Abstract
Introduction:
The use of non-medicinal facilities for correcting processes in various pathological conditions is one of the most urgent problems of modern medicine.
Purpose of the Work:
To study the effect of low-intensity infrared laser radiation on reparative bone formation and angiogenesis in bone regeneration which is formed in treatment of fractures under conditions of transosseous osteosynthesis.
Material and Methods:
A tibia fracture was modeled experimentally in rats in the control and experimental groups. Reposition and fixation of fragments were performed. The fracture zone in the experimental group animals was exposed to the impact of pulsed infrared laser irradiation of low intensity. Animals from the control group underwent the impact simulation. The operated bones were investigated using the methods of X-ray, light and electron microscopy, X-ray electron probe microanalysis.
Results:
It was established that laser radiation exposure sessions activated fibrillogenesis and angiogenesis, accelerated compacting of newly formed bone tissue and increased its maturity while primary fracture healing occurred. Prolonged capillary dilatation and endothelium-dependent vasodilation, intensive capillarogenesis were noted after sessions of laser therapy in bone regeneration. Endothelial outgrowth was formed in the lumen of the vessels forming capillary buds that propagate along the “mother” vessels (endovascular capillarogenesis).
Conclusion:
The data obtained revealed a possible mechanism of laser radiation exposure at the level of a whole organism and proved the effectiveness of its application in clinical practice at the early stages of patient rehabilitation under conditions of transosseous osteosynthesis.
Author Contributions
Academic Editor: Yuksel Aydar, Department of Anatomy, Medical School of Eskisehir Osmangazi University, Eskisehir, Turkey.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2018 Yu. М. Iryanov.et al
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
The use of non-medicinal facilities for correcting reparative regeneration processes in various pathological conditions is one of the most urgent problems in modern medicine. Laser therapy of different types is widely used in clinical practice including traumatology and orthopaedics for treating locomotorium injuries and diseases. It is aimed to provide anesthetizing, anti-edematous, anti-inflammatory and trophic-stimulating effects 1, 2, 3, 4, 5. It also ensures the decrease in medicinal burden in a patient’s organism. The treatment with laser beams is comfortable, non-invasive, aseptic, painless, harmless, and controllable 6, 7, 8, 9, 10, 11. The low-energy laser impact has a multifactorial effect on the organism 12, 13, 14, 15, 16. The activity of the enzymatic systems and the rate of oxygen consumption by tissues increase, the level of peroxide lipid oxidation decreases, oxidation-restoration processes activate, and the concentration of adaptive hormones increases 17, 18, 19. At the same time the mechanism of laser therapeutic impact is largely unclear, and the doses are selected empirically. There is little experimental work in this area and it is mainly performed by X-ray and clinical methods 20, 21. There is no information on the efficacy of low-intensity infrared laser radiation in treatment of fractures under conditions of transosseous osteosynthesis and its influence on morphological features of bone regeneration. It indicates the urgency of the problem and the need for its study. The purpose of this work is to study the effect of low-intensity infrared laser radiation on reparative bone formation and angiogenesis in bone regeneration which is formed in treatment of fractures under conditions of transosseous osteosynthesis.
Material and Methods
The Experimental Procedure
We performed experiments on 32 adult Wistar male rats weighing 340-390 g in the control and experimental groups (16 animals each). Animal care, surgical procedures and euthanasia of animals were performed according to the guidelines of the RF Ministry of Health and those of “European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes”.
We modeled a tibia fracture in the shaft middle third in a closed way under general anesthesia, performed reposition and fixation of fragments using a device worked out by us 22, 23, 24, 25. One day after surgery the animals in the experimental group underwent the impact of low-energy pulsed infrared laser irradiation using a therapeutic laser device (serial device of laser therapy "UZOR-A-2K", country of manufacture: Russia, "BINOM"), wave length within the near-infrared range (0.89±0.02 µm), pulse frequency – 150 Hz, power – 4 W, pulse duration – 110-160 ns. The exposure of the fracture zone was performed in a pulse mode locally for 10 minutes. The distance between the emitter and the skin was one (1) mm. The sessions of irradiation exposure were repeated in one day and performed at 7 and 14 days after surgery. This technique of laser therapy for bone fractures was developed by the manufacturer of the apparatus. The animals of the control group underwent exposure simulation when the therapeutic laser device was switched off. Eight (8) animals were used for each time point. We took X-rays immediately after surgery and during treatment.
