Abstract
Background:
The diagnosis and treatment outcomes of Non- Hodgkin Lymphoma’s (NHL) in resource poor countries in the absence of routine molecular studies and immunohistochemistry is challenging.
Methods:
A retrospective review of case folders of NHL patients aged13 years and above. Information obtained from the case folders included age, sex, histological subtype, subtypes using the Working Formulation and WHO classifications. Treatment given and follow up information were also evaluated.
Results:
A total of 279 cases of NHL were identified within the study period. The mean age of the patients was 48.8 ± 17.0 years. The male to female ratio was approximately 1.5:1. The majority of cases seen (53%) were diffuse large B- cell lymphoma. Chronic lymphocytic leukaemia/ small lymphocytic lymphoma (22.2%) was the next most common subtype. Other sub types seen, in order of frequency, included diffuse mixed cell lymphoma (6.4%), gastric lymphomas (3.9%), mediastinal B- cell lymphoma (2.9%), Burkitt’s lymphoma (1.8%), splenic marginal zone B-cell lymphoma (1.1%), lymphoblastic lymphoma (1.1%), mucosa- associated lymphoid tissue (MALT) type B- cell lymphoma (0.7%) and follicular lymphoma (0.7%).
Conclusion:
This study provides an overview of the distribution of NHL subtypes and their outcomes in a resource constrained setting. Immunohistochemistry, cytogenetics and specific molecular studies which are important in characterization of NHLs, should be made affordable and accessible in low income countries.
Author Contributions
Academic Editor: Krzysztof Roszkowski, Nicolaus Copernicus University, PL. Department of Oncology, Radiotherapy and Gynecologic Oncology
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2017 Dei-Adomakoh YA, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Non- Hodgkin lymphomas (NHL) are clonal malignant diseases that arise from the lymphoid system1, 2It accounts for 4% of all cancers and represents approximately 10% of all childhood cancers1. It accounts for 14–19 cases per 100,000 population in the West.1, 2 During the past few decades, incidence rates of NHL have risen3 with an estimated rate of increase of 3-4% per annum In Europeans.4, 5This has been attributed to an increase in mean age of the population, improvements in diagnosis, the HIV pandemic and immunosuppressive therapy.6 NHL is found predominantly in males with a male to female ratio of 3:2.7, 8
Especially in Sub-Saharan Africa, NHL classification, diagnosis and treatment present with a lot of challenges. Some of the classification schemes used include: the Rappaport, Lukes-Collins, Kiel, Working Formulation, REAL and World Health Organization (WHO). These classifications provide clinicians with diagnostic and prognostic information for the various subtypes of NHLs. For this study the working formulation and WHO classifications (few cases) were used.
Korle - BuTeaching Hospital (KBTH) where the study was conducted,is the largest hospital in Ghana with a bed capacity of 2000 and one of two major tertiary referral centers in the country. The Haematology department renders outpatient, inpatients and day care services to patients with a wide range of haematological disorders. On the average 50 patients are attended to on out patient basis weekly. The department has over 6000 patients in its database.
The stimulus for this study was the fact that Subtypes and treatment outcomes of adolescent and adult NHL in the foremost referral hospital in Accra, Ghana has not been done before.
Materials and Methods
A retrospective review of case folders of all patients aged13 years and above, diagnosed with NHL between January 1, 2008 and October 31, 2013, at the haematology department of the KBTH, Accra, Ghana.
Data Collection
Detailed history and clinical information relating to age, sex, histological subtype, subtypes using the working formulation, immunohistochemistry (where available), treatment given and outcomes were retrieved from case folders of eligible study participants.
Data Analysis
Data were captured using Microsoft Excel 2007 Database. Analysis was done with Statistical Package for the Social Sciences (SPSS) software version 20. Data were analyzed by simple descriptive statistics (i.e., proportions, ratios and percentages) and summarized in tables.
