Abstract
Some strains of Foot and mouth disease virus (FMDV) are endemic in Egypt. The present study was performed on cattle and buffaloes (ages: 3 months up to 1.5 years old, of years 2015 and 2016), which were suffering foot and mouth disease (FMD). Sera and tissues samples were tested by different techniques including serum and virus neutralization tests (SNT, VNT), virus isolation and identification by tissue culture methods, Enzyme linked immune-Sorbent Assays (ELISA); and by the pathological and hematology techniques. The results showed the predominance of FMDV serotype O with the presence of serotypes SAT2 and A. The results showed the pathologic picture of FMD was similar regardless its specific subtypes, as apparently the studied strains produces same pathological and hematological changes. Microscopic examination reveals severe hydropic degenerations and necrosis in most affected organs, accompanied by significant changes in blood parameters which indicate severity and direct effects of FMDV on the hematopoietic system. These findings indicates the mode of pathogenesis of FMD virus in its way to exhibits the characteristic symptoms of illness. However, the investigation showed the presence of FMDV type O, A and SAT2 in the studied areas of delta governorates. It is important to focus on producing of vaccines which have only these serotypes as solution to get rid of the endemic behavior of FMDV in delta of Egypt.
Author Contributions
Academic Editor: Xintao Hu, National Cancer Institute
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2017 Samia Ahmed Kamal, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Foot and mouth disease (FMD) is an old and well known acute illness that characterize by fever, local and systemic lesions and short incubation periods (3-6 days). Foot and mouth disease virus (FMDV) (RNA genome of family Picornaviruses, genus Aphthovirus) cause high morbidity and low mortality of infected animals (cattle, buffaloes, swine, sheep, goats, and many other cloven-hoofed ruminants, wild ruminants of all species of deer and antelope as well as elephant, and giraffe). However, human being and higher primates are accidentally infected with non-fundamental outcomes. Carriers' animals are present and infections need only direct contact with infectious materials. FMD mortality rates are high in young animals that exceed 50%resulting from cardiac stroke (sudden deaths) but lower to 5% among adults. The pathological lesions of FMD develop 2-14 days post infection. The seven antigenic serotypes of FMDV have been identified by the cross immunity tests, unless they possess certain general structure that make them all cross react to variable strength in complement fixation tests. There is also a wide variation in serological specificity within each serotype. Each of FMDV serotypes appears to consist of several subtypes. FMDV capsid constructed of four distinctive types of structural proteins (SP; VP1- V4). However, other proteins were detected as results of infection and propagation of viruses refers to them as non-structural proteins (NSP) which have certain values in differentiating between infected and vaccinated subjects (DIVA). Experimental studies on susceptible cattle classify FMDV by serological procedures on blood to seven serotypes (strains): O, A, Asia-1 and the South African Territories (SAT) (types: SAT1, SAT2 and SAT3) , and type C. Subsequent analysis by reverse transcriptase (RT) polymerase chain reactions (PCR) technology (RT- PCR) showed the presence of subtypes for each serotypes (Coetzer & Tustin, 2004; Fauquet et al., 2005; World Organization for Animal Health, OIE, 2016; Alexanderson et al., 2002; Bastos et al., 1999; Beard & Mason, 2000).
FMD infected cattle showed a significant decrease (P<0.01) in the erythrocytes count (RBCs) and hemoglobin (Hb) content (Mohapatra et al., 2005; Dhanda and Gopalkrishna, 1948). However, it was reported that RBCs count in the FMD infected group was significantly decrease (P<0.05), while MCV values were significantly higher; these findings may indicate anemia (Gurbuz et al., 2004). Andin FMD infected sheep showed significant increase in PCV (Al-Rukibat et al., 2015). Also, found that PCV was higher in FMD infected animals than in healthy ones (Elitok et al., 2005).
The aims of this study are to investigate the current situation of FMD in cattle and buffalo in Egypt, to investigate clinical and histopathology of FMDV and showing new aspects that may help in controlling this disease.
Materials and Methods
Animals:
1-Animals enrolled in virology studies: The present study performed on 100 apparently sick cattle and buffaloes from different ages (from 3 months up to 1.5 years old) in different localities in delta governorates.
