Abstract
Background
The genetic material of the genetically modified crop has been altered to develop the necessary insect resistance features by introducing genes from the Bt (Bacillus thuringiensis) bacterium. The objective of this study was to find smuggled GM Bt crops in the Metema farming area and examine its environmental effects.
Method
An experimental; Completely Randomized Design (CRD) was used to collect crop samples in the study area. The CTAB (Cetyltrimethyl ammonium bromide) technique was used to isolate DNA from all transported samples, and the purity was determined using a Nano Drop spectrophotometer. Conventional PCR with particular primers for different Bt gene events was used to detect the presence of genes. Furthermore, utilizing Bt cotton specific primer sets, the prevalence of GM cotton was measured, and amplified fragments were confirmed using agarose gel electrophoresis.
Result
The PCR results revealed that 15 (33.3 percent) of the samples were Bt cotton smuggled from Sudan. The PCR assay also revealed the presence of GM maize. Moreover, the effects of GM genes on the environment were studied in diseased samples, and no transgenes were found. Furthermore, domestic and indigenous crops were used to determine horizontal gene transfers of GM genes to other crops, and the transgene was not found in any of the samples analyzed. Conclusion: In the current study, 28 (13.4%) of the 209 (100%) total analyzed samples were GM crops which indicated the presence of unauthorized GM seeds in the study area. Environmental impact studies and horizontal gene transfer data similarly revealed that the Bt gene was not transferred to other crops and had no harmful environmental effects. For a better understanding of the Impact of imported unauthorized GM seeds, more additional detection of GM events should be done by expanding the sampling site and sample types.
Author Contributions
Academic Editor: Wan Fatein Nabeila Wan Omar, Kulliyyah of Medicine, International Islamic University Malaysia.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2023 Nega Berhane, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Background
Currently feeding the worlds alarmingly growing population has become difficult task. Modern biotechnology, which uses rDNA technology/genetic engineering to improve food quantity and quality, reduce the use of some agricultural pesticides, adjust the natural features of crops, improve dietary value, and even extends shelf life, has emerged as a potent weapon. 11. Meanwhile, the introduction of genetically modified crops in modern biotechnology has been a well thought-out new scientific breakthrough in agriculture 20. In view of this, insect resistance crops have been genetically engineered to combat extraordinarily insect damage. These crops were enabled to the production of a crystal protein with insecticidal properties by incorporating genes from the bacterium Bacillus thuringiensis 15. The goal of this investigation was to find out if Ethiopian farmers were using unapproved genetically modified seeds without the knowledge of the regulating authority.
Bacillus thuringiensis is an aerobic and gram-positive bacterium and forms endospores with crystal proteins or δ-endotoxins. These proteins are selectively toxic to different species of insects. Accordingly, Bt is an important microbial entomopathogen for the biological control of agricultural insects and a key source of genes for developing transgenic crops having the potential to combat insect attack 16.
Bt crops are plants genetically engineered to contain the endospore toxins of the bacterium, but to be resistant to certain insect pests. GM cotton and maize are crops in which transgene from the bacterium Bt, that control insect pests, have been integrated into the genome of the plant through genetic engineering, which makes it resistant to insect and stimulates the plant to produce a toxin that kills the insects 16. With the advancement in the field of genetic engineering, the genes responsible for crystalline δ-endotoxin production in the bacterium is transferred to plants via Agro bacterium mediated transformation with Ca MV (Cauliflower mosaic virus) 35S promoter 22. Bt δ-endotoxin produced in transgenic plants, when enters into the gut system of insects (having alkaline condition), gets activated into protoxin and binds to the specific receptor sites of the gut. The toxin ruptures the gut wall and later causes paralysis and death 21. Currently it becames a common practice to transfer Bt genes into different plants for modification of Bt crops.
GM, cotton, and maize are genetically modified crops in which the trans genes from the bacterium Bt, that control insect pests, has been integrated into the genome of the plant through genetic engineering, which makes it resistant to insects and stimulates the plant to produce a toxin that kills insects 16. Bt cotton and Bt maize are genetically modified crops widely cultivated in whole over the world, including Sudan.