Results
Out of a total of 279 adolescent and adult cases of NHL patients folders reviewed, the mean overall age was 48.8 ± 17.0 years with minimum age of 13 years and maximum 87 years. There were 156 males (55.9 %) and 123 females (44.1 %) with a male to female ratio of approximately 1.5:1. The age and sex distribution of the adolescent and adult NHL is shown in Figure 1 and this showed the most vulnerable age bracket of NHL in both sexes to be 50-69 years. The majority of cases seen (53%) were diffuse large B- cell lymphoma. Followed by chronic lymphocytic leukaemia / small lymphocytic lymphoma (22.2%). Diffuse mixed cell (6.4%) was the third most common and the least common NHL being mycosis fungoides (Table 1). Intermediate grade NHLs constituted the greatest proportion of all NHLs using the working formulation (Figure 2).
Figure 1.Sex and age distribution of the NHL patients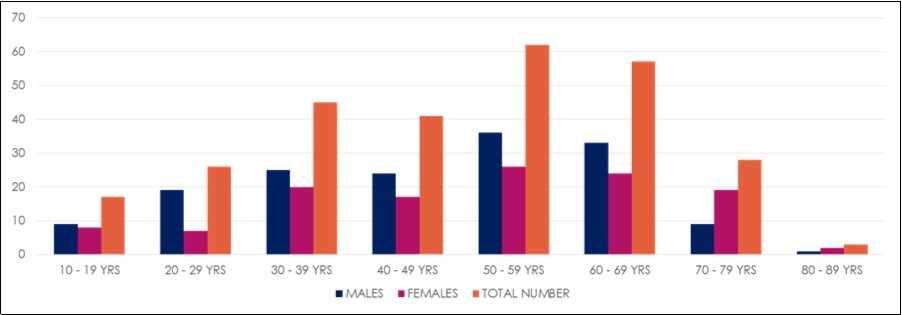
Figure 2.Distribution of the histologic category of NHL according to the Working Formulation
| Subtypes of NHL | Sex | Total | |
|---|---|---|---|
| Male | Female | ||
| Diffuse Large Cell NHL | 85 | 64 | 148 |
| Splenic Marginal Zone Lymphoma | 2 | 1 | 3 |
| Diffuse Mixed | 9 | 9 | 18 |
| Burkitts Lymphoma | 2 | 3 | 5 |
| Lymphoblastic Lymphoma | 3 | 0 | 3 |
| Diffuse Small Cell NHL | 1 | 5 | 6 |
| Mediastinal NHL | 7 | 1 | 8 |
| Chronic Lymphocytic Leukemia | 20 | 22 | 42 |
| Follicular Large Cell With Diffuse Areas | 1 | 1 | 2 |
| Gastric Lymphoma | 5 | 6 | 11 |
| Small Cell Lymphoma | 15 | 5 | 20 |
| Mycosis Fungoides | 1 | 0 | 1 |
| Others | 5 | 4 | 9 |
| Total | 156 | 123 | 279 |
The age bracket shows 10 year-age intervals cross-tabulated against the frequency of new cases of Non- Hodgkin lymphoma per age group (Figure 1). Whereas significant cases (43 cases; 15.4%) were in the young population (below 30 years of age); the incidence increased gradually in the adult population with over half the cases (148 cases) falling between the ages of 30 and 69 years; after which there was a drastic decline in the elderly (69 years and above). There was a peak incidence at age interval of 50−69 years Figure 3 shows an increasing trend in number of NHL cases diagnosed over 5 years.
Figure 3.Year diagnosed and sex specific ratio of NHL patients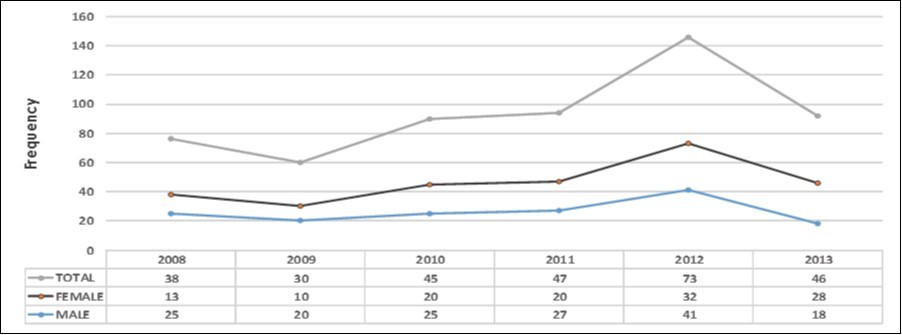
Based on the Working Formulation classification with broad histologic categories of low-, intermediate-, and high-grade lymphomas, this study showed that out of the 279 patients, 83 (29.7%) had low grade NHL, 170 (60.7%) intermediate and 26(9.4%) had aggressive/ high grade NHL (Figure 2).Though, immunohistochemistry is not routinely done for patients due to the cost and lack of facilities, seventeen (6%) cases were done and report-based initial diagnosis and immunohistochemistry interpretations were correct for most cases (Table 2). However four required a change in diagnosis and treatment.