Samples for virological investigation; a total of 300 samples (sera, tissues) were collected from cattle and buffaloes, 60 buffaloes and 40 cattle ages 3 months up to 1.5 years, animals included in this study were not vaccinated against FMD.
2- Animals enrolled in clinical pathology studies: Fifty cattle aged between 1 and 1.5 years were used in this study and divided into two groups. First group (gp-1) forty (40); diagnosed cattle with FMD. The second group 10 cattle were tested free from FMD were used as control.
Samples for haematology; peripheral blood samples collected from the jugular vein into EDTA treated tubes used to establish total red blood cells (RBCs), haemoglobin concentration (Hb), packed cell volume (PCV), erythrocyte indices mean corpuscular volume (MCV) mean corpuscular haemoglobin (MCH) and mean corpuscular hemoglobin concentration (MCHC), total white blood cell and differential leucocytic count manually (Feldman et al., 1986).[12]
Statistical analysis:
The obtained data were analyzed by using t- student test according to SPSS 14 (2006).[13]
3- Histopathology: Tissues specimens collected (5 mm diameter, kept in 10 % formalin solution) from the Dead animals that suffering FMD were undergoing pathological examination. Only the positive FMD tissue specimens were undergoing pathological tests. However, about 50 tissues samples were collected from dead cattle and buffaloes aged 6 months to 1year old. These samples are liver, tongue, heart, kidneys, skin, spleen, and intestine. The samples were processed to prepare 5 micron thickness of histopathological slides, and then stained by H&E (Hematoxlyin and eosin), special stains (Hematoxylin and fuchsin). The slides were examined by light microscope. The procedures were referenced according to (Clayden, 1971), (Lillie &Fullmer, 1976).
3- BHK Cell lines: Darby hamster kidney cells (BHK-21) were prepared and provided by VACSERA Egypt. The BHK cells were used for adaptation of FMDV, virus titration as well as serum neutralization test (SNT). Figure 14
6- Tissue specimens for FMDV isolation in tissue culture:Skin biopsies, comprising epidermis and dermis of the ulcerated mouth lesions, were collected from local cattle and buffaloes. Also, samples and tissues specimens from dead animals were collected aseptically in 15 ml sterile tubes and stored at -20°C until used. The procedures were performed according to Payment &Trudel, 1993.
5- Infectious FMDV: The viruses used in this study are the locally isolated FMDV strains (O/ ME-SA/ Sharqia-72), (SAT2/V11/ Ghb-12), (A/ Africa/ G-1V) that identified by reference laboratory of FAO (WRLFMD) in the Institute for Animal Health, Pirbright, England.
6- Titration of FMDV and determination of infectious dose (ID50): Titration of the virus was according to Reed and Muench’s method. (Reed and Muench, 1938)
7- Virus Neutralization test:This test was used to determine the neutralizing antibodies of FMDV in sera samples of infected animals that able to neutralize FMDV and prevent infection of culture cells. (Payment &Trudel, 1993)
9- Manual ELISA method for Detection of anti-FMDV antibodies in blood of cattle and buffaloes:
this test was performed according to (Payment &Trudel, 1993)
The anti-FMDV antibodies present in serum dilution react with FMDV coated on micro-titer-plates. Unbound reactants are then washed out subsequently, peroxidase conjugated antibodies to (cattle and buffaloes) are added, followed by incubation period and washing, then substrate is added and the peroxidase conjugate bound to the anti- FMDV present in the serum samples reacts with the substrate producing a color. The enzymatic reaction and the resulting color is measured by ELISA reader at 492 nm.
8- PrioCHECK FMDV NS Antibody ELISA Kit (Thermo Fisher Scientific, Inc):It is an ELISA that detects antibodies against the highly conserved non-structural (NS) protein of the FMD virus. The test can therefore be used for all species.
5- FMDV antigen detection and serotyping: Enzyme linked immunosorbent assay (indirect sandwish ELISA): (Instituto Zooproflattico Sperimental della Lombardia dell ‘Emilia Romagna (IZSILER), Brescia, Italy; Ferris et al., 2011.)
The component of indirect sandwich ELISA kit design to detect FMDV serotypes O, A, C, and Asia 1, and SAT-2. {BDSL, IAH, Pirbright, UK; Ferris and Dawson, 1988}.The product is produced by the reference laboratory of FAO, IZSLER: Brescia, Italy.
