Abstract
An 81-year-old male presented to the Emergency Department with urinary retention, subsequent to passing blood clots. A three-way catheter was inserted for continuous bladder irrigation. 48 hours later he deteriorated, with worsening tachypnea and hypoxaemia. Clinical examination and chest x-ray suggested pulmonary odema, managed with intravenous furosemide, and non-invasive ventilation.
His irrigation circuit-chart showed he had received 10 litres Normal Saline via the afferent limb, but only 3 litres recorded at the efferent limb. It was suspected the catheter was adjacent to a vascular-cystic interface, however an urgent contrast CT revealed the irrigating catheter perforating the bladder, being situated in the abdominal cavity (see 3 images). His arterial blood-gas analysis demonstrated the expected normal anion-gap academia, however his Strong Ion Difference calculations, sodium-chloride difference of 13 and a normal albumin level, perfectly demonstrated the expected calculated BE of -13. The catheter was withdrawn, and the patient made a full recovery.
Author Contributions
Academic Editor: Angela Pia Cazzolla, Department of Dentistry and Child Complex Operating Unit of Dentistry at the University of Bari, Italy.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2019 Andrew Lane, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Disorders of the renal tract can often lead to biochemical abnormalities, due the kidneys being the predominant organ of this system. 1 Common disorders of the renal tract often present with a metabolic acidosis due to the renal handling of various electrolytes, and the specific cellular pump mechanisms within the kidney. However, the metabolic acidosis is usually a high-anion gap acidosis due to the accumulation of phosphate and sulphate ions within the plasma, leading to a depletion in serum bicarbonate. We present a patient with an iatrogenic disorder of the genitourinary tract that presented with a severe normal anion gap acidosis, and serum biochemistry that demonstrated the potential use of calculating the strong ion difference.
Case History and SID Discussion
An 81-year-old male presented with to the Emergency Department with urinary retention subsequent to passing blood clots. His urine-flow had been becoming increasingly difficult over the past few months, and he had a recent history of being treated with antibiotics for a urinary tract infection by his General Practitioner. He had a background medical history of benign prostatic hypertrophy, and ischaemic heart disease, although he had not reported any cardiovascular symptoms for many years. He was extremely independent and actively engaged in farming. There was no history of recent trauma to the abdomen or bladder, and his history. It was felt that his presentation was due to haemorrhagic cystitis subsequent to his urinary tract infection 2, since although he did not have coronary artery stents, he was receiving dual antiplatelet therapy. His urinary retention was treated by insertion of a three-way catheter with the aim of performing continuous bladder irrigation to eradicate the bladder of any current blood clots, and his dual antiplatelet therapy was ceased to prevent the formation of further blood clots. It was noted that the insertion was technically difficult with blood being seen at the urethral meatus during the procedure, which was likely due to his worsening benign prostatic hypertrophy, and he was subsequently transferred to the urology ward.
48 hours after admission it was noticed that he was feeling increasingly breathless, and complaining of chest discomfort. He received a Medical Emergency Team review two hours after this documentation for worsening shortness of breath and hypoxaemia, with a respiratory rate of 35 breaths/min and a SpO2 of 91% on 10 litres/minute oxygen via a Hudson mask. Clinical examination revealed bi-basal medium intensity inspiratory crepitations and scrotal odema. His first arterial blood-gas analysis is shown in Table 1, demonstrating a significant normal anion gas acidosis, which was not explained by the 2500 ml of intravenous 0.9% sodium chloride he had received over the previous 48 hours.