Table 2. Comparison of Histological diagnosis by working formulation to immunohistochemistry| NUMBER OF CASES | WORKING FORMULATION | IMMUNOHISTOCHEMISTRY RESULTS | DIAGNOSIS BY IMMUNOHISTOCHEMISTRY |
| 7 | DIFFUSE LARGE CELL | CD20 + | DIFFUSE LARGE B-CELL LYMPHOMA |
| 1 | DIFFUSE LARGE CELL | CD20+; BCL-2+; BCL-6+; CD23+ | FOLLICULAR LYMPHOMA TRANSFOMING TO DIFFUSE LARGE B- CELL LYMPHOMA |
| 1 | DIFFUSE MIXED CELL | CD20+ | DIFFUSE LARGE B- CELL LYMPHOMA |
| 1 | DIFFUSE SMALL CELL | CD20+ | DIFFUSE LARGE B- CELL LYMPHOMA |
| 1 | DIFFUSE LARGE CELL | CD30+; LCA+; CD3-; CD20-; CD79a- | ANAPLASTIC LARGE CELL LYMPHOMA |
| 1 | SMALL CELL LYMPHOMA | CD3-; CD23-; CD20+; CYCLIN-D1-; CD5± | B- CELL SMALL LYMPHOCYTIC LYMPHOMA |
| 1 | MANTLE CELL LYMPHOMA | CD20+ | MANTLE CELL LYMPHOMA |
| 1 | MEDIASTINAL NHL | CD3+; CD7+ | T- LYMPHOBLASTIC LYMPHOMA |
| 1 | DIFFUSE LARGE CELL | CD20-; ALK- | PLASMABLASTIC LYMPHOMA |
| 1 | DIFFUSE LARGE CELL | CD20-; CD10-; BCL-2-; CD3+; Ki67+ | AGGRESSIVE T- CELL LYMPHOMA |
| 1 | DIFFUSE LARGE CELL | CD3 (WEAKLY +); CD5+ | PERIPHERAL T- CELL LYMPHOMA |
| TOTAL: 17 | |||
The study looked at first, second and third year outcomes for patients with NHL in a resource poor setting. Table 3 and Table 4 shows treatment outcomes in the first two years. In the first year two hundred and twenty 220 (78.9%) patients achieved clinical remission, forty eight 48 (17.2%) with poor response, two 2 (0.7%) patients did not respond to first line chemotherapy and were given second line. Figure 4 shows the three year follow up of NHL cases post treatment. Survival rate of NHL in the first year was found to be high (98.6%). Subsequent years showed a decline in follow up cases and thus a decrease in survival rates.