Different countries in the world has practiced planting Gm crops. In 1997, there were 1.7 million ha of GM crops that were grown worldwide. By 2016, there were 185.1 million ha of GM crops grown in 26 countries, 19 of which are developing nations in addition to the seven industrialized nations. Five crops make up the majority of the GM crops, and two of them cotton and corn are resistant to either just insects or to both insects and herbicides. The other three sugar beet, canola, and soybean are herbicide-resistant. In 2016, 99.6 million ha (54%) of GM crops were grown in developing nations, compared to 85.5 million ha (46%) in industrialized nations. Brazil (27%), Argentina (13%), Canada (6%), India (6%), Paraguay (2%), Pakistan (2%), China (2%), and South Africa (1%), along with the United States (72.9 million hectares), expanded the most. This represents 39% of the world's total area. About 117,000 ha were planted in five European nations (Spain, Portugal, Czech Republic, Slovakia, and Romania) in 2015, while 136,000 ha were planted in 2016. Due to onerous government requirements, Romania decided not to plant in 2016 8.
Sudan is exceedingly captivating serious steps to make use of biotechnology for improving agricultural production and the production of transgenic Bt crops 1. Bt Cotton is cultivated in part as a second crop in rotation with engineered maize in rain and irrigated farm lands. Transgenic crops of insect resistant and herbicide tolerant cotton and maize are cultivated in most parts of the country, including on the border of Ethiopia 14.
Ethiopian farmers are highly cultivating cotton and maize around Metema on the border of Sudan and they use the smuggle Bt cotton and Bt maize seeds to protect their crops from insects and to increase the productivity of these crops. Corresponding to this great expectation, these deliberately releases and introduction to the market of genetically modified crops and extensive cultivation of crops might have a harmful effect on the environment 4. Due to these, there is a need to check the availability and environmental impact assessment of the GM crops. Hence, the objective of this study was to detect the existence of an unauthorized GM crop and assess its environmental impact in the Trans boundary areas of Sudan and North Western part of Ethiopia.
Materials And Methods
Study Area
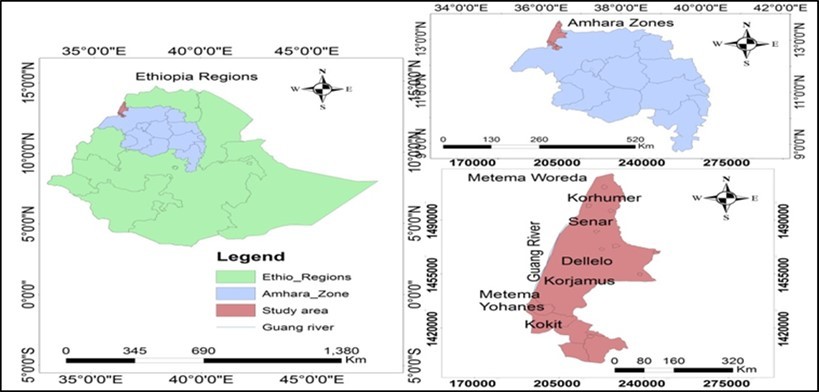
The study was conducted in West Gondar Metema district, which is located about 900 km North West of Addis Ababa and about 160 and 340 km west of Gondar and Bahir Dar towns respectively. It is one of the west Districts of Ethiopia bordering the Sudan. The district has twenty kebeles of which 18 are rural-based peasant administrations. The altitude of the district ranges from 550 to 1608 meters above sea level. Its minimum annual temperature ranges between 220c and 280c. The daily temperature is higher from March to May and sometimes reaches 430c. The District is considerably lowland with exception of some mountaintops 5. The mean annual rainfall ranges from about 850 mms to 1100 mms, with a unimodal distribution. Thus, the rainy months extend from June to the end of September.
The district is known for cultivation of various cereals. About 90% of the district’s cultivated areas of Kokit, Korjamus, Dellello, Senare and Korhumer are covered by predominantly sesame followed by cotton and maize which are currently important marketable crops. (Figure 1 )
Study Design
An experimental; Completely Randomized Design (CRD) was used to assess the availability and environmental impacts of unauthorized GM seeds in the study area and employed.