Methods of the Study
The animals were euthanized 7 and 14 days after surgery. Eight animals were used for each time point. We fixed the operated bones in a 2% solution of paraformaldehyde and glutaraldehyde in phosphate buffer (pH 7.4) for 1 day at a temperature of 4 degrees in Celsius. Then we fixed regenerated bone pieces in a 1% solution of osmium tetroxide for electron microscopy. The specimens were dehydrated in a series of ethanol increasing concentrations, in 100% acetone, and then embedded in paraffin (after decalcification) and araldite (without decalcification). The paraffin-embedded histo-topographic longitudinal sections were stained with hematoxylin-eosin and picrofuchsin by Van Gieson. The morphological analysis and photomicrography of the histological preparations were performed using Stemi2000-C microscope complete with AxioCam ERc5sdigital camera and Zenblue(“Carl Zeiss Micro Imaging GmbH”, Germany) software. The araldite-embedded bones were studied with the characteristic X-ray irradiation of calcium using INCA-200 Energy X-ray electron probe micro analyzer (“Oxford Instruments Analytical”, England). The activity of osteogenesis process was determined by the content of bone tissue structures in the intermediate zone of the regenerated bone. The degree of maturity and strength of the newly formed bone tissue was assessed using the compactness index (ratio of bone tissue and non-mineralized structures). We sawed the blocks in the zone of the regenerated bone to obtain the specimens for electron microscopy, prepared ultra-thin sections of 70-90-nm thickness using “LKB-8800 ultra-microtome” (Sweden), contrasted them with uranyl acetate and lead citrate solutions and studied using JEM-2010(“Jeol”, Japan) transmission electron microscope under the accelerating voltage of 80 kV. Then we treated the specimens with 2% sodium ethylated solution (to remove araldite from the surface), sprayed them with platinum and palladium alloy (in 1:3 ratio) in IB-6 ion vacuum sprayer (“Eico”, Japan) and studied in secondary electrons under the accelerating voltage of 20 kV using JSM-840 scanning electron microscope (“Jeol”, Japan). The number of vessels and their lumen diameter were determined by scanning electronograms in the section area unit of 0.01 mm2for x1000 instrumental magnification.
Methods of a Statistical Analysis
The results of quantitative investigations were processed by variation statistics methods. The significance of differences in the compared parameters was calculated using Student t-test. Differences were considered to be significant for the level of р < 0.05.
Results
A transverse fracture in the middle third of the tibial shaft diaphysis was determined in animals 7 days after the operation (Figure 1). Edges of the fragments had uneven surfaces. There was no bone fusion in the animals of the control group (Figure 1a). The formation of periosteal and endosteal deposits of insignificant extent at the ends of bone fragments was observed. Fine-meshed structures of thin bone trabeculae were formed at a distance of 1-2 mm from a fracture plane and tightly welded to the cortical layer of the fragments. Structures of newly formed bone tissue in the intermediate zone were not detected Figure 1a). A thin layer of trabeculae of coarse-fibrous bone tissue was formed along the endosteal surfaces of the fragments forming an endosteal bone-osteoid fusion that did not penetrate into the intermediate space.
Figure 1.A tibia fracture zone in the animals from the control (a) and experimental (b) groups (3 sessions of laser therapy) 7 days after the operation. Images in the characteristic X-ray radiation of calcium atoms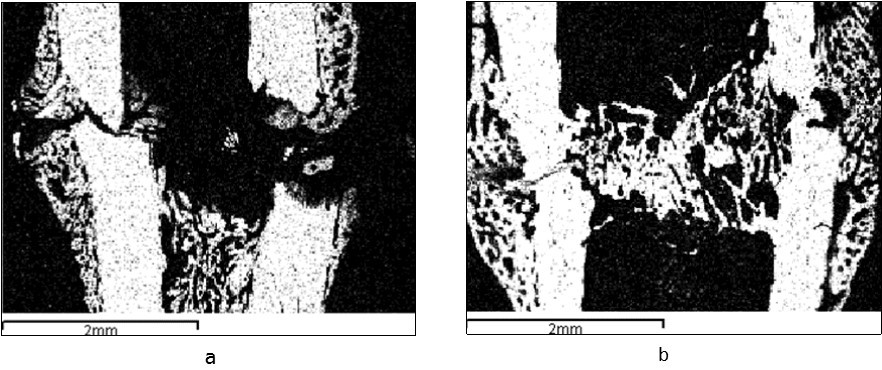
Electron microscopic examination in the area of the bone fragments revealed the foci of alternatively destructive changes and the areas of organized hematoma, infiltrated lymphocytes, neutrophilic granulocytes, monocytes, mast cells, macrophages (Figure 2a, 2b). Bone debris, filaments, fibers and clots of fibrin were revealed in the intermediate and endosteal zones of the regenerated bone; little-differentiated connective tissue with edema signs was formed. Isolated sinusoids with moderate ectasia, parietal micro thrombi, diapedesis of erythrocytes and leukocytes were observed in the regenerated bone. Perivascular cells were sporadic.