Table 3. FIRST YEAR OUTCOME FOR ALL NHL SUBTYPES| TYPE OF CHEMOTHERAPY GIVEN | CHOP | CVP | R-CHOP | CHLORAMBUCIL + PREDNISOLONE | CODOX-M/ IVAC | TOTAL |
| INDOLENT TYPE NHL | ||||||
| CR | 37 | 34 | 2 | 2 | 0 | 75 (26.9%) |
| PR | 3 | 1 | 0 | 0 | 0 | 4 (1.4%) |
| NR | 1 | 0 | 0 | 0 | 0 | 1 (0.4%) |
| LTF | 2 | 0 | 0 | 0 | 0 | 2 (0.7%) |
| TOTAL | 43 (15.5%) | 35 (12.5%) | 2 (0.7%) | 2 (0.7%) | 0 (0.0%) | 82(29.4%) |
| INTERMEDIATE TYPE NHL | ||||||
| CR | 120 | 3 | 9 | 0 | 0 | 132 (47.3%) |
| PR | 32 | 4 | 0 | 0 | 0 | 36 (12.9%) |
| NR | 1 | 0 | 0 | 0 | 0 | 1 (0.4%) |
| LTF | 2 | 0 | 0 | 0 | 0 | 2 (0.7%) |
| TOTAL | 155 (55.6%) | 7 (2.5%) | 9 (3.2%) | 0 (0.0%) | 0 (0.0%) | 171 (61.3%) |
| AGGRESSIVE TYPE NHL | ||||||
| CR | 9 | 0 | 3 | 0 | 1 | 13 (4.65%) |
| PR | 12 | 0 | 0 | 0 | 1 | 13 (4.65%) |
| NR | 0 | 0 | 0 | 0 | 0 | 0 (0.0%) |
| LTF | 0 | 0 | 0 | 0 | 0 | 0 (0.0%) |
| TOTAL | 21 (7.5%) | 0 (0.0%) | 3 (1.1%) | 0 (0.0%) | 2 (0.7% | 26 (9.3%) |
| GRAND TOTAL | 219 (78.5%) | 42 (15.1%) | 14 (5.0%) | 2 (0.7%) | 2 (0.7%) | 279 (100%) |
| TYPE OF TREATMENT GIVEN | CHOP | CVP | R-CHOP | CHLORAMBUCIL + PREDNISOLONE | CODOX-M/ IVAC | TOTAL |
| INDOLENT TYPE NHL | ||||||
| CR | 18 | 4 | 2 | 2 | 0 | 26 (9.3%) |
| PR | 1 | 1 | 0 | 0 | 0 | 2 (0.7%) |
| NR | 0 | 0 | 0 | 0 | 0 | 0 (0.0%) |
| RELAPSE | 3 | 4 | 0 | 0 | 0 | 7 (2.5%) |
| LTF | 21 | 26 | 0 | 0 | 0 | 47 (16.9%) |
| TOTAL | 43 (15.4%) | 35 (12.6%) | 2 (0.7%) | 2 (0.7%) | 0 (0.0%) | 82 (29.4%) |
| INTERMEDIATE TYPE NHL | ||||||
| CR | 47 | 1 | 7 | 0 | 0 | 55 (19.7%) |
| PR | 2 | 0 | 0 | 0 | 0 | 2 (0.7%) |
| NR | 0 | 0 | 0 | 0 | 0 | 0 (0.0%) |
| RELAPSE | 12 | 0 | 2 | 0 | 0 | 14 (5.0%) |
| LTF | 94 | 6 | 0 | 0 | 0 | 100 (35.9%) |
| TOTAL | 155 (55.6%) | 7 (2.5%) | 9 (3.2%) | 0 (0.0%) | 0 (0.0%) | 171 (61.3%) |
| AGGRESSIVE TYPE NHL | ||||||
| CR | 5 | 0 | 2 | 0 | 0 | 7 (2.5%) |
| PR | 0 | 0 | 0 | 0 | 0 | 0 (0.0%) |
| NR | 0 | 0 | 0 | 0 | 0 | 0 (0.0%) |
| RELAPSE | 2 | 0 | 1 | 0 | 0 | 3 (1.1%) |
| LTF | 14 | 0 | 0 | 0 | 2 | 16 (5.7%) |
| TOTAL | 21 (7.5%) | 0 (0.0%) | 3 (1.1%) | 0 (0.0%) | 2 (0.7%) | 26 (9.3%) |
| GRAND TOTAL | 219 (78.5%) | 42 (15.1%) | 14 (5.0%) | 2 (0.7%) | 2 (0.7%) | 279 (100.0%) |
Figure 4.Post-treatment 3-Year Follow-Up of Patients with NHL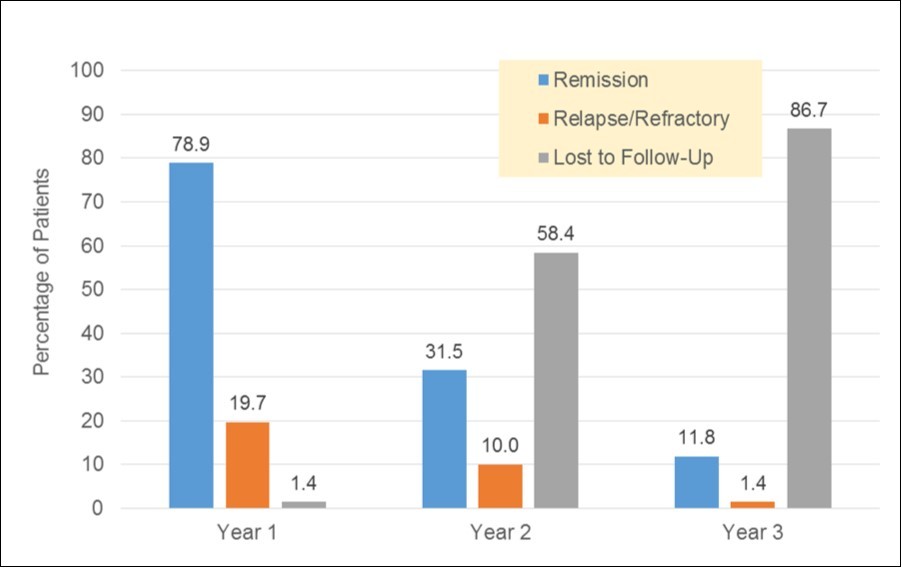
Discussion
In Ghana, NHL has been reported to be quite common among children.9 The incidence of NHL from previous studies in developed and middle income countries is higher compared to Hodgkin's lymphoma (HL).10, 11, 12, 13, 14The is variation in incidence according to age, sex, race, socioeconomic status and histologic subtypes.15 The male preponderance in this study is not different from previous studies.16, 17, 18The sex of an individual is one of the greatest known risks for contracting lymphomas and leukaemias.19It has been reported that the preferential male involvement in lymphoid cancer is most marked in the youngest age group in NHL as well as HL.20 In 1998, an international consortium reviewed pooled patient data and the male sex factor was identified as an adverse prognostic score for advanced lymphomas.20