On inspection of his urinary irrigation chart, calculations suggested that he had received 10 litres entering via the afferent limb, but only 3 litres fluid recorded exiting via the efferent limb of the circuit, although at the time it was unclear if it was not excreted from his urinary tract or if the recording on the ward was incorrect. With calculations difficult to fully ascertain, it was felt that the most likely diagnosis was pulmonary odema due to fluid overload, which was confirmed on chest x-ray, which showed bilateral opacification predominantly in the upper lobe vasculature. His bladder-irrigation was ceased, and was treated with intravenous frusemide and was transferred to the Intensive Care Unit for ongoing treatment of the pulmonary odema with non-invasive ventilation. On arrival in the Intensive Care Unit, his shortness of breath had worsened and a repeat arterial blood-gas (column 2 of Table 1), showed a worsening of his biochemical profile. It was suspected that there was a conduit from the in-dwelling catheter to his systemic circulation, which was initially thought to result from a vascular-cystic interface, similar to the pathogenesis of TURP (transurethral resection of prostate) syndrome. TURP syndrome is a rare but life-threatening complication of TURP procedure, which leads to systemic fluid overload and rapid hyponatraemia, due to absorption of the fluids used to irrigate the bladder during the operation into the prostatic venous sinuses. 3
Table 1. Arterial blood gas analysis: Column 1 Medical Emergency team call at 17:25, column 2 on the Intensive Care Unit at 18:10| Parameter | 1st ABG 17:25 | 2nd ABG 18:10 |
| pH | 7.11 | 7.04 |
| PaO2 (mmHg) | 103 | 39 |
| PaCO2 (mmHg) | 47 | 59 |
| SpO2 % | 98 | 60 |
| HC03- (mmol/L) | 14 | 15 |
| BE | -13 | -14 |
| Na+ (mmol/L) | 142 | 142 |
| K+ (mmol/L) | 4.5 | 4.2 |
| Cl+ (mmol/L) | 117 | 117 |
| BSL (mmol/L) | 7.6 | 8.1 |
| Lactate(mmol/L) | 1.4 | 3.7 |
| Albumin (g/L) | 40 | 40 |
However, a closer look at his arterial blood-gas analysis demonstrated a significant normal anion-gap academia, and his Strong Ion Difference calculations are shown below. Strong ions are ions that dissociate completely at a certain pH in a particular solution. In blood, this is at a pH 7.4. Strong cations are: Na+, K+, Ca2+, Mg2+, whereas strong anions are: Cl- and SO42-. Strong Ion Difference is the difference between the concentrations of strong cations and strong anions which = (Na+ + K+ + Ca2+ + Mg2+) – (Cl– – L-lactate – urate). Abbreviated this = (Na+) – (Cl–), and the overall effect on the base excess is SID – 38 (the normal sodium-chloride difference). The other parameter that has to be considered is ATOT, which is the total plasma concentration of inorganic phosphate, serum proteins and albumin (weak non-volatile acids), and is most affected by the serum albumin concentration. Using the abbreviated forms of the Strong Ion Difference equations, which take into account the effect on the base excess of hyperchloridaemia and hypoalbuminaemia, 4 the calculated base excess is -13, which exactly matches the base excess that was found on the ABG.
Chloride Effect
Chloride effect on Base Excess = Na+ - Cl- - 38
In this case at the first Medical Emergency Team call, the Cl- effect = 142-117-38 = -13
Albumin Effect
Albumin effect on Base Excess = (42-Alb)/4
In this case at the first Medical Emergency Team call, the Albumin effect = (42-42)/4 = 0
An urgent CT abdomen revealed a bladder perforation with the tip of the irrigating catheter situated in the abdominal cavity (Figure 1, Figure 2, Figure 3a,Figure 3b). This was most likely due to over-insertion of the 3-way catheter due to the extra force required because of the benign prostatic hypertrophy. He had therefore been receiving normal saline as ‘peritoneal dialysis’ with normal saline since insertion of the catheter. The catheter was withdrawn, and the patient made a full recovery.