Figure 2.The ultrastructure of the intermediary zone of regeneration 7 days after the operation in the animals of the control group: a – transmission electron microscopy, b – scanning electron microscopy. Increase: a – 6000, b – 2000
Regeneration was clearly visible in the operated bone in the experimental group of animals 7 days after the operation and 3 sessions of laser therapy. It was noted on the entire width of the fragments ends. Volumetric periosteal and endosteal bone-osteoid stratifications grew into the regeneration on the bone fragments ends from the periosteal and endostea surfaces and overlapped diastase (Figure 1b, Figure 3). Bone fusion between fragments was formed (intermediate regeneration). The calcium content in this zone was 15-20 % (Figure 3a). Numerous blood vessels and cells at various stages of osteogenic differentiation were located between the bony beams (Figure 3b, 3c, Figure 4a).
Figure 3.The calcium content (a) and the structure (b, c) of regeneration in the zone of the tibia fracture fusion in the animals of the experimental group 7 days after the operation; a – images in the characteristic X-ray radiation of calcium atoms, b – light microscopy of histological sections, staining by Van Gieson (b) and hematoxylin-eosin (c), lens 10, eyepiece 10
Preosteoblasts, differentiated osteoblasts, fibroblasts, macrophages, extravascular erythrocytes and bundles of collagen fibers were identified in the fusion zone during the electron microscope study (Figure 4a).
Figure 4.Blood vessels in the intermediate zone of regenerated bones 7 days after fracture occurrence: а – a sinusoid in the regenerated bone in the animal from the control group; b, c, d – newly formed capillary terminals (arrows) in the regenerated bone in the animals from the experimental group – 3 sessions of laser therapy; а, b, c – scanning electron microscopy, d – an ultrathin section, transmission electron microscopy, magnification x 8000.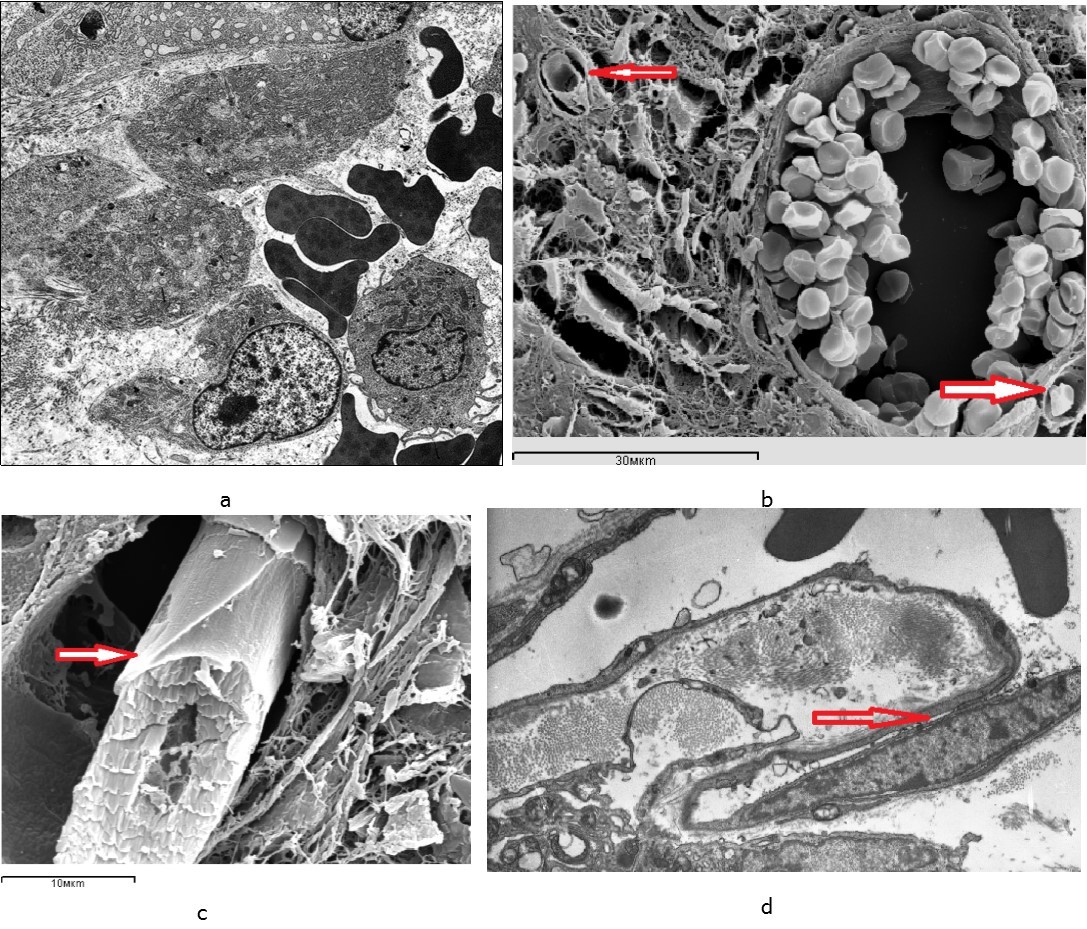
