaira, Martin Escribano J, P. (1998) Treatment of chronic ventilatory failure using a diaphragmatic pacemaker. , Spinal Cord 36, 310.
- 17.Onders R P, Elmo M, Khansarinia S. (1984) Complete worldwide operative experience in laparoscopic diaphragm pacing: results and differences in spinal cord injured patients and amyotrophic lateral sclerosis patients. , N Engl J Med 310, 1150.
- 18.Krieger L M, Krieger A J. (2000) The intercostal to phrenic nerve transfer: an effective means of reanimating the diaphragm in patients with high cervical spine injury, plastic and reconstructive surgery. Plast Reconstr Surg. 105, 1255-61.
- 19.Ministério da Brasil. (2007) Ciência e Tecnologia, Tecnologia Assistiva [texto na Internet]. out. 11], Disponível em: http://www.mct.gov.br/index.php/content/view/ 18622.html , Brasília;
- 20. (2003) Organização Mundial da Saúde, CIF: Classificação Internacional de Funcionalidade, Incapacidade e Saúde. São Paulo: EDUSP;.
- 21.De Santo Madeya, S. (2006) The meaning of living with spinal cord injury 5 to 10 years after the injury. , West J Nurs Res 28(3), 265-89.
- 22.Masini M. (2001) Estimativa da incidência e prevalência da lesão medular no Brasil. , J Bras Neurocirurg 12(2), 97-100.
- 23.Mota Jr SC. (1999) A mudança da qualidade de vida profissional dos politraumatizados vítimas de acidentes de trânsito. Trabalho de conclusão de curso da Associação Brasileira de Medicina de Tráfego (ABRAMET). Maceió, novembro de.
- 24.Wyndaele M, Wyndaele J J. (2006) Incidence, prevalence and epidemiology of spinal cord injury: what learns a worldwide literature survey?. , Spinal Cord 44(9), 523-9.
- 25.Severinghaus J W, Mitchell R A. (1962) Ondine’s curse - failure of respiratory center automaticity while awake. , Clin Res 10, 122.
- 26.Mellins R B, Balfour HH Jr, Turino G M, Winters R W. (1970) Failure of automatic control of ventilation (Ondine’s curse). Report of na infant born with this syndrome and review of the literature. , Medicine (Baltimore) 49, 487-504.
- 27.Urushihara N, Nakagawa Y, Tanaka N, Uemura N.Yoshida A.Ondine's curse and Hirschsprung's disease:neurocristopathicsyndrome.Eur. , J Pediatr Surg 9(6), 430-2.
- 28.Urushihara N, Nakagawa Y, Tanaka N, Uemura N, Surg Yoshida A Eur J Pediatr. (1999) . 9(6), 430-33.
- 29.Hunt C E, Matalon S V, Thompson T R, Demuth S, Loew J M et al.Central hypoventilation syndrome: experience with bilateral phrenic nerve pacing in 3 neonates. , Am Rev Respir Dis 118(1), 23-8.
- 31.Mattera I, Bachetti T, Puppo F, M Di Duca, Morandi F et al. (2004) PHOX2B mutations and polyalanine expansions correlate with the severity of the respiratory phenotype and associated symptoms in both congenital and late onset Central Congenital Hypoventilation Syndrome. , J Med Genet 41, 373-380.
- 32.Repetto G M, Corrales R J, Abara S G, Zhou L, Berry-Kravis E M et al. (2009) Later-onset congenital central hypoventilation syndrome due to a heterozygous 24-polyalanine repeat expansion mutation in the PHOX2B gene. , Acta Paediatr 98, 192-195.
- 33.Weese-Mayer D E, Berry-Kravis E M, Zhou L. (2005) Adult identified with congenital central hypoventilation syndrome – mutation in PHOX2b gene and late-onset CHS [comment]. , Am J Respir Crit Care Med 171-178.