Results
Clinical symptoms:
The present study was performed on 100 clinically diseased cattle and buffaloes of different ages (from three months to 1.5 years old) from different localities in delta governorates years 2015 and 2016. The animals suffered fever (40oC - 41oC), lameness, lacrimation, excessive salivation, bloody mouth discharges, oral erosions, different lesions (vesicles, ulcerations, salivation), foot lesions (ulcerations on the inter-digital space with lameness), teat lesions (vesicles and ulcerations on the teat with difficulty on milking due to pain), and sudden death mostly in young animals. Mortalities were recorded high among cattle and buffaloes less than 3 months old. Figures (1-5).
Figure 1.Animals infected with FMD, showed lameness, arched back, emaciation and general fatigue
Figure 2.buffalo calf (1 year old) showed lacrimation
Figure 3.buffalo calf (1 year old) showed fever
Figure 4.buffalo calf (1 year old) showed salivations tinged with blood
Figure 5.cattle (1 year old) showed lesions on mouth in the form of ulcerations accompanied by excessive salivations.
Post-mortem examination:
Macroscopic lesions of FMD in young animals are in the myocardium, liver, kidneys, intestine, tongue, mouth cavity, nostrils, blood vessels and skin. Lesion of heart are forming whitish streaks on myocardium separated by dark and congested areas in longitudinal shapes giving the pathgnomonic lesion of FMD in the young infected animal. Skin showed vesicles and erosions usually seen in the areas of soft tissues: mouth, muzzle, nostrils, foot, udder, vagina and anal area, base of horns, conjunctiva.
Figures 6.kidneys (dead cattle less than 1 year old) showed severe hydropic degeneration of renal tubular epithelium accompanied by dilated renal tubules, vesicles formations and casts (arrows). (H&E, X 100)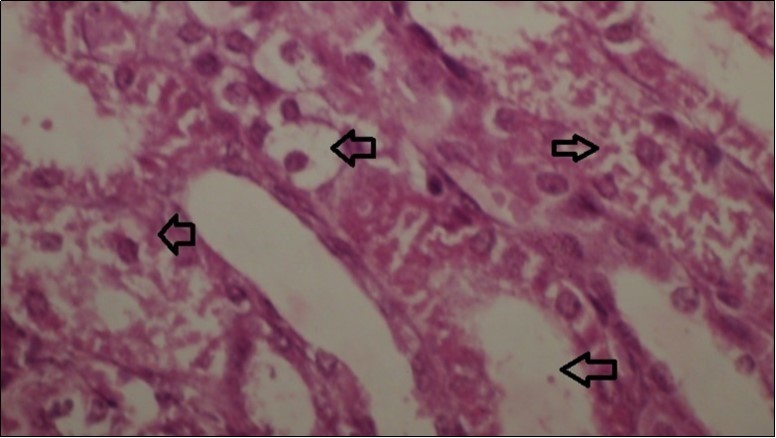
Figures 7.kidneys (dead cattle less than 1 year old) showed severe hydropic degeneration of renal tubular epithelium accompanied by dilated renal tubules, vesicles formations and casts (arrows). (H&E, X 60)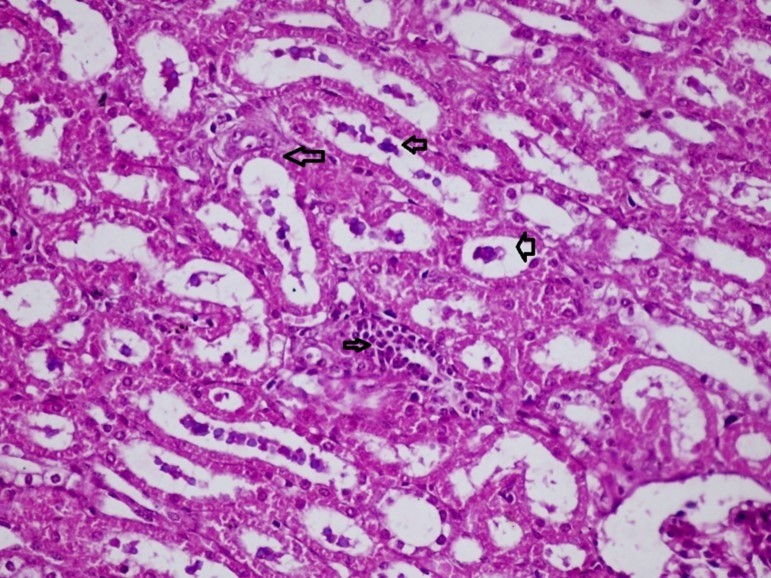