Signs of increased permeability of the endothelial layer were recorded in the vessels of the microcirculatory bed localized in the fusion zone. It was evidenced by the multiplicity of micropinocytic vesicles, the dissociation of endothelial contacts and the appearance of interendothelial pores and slots on the luminal surface. In the lumen of vessels the capillary buds were revealed in the form of endovascular endothelial outgrowths (Figure 4c, 4d). It indicated active processes of reparative angiogenesis. Endovasal endothelial processes had a characteristic tile-like outer layer which had been formed by flat marginal regions of neighboring endotheliocytes (Figure 4c).
The results of electron probe microanalysis (Table 1) evidenced osteogenesis activation and the increase in the maturity degree of newly formed bone tissue in the regenerated bones in the animals from the experimental group comparing with the control one. Thus, the content of bone tissue in the regenerated bones 177.09 % exceeded the parameters in the control group of animals, and the index of compactness exceeded those parameters more than twice (222.23 % higher). In the animals of the experimental group angiogenesis was significantly intensified and confirmed by 173.31% increase in the number of vessels, as well as by 75.90% decrease in their diameter compared with the control group.
Table 1. Characteristics of bone formation and angiogenesis in the intermediate zone of the regenerated bone in the animals of the control (C) and experimental (Exp) groups (М±m)| Parameters | Period of the experiment, days | |||
| 7 | 14 | |||
| C | Ex | C | Ex | |
| Bone tissue, % | 18.38±0.90 | 32.55±1.511 | 28.67±1.52 | 58.16±2.111 |
| Index of compactness | 0.22±0.01 | 0.49±0.021 | 0.40±0.02 | 1.39±0.071 |
| Number of vessels | 1.65±0.07 | 2.86±0.151 | 2.23±0.14 | 4.56±0.171 |
| Vascular lumen diameter, µm | 42.75±2.05 | 32.45±1.511 | 49.65±2.05 | 30.18±1.091 |
Formation of the primary regenerated bone was observed in the control group of animals 14 days after the operation, signs of periosteal and endosteal fusion appeared Figure 5a, 5e). Regeneration in the intermediate zone between the fragments was presented by a newly formed spongy bone from a fine-meshed network of intertwining bone trabeculae of various degrees of maturity (Figure 5c). Fracture healing was characterized by the secondary type with predominantly periosteal and endosteal callus formation (Figure 5e).