Sampling Technique
Quadrant or plot and simple random sampling techniques were implemented to choose crop samples to be tested in the laboratory. Sampling sites of farms were plotted in quadrants and for each quadrant; samples on the basis of simple random sampling technique were collected and recorded in codes. A quadrat is often a square frame that is set directly on top of the plants and is done using ropes. Plots are another name for quadrats. Plot or quadrat-based cover methods are essentially estimations of how much ground a plant will cover in a specific space. Plot-based techniques are not as effective for foliar or basal cover and are used to measure the amount of canopy cover that exists on a location. The majority of plot-based techniques aren't effective for trees or shrubs because they're mostly made to estimate the cover of herbaceous plants.
Sample Collection
In the study area, cotton and maize crops were collected from farm land through the quadrant method during the farming season. All collected crop samples were preserved through standard botanical techniques and transported to the laboratory. To detect the existence of horizontal gene transfer and impact on the environment, sesame, sunflower, sorghum, weeds, diseased crops, and water samples were collected and transferred to the laboratory. All collected samples were tested and analyzed by standard testing procedures.
Extraction and Detection of Genomic DNA from Plant and Water Samples
A total of 210 samples (forty-five cotton, forty-five maize, thirty sunflower, thirty Sesame, thirty sorghum, and thirty weed samples) have been used for DNA extraction. Genomic DNA from all collected samples was isolated by using the Cetyl Trimethyl Ammonium Bromide (CTAB) method with some modifications of 7. The DNA samples were dried and stored at -20 °C for further use. Detection of DNA from water samples were done through Dische Diphenylamine Test according to 6.
Polymerase Chain Reaction
The extracted DNA was used as a template in PCR analysis and Conventional PCR was used for detection of GM genes with a specific set of primers. Cotton common GM gene was amplified with forward primer sequence (F’CACATGACTTAGCCCATCTTTGC and a reverse primer (R,CCCACCCTTTTTTGGTTTAGC ), Maize common GM gene was detected by using primer sequences(F’CGTCGTTTCCCATCTCTTCCTCC and R, CCACTCCGAGACCCTCAGTC), Bt176 gene was amplified by using specific primers (F,GGCCGTGAACGAGCTGTT and R, GGGAAGAAGCCTACATGTTTTCTAA) and the Bt11 gene was detected by specific set of primers (F, GCGGAACCCCTATTTGTTTA, and R, TCCAAGAATCCCTCCATGAG) 9. All the PCR reactions were performed in a final volume of 25 μl reaction mixtures containing 0.5 μl of 10 pmole for each forward and reverse Primer, 2 μl of 2.5 mM of dNTP, 0.4 of 1 unit Taq polymerase, 2 μl of 10x PCR buffer, 1.5 μl of 25mM MgCl2, 15.1 μl double distilled water and finally 3μl of 100 ng template DNA for one reaction for 35 cycles.
The PCR reaction for common Bt cotton gene and maize was carried out using a master cycler gradient (Eppendorf thermal cycler gradient 5331) with the following thermal cycling conditions, Initial denaturation, 95°C for 7 min, Final denaturation, 94°C for 1 min, Annealing, 59°C for cotton and 54°C for maize for 45sec, extension, 72°C for 30 sec, the final extensions was 7 min at 72°C and final hold at 4°C. On the other hand, thermal cycler conditions for Bt-11 and Bt-176 were as follows: Initial denaturation at 95°C for 4 min, denaturation at 95°C for 50 sec, primer annealing for 45 sec, 52°C for Bt-11 and 54°C for Bt 176 primers, primer extension at 72°C for 50 sec; and a final elongation at 72°C for 5 minutes 10. The PCR product was subjected to gel electrophoresis containing 2% agarose.
Data Analysis
The data were analyzed using the SPSS (statistical package software for social science) software package version 20 for window. SPSS is a software program used by researchers in various disciplines for quantitative analysis of complex data. Occurrence and frequency of transgene were analyzed via descriptive statistics of cross tabulation and frequency for each treatment in their corresponding experimental unit.