Figure 8.spleen (dead cattle less than 1 year old) showed severe depletion of lymphocytes with necrosis of endothelial lining or splenic arterioles (arrows). (Hematoxylin and fuchsin X 60)
Figure 9.Tongue (dead cattle less than 1 year old) showed vesicular nuclei of stratum corium epithelium which suffering hydropic degeneration. Esinophlic intranuclear inclusions were seen surrounded by hallow zone(arrows). (Hematoxylin and fuchsin X 60)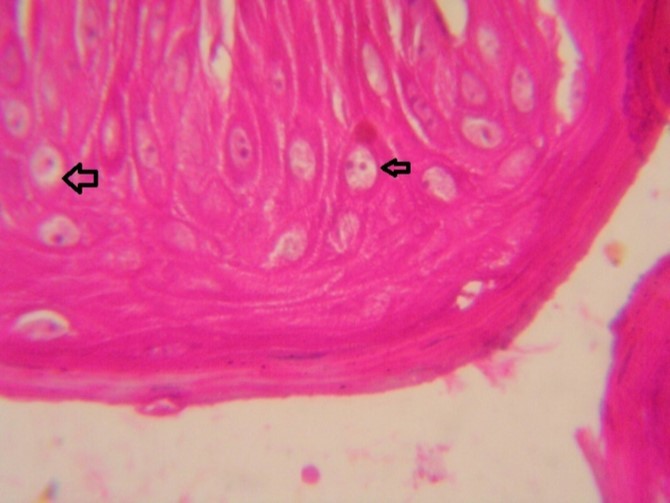
Foot and mouth disease virus (FMDV):
A field strains (serotypes O, A& SAT 2) of FMDV were isolated during the outbreaks of the disease in Egypt by Animal Health research Institute, Egypt. These virus strains were identified by the FAO World Reference Laboratory for Foot-and-Mouth Disease (WRLFMD) of The Pirbright Institute, England. They were used for cell culture adaptation and propagation, serum neutralization test and as reference guide of other tests.
Figure 10.Heart (dead cattle less than 1 year old) showed area of extravasated blood with few inflammatory WBCs (arrows). (Hematoxylin and fuchsin X 40)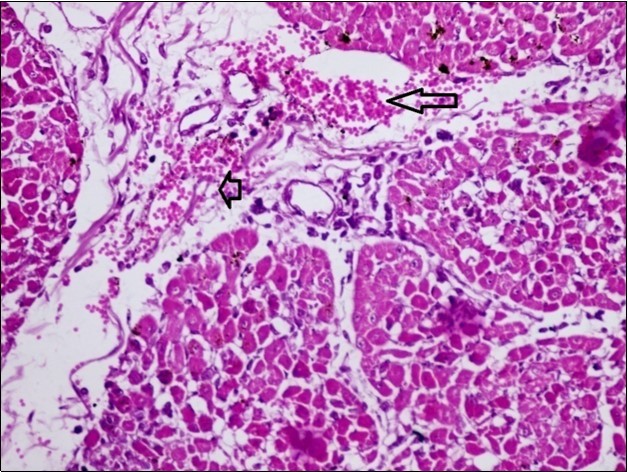
Figure 11.heart (dead cattle less than 1 year old) showed vesicular nuclei of myocytes which suffering hydropic degeneration. Some inflammatory cells (neutrophils, esinophils, lymphocytes) substitute an area of necrosis inside myocardium bundles (arrows). (H &E, X 40)
Reed &Muench methods: calculating the 50 % endpoint of virus activity (TCD50):
The data in table (1) illustrates the procedure used in the Reed-Muench method for calculating the accumulated values of infected and uninfected cell culture tubes. The dilution which would be expected to yield 50% positive (CPE) tubes is seen to lie between 10-3 and 10 -4 and will, in fact, be located at the proportionate distance from 10-3. The necessary proportionate distance (PD) of the 50 % infectively end point is obtained. The dilution of reference virus selected for the neutralization test is usually 100 times stronger than the 50% end point.