In the experimental group of animals the bone fragments were connected by periosteal-endosteal bone structures in the form of vertical brackets 14 days after the operation (Figure 5b, 5f). Periosteal stratifications of 1.5-2 mm in length compactized and integrated the fragmental ends as a fusiform coupling. The regenerated bone in the intermediate zone was presented as spongy bone tissue tightly soldered with fragmental ends and overlapping the diastasis (Figure 5d). The formation of a new bone cortical layer was observed. Fracture healing occurred by the primary type. Fragmental ends were connected by the secondary osteons of lamellar bone tissue of different maturity degree with compactization scenes. In the area of damage bone thickening was observed due to the persistent periosteal layers of 1.5-2 mm which were compacted, and the spindle-shaped “clutch” united the ends of the fragments (Figure 5f). According to the data of electron probe microanalysis, 14 days after fracture occurrence and after six sessions of the exposure to laser irradiation the content of bone tissue in the regenerated bones 202.86 % increased, the index of compactness 347.51 % increased, and the number of vessels 294.5 % increased with 60.8 % decrease in their diameter comparing with the parameters in the animals of the control group (Table 1).
Figure 5.The rat tibias 14 days after surgery: а, c, e – the bones of the animals from the control group; b, d, f – the bones of the animals from the experimental group; а, b – X-rays; c, d – light microscopy of histological sections, staining by Van Gieson; e. f - images in the characteristic X-ray radiation of calcium atoms, lens 5, eyepiece 5; g - scale of calcium concentration in micro-sections (pixels) of the image.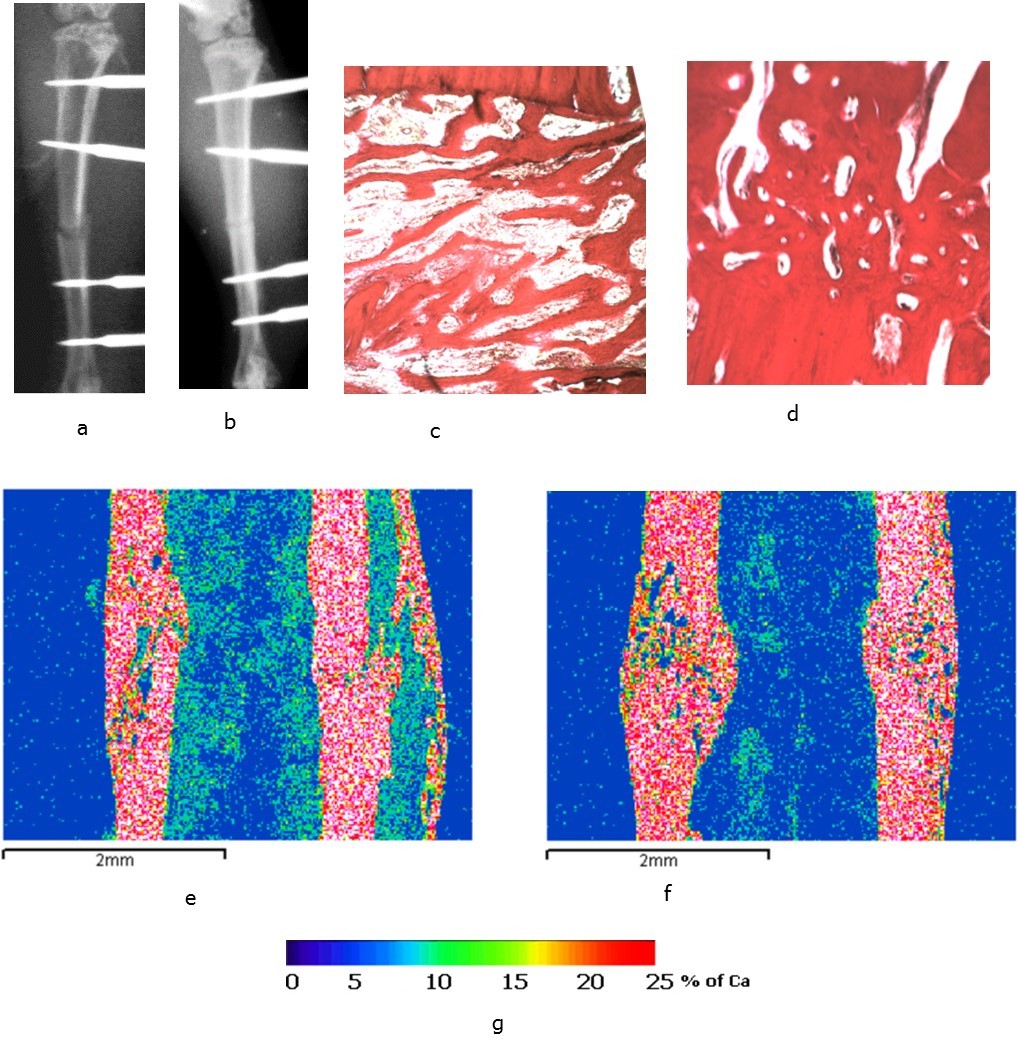
Discussion
Clinical and experimental-morphological studies by Academician G.A. Ilizarov and his students 26, 27 proved not only the possibility of a rapid process of bone tissue restoration under conditions of transosseous osteosynthesis but also laid foundations for further looking for ways to correct reparative processes in bone regeneration. According to our studies, one of the approaches to optimization of reparative osteogenesis and angiogenesis is a low-intensity infrared laser radiation method. The maximum of laser irradiation transmission by the skin is within the wavelength range of 0.8-1.2 µm. The exposure to infrared laser beams is especially effective because they penetrate well into the damaged soft tissues and reach the zone of bone fracture and the regenerated bone subjected to direct irradiation impact 3. The stimulating effect of laser radiation on reparative bone formation and angiogenesis may not be direct but mediated and realized with complex systems of autocrine, paracrine, neuroendocrine and immune regulation. In this case the mast cells are the primary cell target in bone regeneration as well as in the damaged soft tissues. Stimulation of cell secretory activity is an important amplifying mechanism in the systemic