Results
Different crop samples were collected from the trans boundary areas of Ethiopia and Sudan for detection of genetically modified crops, assessment of its environmental impact, and the prevalence of horizontal gene transfer. A total of 210 samples of smuggled and domestic crops of cotton, maize, sunflower, sorghum, weeds, and sesame were collected from Metema farming areas of Kokit, Korjamus, Dellelo, Senare and Korhumer. The Isolated DNA quality and quantity was determined with 1% Agarose gel electrophoresis (Figure 2).
Figure 2.Extracted DNA of samples on 1% agarose gel electrophoresis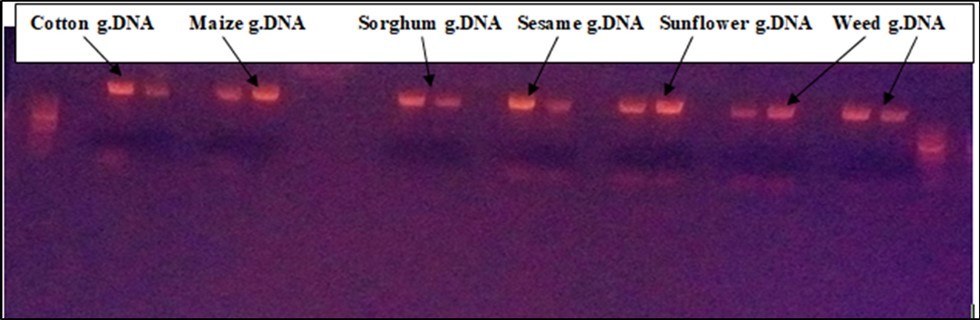
The environmental DNA from water bodies was checked by Dische Diphenylamine Test and 3(100%) clear colored result was observed from water collected from Guang River and blue color was observed from the control of DNA samples (Table 1).
Table 1. The Prevalence of Environmental DNA from water samples| Water samples | Availability of DNA | Total | |
| Yes | No | ||
| Top | 0(0.0%) | 1(33.3%) | 1(33.3%) |
| Middle | 0(0.0%) | 1(33.3%) | 1(33.3%) |
| Deep | 0(0.0%) | 1(33.3%) | 1(33.3%) |
| Total | 0(0.0%) | 3(100%) | 3(100%) |
PCR analyses were carried out for different transgenes of cotton GM gene, maize GM gene, Bt-176 gene, and Bt-11 gene with their corresponding plant-specific primers to confirm the presence of GM crops. A product of 277 bp was observed from smuggled cotton samples as shown below in Figure 3. Out of 45 cotton samples, 15(33.3%) of them were PCR positive and 30(66.7%) were PCR negative (Table 2).
Figure 3.PCR products of common Bt cotton gene.
| Cotton samples | Sampling sites | Occurrence of Bt gene | |||||||
| Kokit | Korjamus | Dellello | Senare | Korhumer | Total | Yes | No | Total | |
| Smuggld | 3(6.7%) | 3(6.7%) | 3(6.7%) | 3(6.7%) | 3(6.7%) | 15(33.3%) | 15(33.3%) | 0(0.0%) | 15(33.3%) |
| Domestic | 3(6.7%) | 3(6.7%) | 3(6.7%) | 3(6.7%) | 3(6.7%) | 15(33.3%) | 0(0.0%) | 15(33.3%) | 15(33.3%) |
| Diseased | 3(6.7%) | 3(6.7%) | 3(6.7%) | 3(6.7%) | 3(6.7%) | 15(33.3%) | 0(0.0%) | 15(33.3%) | 15(33.3%) |
| Total | 9(20.0%) | 9(20.0%) | 9(20.0%) | 9(20.0%) | 9(20.0%) | 45(100.0%) | 15(33.3%) | 30(66.7%) | 45(100.0%) |
On the other hand PCR amplification was conducted for maize GM gene by specific set of primer sequences of (F’CGTCGTTTCCCATCTCTTCCTCC and R,CCACTCCGAGACCCTCAGTC). A product of 226bp was observed (Figure 4) of them 14(31.8%) were seed of smuggled maize showing positive PCR product and 30(68.2%) of total maize samples were PCR negative (Table 3).