Table 1. Virology Results| Sample no. | animal | Serological tests | FMDV Isolation On BHK | FMDV-Antigen Detection (Ezler) | symptoms | |||||||||
| serum | tissues | cattle | buffaloes | SNT | ELISA Anti-FMDV | ELISA Priocheck | ||||||||
| Anti-NSP | O | A | SAT-2 | |||||||||||
| +ve | -ve | +ve | -ve | +ve | -ve | |||||||||
| 10 | 6 | + | 10 | 10 | 8 | 2 | 5 | + | Fever, off food, ulcers on gum, sudden deaths | |||||
| 15 | 11 | + | 8 | 8 | 3 | 12 | 10 | + | salivations, ulcer on mouth | |||||
| 19 | 15 | + | 18 | 10 | 9 | 7 | 12 | 11 | + | Fever, off food, ulcers on gum, sudden deaths | ||||
| 1 | 1 | + | 1 | 1 | 1 | 1 | + | fever | ||||||
| 33 | 13 | + | 22 | 29 | 17 | 16 | 13 | + | Fever, off food, ulcers on gum, sudden deaths | |||||
| 12 | 9 | + | 9 | 9 | 5 | 8 | 9 | + | Fever, off food, ulcers on gum, sudden deaths | |||||
| 21 | 21 | + | 21 | 21 | 11 | 16 | + | Salivations, lamness, arched back, off food | ||||||
| Control | Control | 10 | 10 | + | + | 4 | 6 | 10 | + | + | + | Reference confirmed infection | ||
| +ve | +ve | |||||||||||||
| Control | Control | 10 | 10 | - | - | - | - | - | - | - | - | - | - | Reference confirmed free |
| -ve | -ve | |||||||||||||
Viral isolation results:
Samples used for isolation were collected from animals which showed severe clinical symptoms and FMDV positive infections with FMDV by using various serological examination. FMDV isolation trials from tissue samples were using BHK-21 cell lines. All samples were collected during active infection and from feverish animals (i.e. during circulation of viruses in blood; vireamia). Tissue culture infected cells showed cytopathic effects (CPE) in the form of rounding of cells, abnormalities on cytoplasm and cytolysis.
Figure 12.liver (dead cattle less than 1 year old) showed hepatocytes suffering hydropic degeneration. And necrosis (H&E, X 60).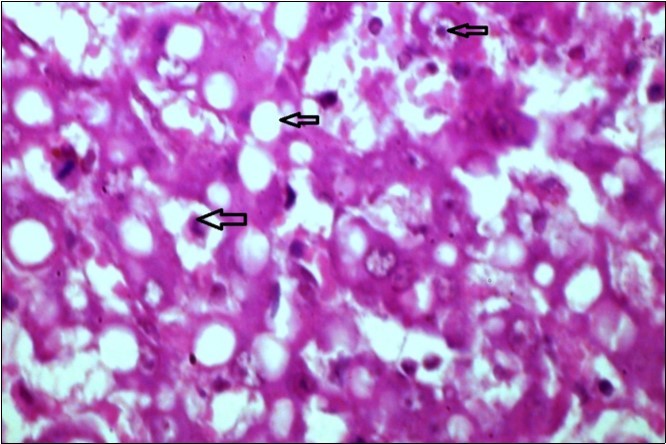
Figure 13.Small intestine (dead cattle less than 1 year old) showed hydropic degeneration, congested blood vessels, inflammatory cells (H&E, X 30).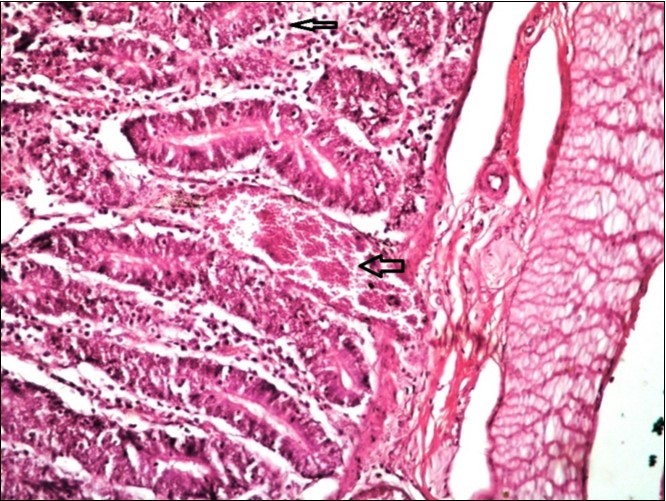
Figure 14.FMDV infected BHK-21 cells culture and Cyto-Pathic Effect (CPE) in the form of cells rounding and detachment, granularity of the cytoplasm and complete cell lysis.