response of the organism to the action of low-intensity laser radiation. The analysis of the data obtained evidences the fact that the process of reparative osteogenesis during bone fracture healing with transosseous osteosynthesis under the impact of infrared laser irradiation occurs considerably more actively than that in the control group. This manifests itself in decreasing severity of inflammatory process, intensifying microcirculation and fibrillogenesis, as well as in the earlier regenerated bone formation and bone union achievement, acceleration of the processes of reorganization and compactization of newly formed bone tissue, the increase in its maturity degree. The intermediate regenerated bone is formed just 7 days after surgery and laser therapy session. The periosteal, intermediate and endosteal bone union is determined after 14 days. The fracture healing occurs by the primary type. After laser therapy sessions the prolonged intensive capillarogenesis is noted in bone regeneration formed with transosseous osteosynthesis. Stimulation of the endothelial outgrowths formation in the lumen of the vessels forms the capillary buds. They propagate along the “mother” vessels for considerable distances not meeting the resistance of perivascular tissue structures. This method of formation and capillary growth directly in the lumen of pre-existing vessels is called “endovasal capillarogenesis”. It was first described by us in the study of bone wound healing 28, 29, 30, 31, 32, 33. It is one of the stages of regenerative angiogenesis and provides accelerated regeneration and oriented growth of newly formed vessels.
Conclusion
The findings revealed a possible mechanism of the laser irradiation impact at the level of the whole organism. They confirm the efficiency of its use in clinical practice at the early stage of the patient rehabilitation under transosseous osteosynthesis.
Acknowledgements
We are thankful to the staff of our institutions for the assistance in carrying out the experiments and care for the animals at all the stages of our work.
References
- 1.Bidar M, Moushekhian S, Gharechahi M, Talati A, Ahrari F et al.The Effect of Low Level Laser Therapy on Direct Pulp Capping in Dogs. , J Lasers Med Sci.2016Summer.Epub2016Jul18 7(3), 177-183.
- 2.Eshghpour M, Ahrari F, Najjarkar N T, Khajavi M A. (2015) Comparison of the effect of low level laser therapy with alvogyl on the management of alveolar osteitis. Med Oral Patol Oral Cir Bucal.[PMC free article][PubMed][Cross Ref]. 20(3), 386-392.
- 3.Moosavi H, Maleknejad F, Sharifi M, Ahrari F. (2015) A randomized clinical trial of the effect of low-level laser therapy before composite placement on postoperative sensitivity in class V restorations. Lasers Med Sci. [PubMed] [Cross Ref] 30(4), 1245-1249.
- 4.Moosavi H, Maleknejad F, Sharifi M, Ahrari F. (2015) A randomized clinical trial of the effect of low-level laser therapy before composite placement on postoperative sensitivity in class V restorations. Lasers Med Sci. [PubMed] [Cross Ref]. 30(4), 1245-1249.