Table 3. The prevalence of Bt gene based on samples and Sampling site using cross tabulation.| Maize samples | Sampling sites | Occurrence of Bt gene | |||||||
| Kokit | Korjamus | Dellello | Senare | Korhumer | Total | Yes | No | Total | |
| Smuggled | 2(4.5%) | 3(6.8%) | 3(6.8%) | 3(6.8%) | 3(6.8%) | 14(31.8%) | 14(31.8%) | 0(0.0%) | 14(31.8%) |
| Domestic | 3(6.8%) | 3(6.8%) | 3(6.8%) | 3(6.8%) | 3(6.8%) | 15(34.1%) | 0(0.0%) | 15(34.1%) | 15(34.1%) |
| Diseased | 3(6.8%) | 3(6.8%) | 3(6.8%) | 3(6.8%) | 3(6.8%) | 15(34.1%) | 0(0.0%) | 15(34.1%) | 15(34.1%) |
| Total | 8(18.2%) | 9(20.5%) | 9(20.5%) | 9(20.5% | 9(20.5%) | 44(100.0%) | 14(31.8%) | 30(68.2%) | 44(100.0%) |
PCR for transgene Bt-176 was run using a specific set of primer sequences (F,GGCCGTGAACGAGCTGTT and R,GGGAAGAAGCCTACATGTTTTCTAA). The PCR product obtained was checked on 2% agarose gel electrophoresis, with a 100 bp ladder ran parallel to check the size of amplicon. A product of 211bp was observed (Figure 4, Figure 5, Figure 6). From overall, 14(31.8%) were smuggled Bt maize and 30(68.2%) maize samples showed no PCR amplification product.
PCR for another transgene Bt-11 was conducted using a specific set of primer sequences (F, GCGGAACCCCTATTTGTTTA, and R, TCCAAGAATCCCTCCATGAG). The PCR product was checked on 2% Agarose gel electrophoresis with a 100bp ladder and 189 amplification products was observed (Figure 6). Smuggled Bt maize of 14(31.8%) were detected and 30(68.2%) of total maize samples showed no PCR product (Table 4).
Figure 4.PCR products of common Bt maize gene.
Figure 5.PCR products of Bt-176 specific gene
Figure 6.PCR products of Bt-11 specific genes.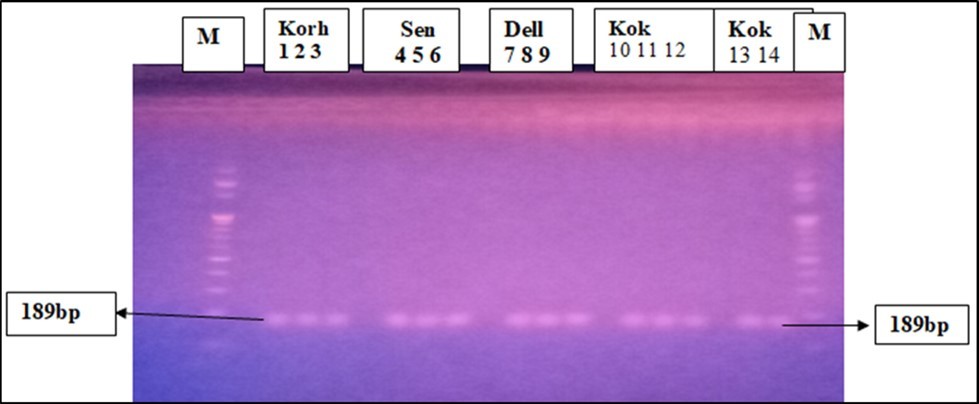
| Samples | Occurrence of Bt gene | Total | |
| Yes | No | ||
| Cotton | 15(7.2%) | 30(14.4%) | 45(21.5%) |
| Maize | 13(6.2%) | 31(14.8%) | 44(21.1%) |
| Sorghum | 0(0.0%) | 30(14.4%) | 30(14.4%) |
| Sesame | 0(0.0%) | 30(14.4%) | 30(14.4%) |
| Sunflower | 0(0.0%) | 30(14.4%) | 30(14.4%) |
| Weed | 0(0.0%) | 30(14.4%) | 30(14.4%) |
| Total | 28(13.4%) | 181(86.6%) | 209(100%) |
DNA extracted from domestic cotton, maize, sesame, diseased plants, weeds and sorghum samples were run on PCR with all four set of primers and there were 0 (0.0%) amplification products from all set of primers except smuggled cotton and maize samples (Table 4).