The cultures showed CPE were subjected to test by antigen detection ELISA (IZLER), the results were shown in table (2).
Table 2. Determination of virus titer (TCD50): Reed and Muench method| Virus dilutions | Infected culture | Uninfected culture | Accumulated values | Ratio infected | Percent infected | |
| infected | Uninfected | |||||
| 10-1 | 6 | 0 | 19 | 0 | 19/19 | 100 |
| 10-2 | 6 | 0 | 13 | 0 | 13/13 | 100 |
| 10-3 | 4 | 1 | 7 | 1 | 7/8 | 87 |
| 10-4 | 3 | 3 | 2 | 4 | 2/6 | 33 |
| 10-5 | 0 | 5 | 0 | 9 | 0/9 | 0 |
Conventional ELISA:
This test performed to detect anti-FMDV antibodies in sera samples. The results were shown in table (2). However, it was observed that sera gives positive reaction in this test is sometimes but not always gives positive reaction with PrioCHECK ELISA.
Virus neutralization test (SNT):
Results were shown in table (2). It was observed that the Sera samples which exhibits positive reactions by conventional ELISA were giving positive reactions by SNT test.
The PrioCHECK FMDV NS ELISA:
It is an ELISA that detects antibodies against the highly conserved non-structural (NS) protein of the FMD virus. The test can therefore be used for all species. The positive reactions mean the presence of infectious agent of FMDV. The indicate anaemia. Al-Rukibat et al., (2015) recorded significant increase in PCV in FMD infected sheep. Also, Elitok et al., (2005) found that PCV was higher in FMD infected animals than in healthy free ones. However, from our histopathology of kidneys of infected animals it was observed that it suffers degenerations and focal necrosis in both cortex and medulla, and as erythropoietin is a vital substance produced by the kidneys and is responsible for synthesis of RBCs (erythropoiesis), one could explain main reason for anemia in FMD infected animals (Jubb et al., 1991).
Table 3. Erythrogram of affected cattle with FMD (mean values ±SE)| Parameters | FMD infected group | Control group |
|---|---|---|
| RBCs (×106/Ml) | 7.11±0.13* | 10.71±0.21 |
| Hb (g/dl) | 8.11±0.23* | 12.17±0.13 |
| PCV (%) | 24.93±0.81* | 36.9±1.16 |
| MCV (Fl) | 35.07±1.17 | 34.45±1.17 |
| MCH (Pg) | 11.41±0.58 | 11.36±0.58 |
| MCHC (g/dl) | 32.54±0.31 | 32.28±031 |
| Parameters | FMD group | Control group |
| WBCs | 12.30 ± 0.27* | 10.15±0.36 |
| Neutrophils | 2.76 ± 0.40 | 3.51±0.18 |
| Lymphocytes | 8.97±0.33* | 6.37±0.34 |
| Monocytes | 0.30±0.02 | 0.32±0.08 |
| Eosinophils | 0.27±0.02 | 0.31±0.05 |
| Basophils | 0±0 | 0±0 |
Leucogram of FMD affected animals showed leukocytosis with lymphocytosis. Our work is in accordance with Mohapatra et al., (2005) who stated the same results in all ages of infected animals with FMD. Elitok et al., (2005) 11 found that leucocyte count in FMD infected sheep was higher than normal level (over 10.000 cells/ul), mean neutrophil count (22.2%) less than control (34.7%), lymphocytosis was (75.4). Al-Rukibat et al., (2015) mentioned that there was a significant increase in eosinophil percentage in FMD positive sheep. The increase in total leucocytes count was mainly due to increase in lymphocytes; and this findings are in agreement with Ghanem & Abd El-Hamid (2016).