In this study, majority of the patients (60.0%) were males. There was paucity of cases above the age of 69 years. This is however not surprising because the life expectancy in the general population in Ghana is averagely 65.75%.21This may be responsible for the decline in incidence above 69 years.
A significantly higher incidence has been reported among adolescents and young adults in some industrialized countries22, 23whereas less developed countries still show high rates in childhood23and now adolescent and young adults. There were only five cases of Burkitts lymphoma during the study period probably because patients were adolescents and adults, whereas a study done by C. Segbefia et al24showed 30.7% of childhood malignant neoplasms being Burkitts lymphoma.
These observations emphasize the need to identify the risk factors for NHL in the young and follow them up into adulthood. In a study carried out in Ghana on malignant neoplasms with ethnicity and characteristics of NHL in children, it was observed that Non-Hodgkin's diffuse cell type formed the most common lymphoma (53.3%) and the most common pediatric malignancy (29.6%).9 Even though the Working Formulation is now obsolete, the Intermediate grade of NHL was found to be the most common type of NHL; with the Diffuse Large Cell and Diffuse Mixed Cell types being the most common.
In terms of the epidemiologic risk factors such as comorbidities like HIV, though it was not routine to conduct this test because of the low prevalence in Ghana, in the last five years it has gradually been incorporated into our diagnostic workup for NHL.
The Working Formulation may still be a good source of diagnostic and prognostic information and a tool for treatment planning in countries where immunohistochemistry is not available or unaffordable.
The major limitations were unavailability of immunohistochemistry and molecular studies to better characterize NHL, poor documentation, poor follow up data after the first year. Another limitation was the fact that the sample size was relatively small to be representative of Ghana plus the study was only done in one area in Ghana – the major referral and teaching hospital in Accra (KBTH) to arrive at a definitive conclusion.
In conclusion, this study provides an overview of the distribution of NHL subtypes and their outcomes in adolescent and adult patients in a resource constrained setting. Patient loss to follow up in the 2nd and 3rd year post treatment was very high and the reasons for this should be explored in a future study. Immunohistochemistry, cytogenetics and specific molecular studies, which are important in characterization of NHL, should be made affordable and accessible in low-income countries.