Discussion
Before the development of Bt cotton, pest impact on cotton growers was significant. Farmers were losing a lot of their cotton due to H. virescens and the pink bollworm, Pectinophora gossypiella, because of synthetic insecticide resistance. Nineteen four persont of the cotton grown in the USA is genetically engineered 8.
According to a research by the University of California, the average cost reduction for pesticides used in Bt cotton fields from 1996 to 1998 was between 25 and 65 dollars per acre. During the same time period, it was also projected that the yield was 5% higher than that of traditional cotton. Additionally, Bt cotton dramatically reduced the need for foliar treatments to combat other cotton pests, which in turn reduced the price of pesticides 16. The first Bt cotton to be sold in the USA was Bollgard cotton, a Monsanto Company brand, in 1996. It was manufacturing the Cry1Ac toxin, which was highly active against pink bollworm and tobacco budworm. Farmers in the Western Cotton Belt of the USA mainly used bt cotton to combat pink bollworm, while farmers in the Mid-South and South-East used it to combat tobacco budworm, fall armyworm, Spodoptera frugiperda, and S. exigua to a lesser extent (Stewart 2007).
The present study provides a simple and reliable conventional PCR method for detecting genetically engineered crops and its environmental impact assessment. In this study, the presence of horizontal gene transfer was also assessed. For PCR analysis, qualified and quantified DNA was extracted from different crop samples and the occurrence of Bt gene from the extracted DNA was checked by a different specific set of primers. Dische Diphenylamine Test was conducted to detect the DNA from water collected from Guang river, and the result showed that there were no Environmental DNA from water bodies. For the achievement of research objectives, different PCR analyses were carried out using specific primers of common Bt cotton, common Bt maize, Bt-11, and Bt-176.
In the present study, the presence of Bt cotton was confirmed by plant-specific primers of common Bt cotton gene . The results showed that a PCR product with 277bp was observed after PCR reaction. The result of this revealed that 15(33.3%) were bt cotton from overall 45(100%) cotton samples. However, the Bt gene was found in the smuggled cotton other than 0(0.0%) of domestic and diseased cotton samples of 15(33.3%) for each. This result was in line with 24; 3 studies of PCR based detection of transgenic cotton seed samples and also agree with 18 PCR based detection of transgenic rice. The results of this study indicates that Bt cotton is Smuggled from Sudan and cultivated in the all studied areas of Kokit, Korjamus, Dellello, Senare, and Korhumer on Metema, Ethiopia.
In this study, the PCR assay was carried out, primers specific to inserted genes of common Bt maize were used, and 226bp were observed from PCR products. The result implies that 14(31.8%) were Bt maize from a total of 44(100.0%) maize samples and the Bt maize were smuggled from Sudan. However, 30(68.2%) of Domestic and diseased maize samples have 0(0.0%) Bt gene. This result was also in agreement with previous reports, Detection of Genetically Modified Maize by Polymerase Chain Reaction (12; Payam et al., 2020; 10. The results of this study also revealed that Bt Maize is smuggled from Sudan and cultivated in the studied areas of Ethiopia.
In the present study, PCR assay was conducted for a specific gene of primers specific to inserted genes in the event 176 GM maize. Bt-176 genes were observed with 211bp in smuggled maize samples and 14(31.8%) had Bt gene from overall samples of 44(100.0%) maize and 30(68.2%) of Domestic and diseased maize samples have 0(0.0%) cry genes. Likewise, other scholars also reported Detection of Genetically Modified Maize in Processed Foods by Polymerase Chain Reaction (Maher et al., 2013; 10; 13. The result of this study indicates that event 176 GM maize is smuggled from Sudan and cultivated in the studied areas of Ethiopia.