Conclusions
It could conclude that the virology investigations show the predominance of FMDV serotypes O, A, SAT2. However, the clinical and pathological studies showed the mode of pathogenesis of FMD inside infected animals.
References
- 2.Fauquet C, Fauquet M, M A.. 2005.Virus Taxonomy: VIII Report of the International Committee on Taxonomy of Viruses .
- 4.Alexandersen S, Zhang Z, A I Donaldson. (2002) Aspects of the persistence of foot-and-mouth disease virus in animals—the carrier problem. , Microbes Infect 4, 1099-1110.
- 5.A D Bastos, H J Bertschinger, Cordel C, Vuuren C D van, Keet D et al. (1999) Possibility of sexual transmission of foot-and-mouth disease from African buffalo to cattle. , Vet. Rec 145, 77-79.
- 6.C W Beard, Mason P. (2000) Genetic determinants of altered virulence of Taiwanese foot-and-mouth disease virus. , J. Virol 74(2), 987-991.
- 7.Mohapatra A P K, A K Kundu, P C Bisoi, B M Prusty. (2005) Haematological and biochemical changes in crossbred cattle affected with Foot and Mouth disease. Indian veterinary journal. 82(2), 141-144.
- 8.M R Dhandra, U R Gopalakrishna. (1948) Foot and Mouth disease of animals in India. INAR. , New Delhi
- 9.Gurbuzg H B G, G H Vehbi, Mehmet C T L. (2004) Alterations in some hematological parameters in cattle suffering from foot and mouth disease. , Turk J Vet Anim Sci 28, 723-727.
- 10.R K Al-, Hananeh W, Athumneh H. (2015) Comparative study of Foot and Mouth disease in apparently healthy Awassi sheep using different diagnostic tests in Jordan. , Veterinary Science Development 5, 5633-28.
- 11.Elitok B, Ranany Z, O M Elitok, Unver O. (2005) Haematological, biochemical and histopathological Aspects of spontaneous ovine and caprine foot and mouth disease. Indian Vet. , J 2(2), 137-140.
- 12.B F Feldman, L J Zink, N C Jain. (1986) Schalm’s Veterinary Hematology. Text book. Lippincott Williams.
- 13.Levesque R. (2007) SPSS Programming and Data Management; A guide for. SPSS and SAS users (4thEd.) , Chicago, Illinois: 1-56827.
- 14.E C Clayden. (1971) . Practical section cutting and staining. 5th ed., Churchill Livingstone , Edinburgh and London .
- 15.R D Lillie, H M Fullmer. (1976) Histopathology Technique and Practical Histochemistry.4th edition. , NY., USA
- 17.L J Reed, Muench H. (1938) A simple method of estimating fifty percent endpoints. , The American journal of hygiene 27, 493-497.
- 20.G, R H Fagg. (1965) Isolation of variants during passage of a strain of Foot and Mouth Disease virus in partly immunized cattle. , J. Hyg 63, 357.
- 21.G. (1965) Isolation of variant strains of foot and mouth disease virus propagated in cell cultures containing antiviral sera. , J. Gen. Microbiol 41, 135.
- 22.Davie J.1962.The classification of subtype variants of the virus of Foot- and-. , Mouth disease. Bull. Office Intern. Epiz 57, 962.
- 24.A R Habashi, Samia Ahmed Kamal, S A Ahmed, Basyouni Y, B I Agag. (2012) Epidemiological profile for the prevalence of Foot and Mouth disease virus. , Zag. Vet. J 40, 33-40.
Cited by (1)
- 1.Kim Mun-Hyeon, Yun Seon-Jong, Kim Yeon-Hee, Lee Hyang-Sim, Kim Ji-Yeon, et al, 2020, Evaluation of Quality Control Methods for Foot-And-Mouth Disease Vaccines by High-Performance Liquid Chromatography, Pathogens, 9(3), 194, 10.3390/pathogens9030194