Authors Contributions:
YDA, EQ, EA, AA, CS and JKA, all contributed in data collection, analysis and manuscript write up
Acknowledgements
We are grateful to the Department of Haematology and the patients for use of medical records.
References
- 1.Carbone A. (2003) Emerging pathways in the development of AIDS-related lymphomas (review). The Lancet Oncology Vol.,4. 22-29.
- 2.Jaffe E S, Harris N L, Stein H.Isaacson PG(2008) Classification of lymphoid neoplasms: the microscope as a tool for disease discovery. , Blood 112(12), 4384-4399.
- 3.Hartge P, Devesa S S, Fraumeni JF Jr. (1994) Hodgkin’s and non-Hodgkin’s lymphomas. Cancer Surv;19-20:. 423-53.
- 4.Clarke C A, Glaser S L. (2002) Changing Incidence of Non-Hodgkin Lymphomas inthe United States. , Cancer 94(7), 2015-2023.
- 5.Marcos-Gragera R, Pollán M, Chirlaque M D, Gumà J, Sanchez M J et al.the Non-Hodgkin’s Lymphoma Working Group. (2010)Attenuation of the epidemic increase in non-Hodgkin’s lymphomas in Spain. Annals of Oncology Volume 21,Issue suppl 3Pp.iii90-iii96.
- 6.CRJ Singer, Baglin T, Dokal I. (2009) . Lymphoma Chapter 5 Oxford Handbook of Clinical Hematology 180-189.
- 7.Walker B R, Colledge N R, Ralston S H, Penman I D. (2014) . Hematological malignancies Davidson’s Principles and Practice of Medicine 22nd edition published by Elsevier .
- 8.Farcet J-P, Gaulard P, Marolleau J-P, J-P Le Coudic, Henni T et al. (1990) Hepatosplenic T-cell lymphoma: sinusal/ sinusoidal localization of malignant cells expressing the T-cell receptor gd. , Blood 75, 2213-9.
- 9.Gyasi R K, Tettey Y. (2007) Childhood Deaths from Malignant Neoplasms in. , Accra Ghana Med J 41(2), 78-81.
- 10.Efrosa I, Miron I, Tansanu I. (2010) Statistical evaluation of clinical characteristics and therapeutic management of Hodgkin’s Disease in children over a 10 year period. Rev Med ChirSoc Med NatlLasi;. 114, 111-4.
- 12.Aziz Z, Rehman A, Akram M, Saeed A. (1999) Non-Hodgkin's lymphoma in Pakistan: Clinico-pathological profile of 175 patients. , J Pak Med Assoc 49, 11-5.
- 13.Adelusola K A, Adeniji K A, Somotum G O. (2001) Lymphoma in adult Nigerians. , W Afr Med J 20, 123-6.
- 14.Diop S, Deme A, Dangou J M, Ndiaye F S, Toure A O et al. (2004) Non-Hodgkin’s lymphoma in Dakar: Study of 107 cases diagnosed between1986and1998. , Bull SocPatholExot 97, 109-12.
- 15.Omoti C E, NKD Halim. (2005) Adult lymphomas in Edo state, Niger Delta region of Nigeria-clinico-pathological profile of 205 cases. , Clin Lab Haem 27, 302-6.
- 16.Nakatsuka S, Aozasa K. (2006) Epidemiology and pathologic features of Hodgkin’s lymphoma. , Int J Hematol 83, 391-7.
- 17.Oluboyede O A, Olaofe W A, GJF Esan. (1977) Non-Hodgkin’s Lymphoma in Ibadan. , Nigeria. J Natl Med Assoc 69(7), 499-502.
- 18.Bodis S, Kraus M D, Pinkus G, Silver B, Kadin M E et al. (1997) Clinical presentation and outcome in lymphocyte-predominant Hodgkin’s disease. , J ClinOncol 15, 3060-6.
- 19.Cartwright R A, Gurney K A, Moorman A V. (2002) Sex ratios and the risks of hematological malignancies. , Br J Haematol 118, 1071-7.