In this work, PCR analysis was conducted using specific gene primers specific to inserted genes in the event 11 GM maize. Bt gene of Bt-11 was observed and had 189bp in smuggled maize samples and 14(31.8%) had Bt gene from overall samples of 44(100.0%) maize and 30(68.2%) of Domestic and diseased maize samples were 0(0.0%) Bt gene. This result was in agreement with other researchers of PCR-based detection of genetically modified soy and maize products (Payam et al., 2020; Maher et al., 2013; 10; 13. The result of this study shows that Event 11 GM maize is smuggled from Sudan and cultivated in the studied areas of Metema woreda.
In the present study, Environmental impacts of unauthorized GM genes were assessed from all diseased samples with all four sets of primers and there were 0.0% Bt genes. The result of this study is in agreement with the previous report, Ecological safety evaluation of transgenic cotton and Ecosystem Impacts of Pesticide Reductions 25; 19. The result of this study implies that the Bt gene might have no negative environmental impacts.
In this study, horizontal gene transfers of unauthorized GM genes were determined from samples of domestic and indigenous crops with all four sets of primers and there were 0.0% Bt genes. However, according to 2. Horizontal Gene Transfer occurred between Plants. In addition to these, Dische Diphenylamine Test showed that DNA was not detected in water and implies, the transgene might not transfer through the water. The results of this study declared that there is no horizontal GM gene transfer to other crops on the study area of Ethiopia.
In general, after detection of genetically modified crops, a polymerase chain reaction (PCR) assay with primers specific to inserted genes was performed in this study. According to PCR results, 28 (13.4%) of the 209 (100%) tested specimens revealed a positive response to the Bt gene primer. The results showed 28 (13.4%) were Bt crops and 181(86.6%) were non-GM from overall sample. The results of the present study confirmed that transgenic Bt crops of cotton and maize are smuggled from Sudan and cultivated in the studied areas of Ethiopia.
Conclusion
In conclusion, the findings of the current analysis showed that a total of 29 GM samples of cotton and maize crops were found. From smuggled samples, 15 different varieties of GM Bt cotton were developed and grown in the study locations. On the other hand, smuggled samples revealed the presence of 14 GM maize crop samples. For the purpose of preventing the entry of unapproved GM crops, additional testing for the identification of GM crops and products is beneficial. According to the current study, GM genes were not discovered using PCR analysis of sick plant samples. Similar to this, other samples of indigenous plants were examined, and no transgenic were found in the tested samples. However, Ethiopian biosafety declaration requires control over, identification of, and avoidance of cultivation of Unauthorized GM seeds in the market due to various degrees of assurance regarding environmental dangers and gene flow of GM genes. The prompt discovery of genetically modified crops that Ethiopia has not yet given the go-ahead to import will benefit from this endeavor, in the end.
Authors’ Contributions
Nega Berhane has developed the proposal of the research and wrote the manuscript; Mulu Muchie has conducted the laboratory work. Zewdu Teshemo conducted the statistical analysis.. All authors read and approved the final manuscript. Senior authorship is shared equally
Authors’ Information
The authors are faculties at University of Gondar
Funding
The research and technology Transfer Vice president office, University of Gondar has funded this research
Declarations
Ethics approval and consent to participate
Ethical approval and consent of participation is not applicable for this study
Consent for publication
Not applicable
Acknowledgements
The Authors are thankful to the Vice president for research and Technology transfer University of Gondar for providing financial assistance. We are also very much thankful to the laboratory technicians of Institute of Biotechnology for the technical assistance provided for the laboratory activities. More additional detection of GM events should be done by broadening the sampling site and sample types for a better understanding of the impact of imported unlawful GM seeds.
References
- 1.N A Abdallah. (2014) The story behind Bt cotton: Where does Sudan stand?. Biotechnology in Agriculture and the Food Chain,5(4): 241-243.
- 2.Alessandra P, R Pascal Aurora, Daniele S, Timothy M V D, Jean-Miche M. (2009) . Visual Evidence of Horizontal Gene Transfer between Plants and Bacteria in the Phytosphere of Transplastomic Tobacco. Applied and environmental microbiology,;75(10): 3314-3322.