Figure 1.coronal plane CT scout image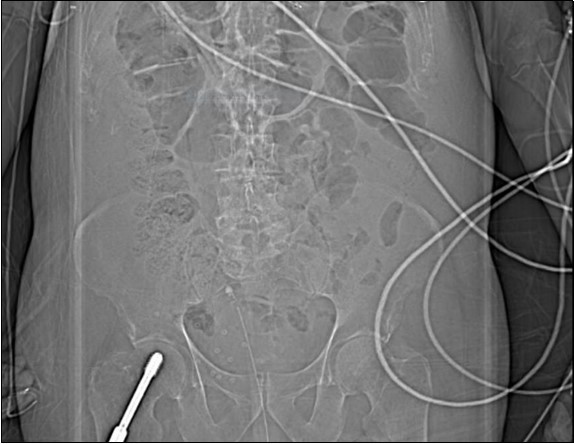
Figure 2.coronal plane CT image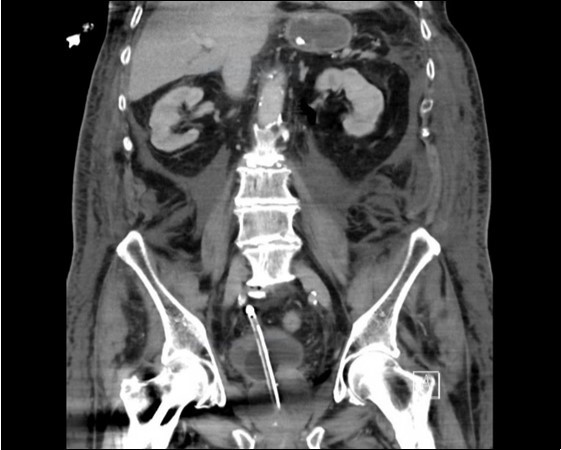
Figure 3a.horizontal plane CT image 2
Further SID Context
The understanding and analysis of acid-base homeostasis has evolved substantially since William Brooke O’Shaughnessy first suggested using intravenous normal saline to treat victims of cholera in the 1830s. 5. For many years the differences in acid-base analytical techniques between the Boston and Copenhagen groups has led to numerous heated debates, and has been called the great transatlantic acid-base debate. 6
Peter Stewart’s book ‘How to Understand Acid-Base’ suggested a new paradigm of how to analyse acid-base disturbances, however despite its popular use in certain groups it has never gained widespread acceptance, and is poorly understood. 7 His approach to acid-base homoeostasis was introduced in 1981 and offers better insight into the analysis of any acid-base system. Development of acidosis by dilution of extracellular fluid with normal saline is an important clinical example in acid-base theory because it does not involve the relocation of a traditional acid, yet significant metabolic acidosis can ensue, and this can be predicted and even quantified by using Stewart’s calculations. Yet despite this obvious theoretical benefit to the explanation of acid-base understanding, the strong ion difference has never really taken off in clinical practice, and remains an approach that appears to be left in the laboratory, due the many clinicians finding it difficult to interpret. 8
Another calculation that can be used is the estimation of the volume of fluid that reached the extracellular fluid compartment, which can be achieved with stoichiometry. Given that the patient weighed 80 Kg and was of average build, his initial extracellular fluid compartment can be estimated at 15.2 litres, comprising of a normal chloride of 98 mmol/L. That the infusion of 0.9% Normal Saline (Chloride concentration of 150 mmol/L) altered the concentration to 117 mmol/L, it can be computationally used to estimate the total normal saline dose to be between 9 and 10 litres (lowering this figure is the amount of saline actually given IV, but this is balanced by the amount of fluid removed from the circulation and the fluid not having entered, but still in the peritoneum).
These two simple calculations should remind clinicians of the utility of simple mathematical calculations to explain complex physiological derangement, which can assist with diagnosis and tailoring of management.
References
- 1.Gowda S, Desai P, Kulkarni S. (2010) Markers of renal function tests. , North American Journal of Medical Science 2(4), 170-173.
- 2.Avidor Y, Nadu A, Matzkin H. (2000) Clinical significance of gross hematuria and its evaluation in patients receiving anticoagulant and aspirin treatment.Urology.55(1):. 22-24.
- 3.Rassweiler J, Teber D, Kuntz R.et al.2006. Complications of transurethral resection of the prostate (TURP) Incidence, management, and prevention. , Eur. Urol 50(5), 969-79.
- 5.Baskett T F. (2002) William O'Shaughnessy, Thomas Latta and the origins of intravenous saline. , Resuscitation. Dec 55(3), 231-4.