- 3.Alka D, S K Tank. (2014) PCR based detection of transgenes cry1ac and cry2ab in mon-15985(bg-ii) bt-cotton in gujarat samples. , Asian Journal of Science and 5(7), 419-422.
- 4.J C Anthony, R G Travis, Jan-Peter N. (2003) The release of genetically modified crops into the environment and Overview of ecological risk assessment. The Plant Journal.33:.
- 5.Bosena T, Bekabil F, Berhanu G, Dirk H. (2011) Factors Affecting Cotton Supply at the Farm Level in Metema District of Ethiopia. , Journal of Agriculture, Biotechnology & Ecology.4 1, 40-51.
- 9.Koppel R, Zimmerli F, Breitenmoser A. (2010) Multiplex real-time PCR for the simultaneous detection and quantification of DNA from three transgenic rice species and construction and application of an artificial oligonucleotide as reference molecule. , European Food and Research Technology 230, 731-736.
- 10.Lih-ching C, Yen-ling C, Jei-hwa Y, yang Daniel-, C.(2001).Detection of Four Types of Genetically Modified Maize by Polymerase Chain Reaction and Immuno-Kit Methods. , Journal of Food and Drug Analysis 9(1), 50-57.
- 11.J S Manjunath, Chandan K, Krishna H, Aparna G. (2018) Genetically modified crops:. , Journal of Pharmacognosy and Phytochemistry 7(1), 2405-2410.
- 12.R B Maria, Renata T B F, Rafael L-F, Paola C-L, Déborah M V G. (2013) Detection of genetically modified maize events in Brazilian maize-derived food products. , Food Science and 33(3), 399-403.
- 13.Maryam R, Mehrangiz M, Hossein R, Hossein V, Mahmoud A. (2013) Detection of Genetically Modified Maize in Processed Foods Sold Commercially in Iran by Qualitative PCR. , Iranian Journal of Pharmaceutical Research.12 1, 25-30.
- 14.Mathur V, Javid L, Kulshrestha S, Mandal A, Reddy A. (2017) A. World Cultivation of Genetically Modified Crops: Opportunities and Risks. Sustainable Agriculture Reviews. 45-87.
- 15.Metz M. (2003) Bacillus thuringiensis: a cornerstone of modern agriculture. , Journal of new seed 5(1), 2-3.
- 16.Mohamed S, Tawfik A. (2018) Genetically engineered (modified) crops (Bacillus thuringiensis crops) and the world controversy on their safety. , Abbas Egyptian Journal of Biological Pest Control 28, 52.
- 17.Z L Jinshui, Donghai P, Ming S. (2018) Whole genomic analysis of Bacillus thuringiensis revealing partial genes as a source of novel Cry toxins. , Applied Environmental Microbiology 8414, 00277-18.
- 18.Molaee A, J K Gholamreza, Setareh A K A, Sassan R. (2019) A simple and accurate PCR method for detection of genetically modified rice. , Journal of Environmental Health Science and Engineering.17: 847-851.
- 19.C V Prakashan, V K, Matin Q. (2014) Bt Cotton and Ecosystem Impacts of Pesticide Reductions. Global Food Discussion Papers. No.41
- 20.Ruchir R. (2018) The impact of Genetically Modified (GM) crops in modern agriculture: A review. Biotechnology in Agriculture and the Food Chain 8(4), 195-208.
- 21.Sanahuja G, Raviraj B, R M Twyman, Capell T, Christou P. (2011) Bacillus thuringiensis: A century of research, development and commercial applications. , Plant Biotechnology Journal 9, 283-300.
- 22.K B Santos, Neves P, A M Meneguim, B dos Santos R, Santos dos et al. (2009) Selection and characterization of the Bacillus thuringiensis strains toxic to Spodoptera eridania (Cramer), Spodoptera cosmioides (Walker) and Spodoptera frugiperda (Smith)(Lepidoptera: Noctuidae). , Biological Control 50(2), 157-163.