- 5.Heravi F, Ahrari F, Mahdavi M, Basafa S. (2014) Comparative evaluation of the effect of Er:YAG laser and low level laser irradiation combined with CPP-ACPF cream on treatment of enamel caries. J Clin Exp Dent. [PMC free article] [PubMed] [Cross Ref]. 6(2), 121-126.
- 6.Holden P K, Li C, Da Costa V, Sun C H, Bryant S V et al. (2009) The effects of laser irirradiation of cartilage on chondrocyte gene expression and the collagen matrix. Lasers Surg Med. 41(7), 487-491.
- 7.Heravi F, Moradi A, Ahrari F.The effect of low level laser therapy on the rate of tooth movement and pain perception during canine retraction. Oral Health Dent Manag. [PubMed] 13(2), 183-188.
- 8.P De Coster, Rajasekharan S, Martens L. (2013) Laser‐assisted pulpotomy in primary teeth: a systematic review. Int J Paediatr Dent. [PubMed] 23(6), 389-399.
- 9.Fernandes A P, Lourenço Neto N, Teixeira Marques NC. (2015) Clinical and radiographic outcomes of the use of Low‐Level Laser Therapy in vital pulp of primary teeth. [PubMed] , Int J Paediatr Dent 25(2), 144-150.
- 10.NL Marques NC Neto, C de Oliveira Rodini. (2015) Low-level laser therapy as an alternative for pulpotomy in human primary teeth. Lasers Med Sci. [PubMed] [Cross Ref]. 30(7), 1815-1822.
- 11.Mordon S. (2009) Cartilage reshaping by laser in stomatology and maxillofacial surgery. Rev Stomatol Chir Maxillofac. 105(1), 42-49.
- 12.Foulad A, Ghasri P, Garg R, Wong B. (2010) Stabilization of costal cartilage graft warping using infrared laser irirradiation in a porcine model. Arch Facial Plast Surg. 6, 405-411.
- 13.Ferriello V, Faria M R, Cavalcanti B N.The effects of low-level diode laser treatment and dental pulp-capping materials on the proliferation of L-929 fibroblasts. J Oral Sci. 2010; 52(1): 33–38. doi: 10.2334/josnusd.52.33. [PubMed] [Cross Ref].
- 14.Madani A S, Ahrari F, Nasiri F, Abtahi M, Tuner J.Low-level laser therapy for management of TMJ osteoarthritis. Cranio. 2014; 32(1): 38–44. doi: 10.1179/0886963413z.0000000004. [PubMed] [Cross Ref] .
- 15.Suzuki M, Ogisu T, Kato C, Shinkai K, Katoh Y. (2011) Effect of CO2 laser irradiation on wound healing of exposed rat pulp. Odontology. [PubMed] [Cross Ref] 99(1), 34-44.
- 16.Yazdanfar I, Gutknecht N, Franzen R. (2015) Effects of diode laser on direct pulp capping treatment. Lasers Med Sci. [PubMed] 30(4), 1237-1243.
- 17.Golpayegani M V, Ansari G, Tadayon N, Shams S, Mir M. (2009) Low-level laser therapy for pulpotomy treatment of primary molars. J Dent Tehran Univ Med Sci. 6(4), 168-174.
- 18.AlGhamdi K M, Kumar A, Moussa N A. (2012) Low-level laser therapy: a useful technique for enhancing the proliferation of various cultured cells. Lasers Med Sci. [PubMed] [Cross Ref] 27(1), 237-249.
- 19.Jahanbin A, Ramazanzadeh B, Ahrari F, Forouzanfar A, Beidokhti M.Effectiveness of Er:YAG laser-aided fiberotomy and low-level laser therapy in alleviating relapse of rotated incisors. [PubMed] [Cross Ref] , Am J Orthod Dentofacial Orthop 146(5), 565-572.
- 20.Ezzati A, Bayat M, Khoshvaghti A. (2010) Low-level laser therapy with a pulsed infrared laser accelerates second-degree burn healing in rat: a clinical and microbiologic study. Photomed Laser Surg. [PubMed] [Cross Ref]. 28(5), 603-611.
- 21.Sobol E, Shekhter A, Baum O Guller, Baskov A. (2011) Laser-induced regeneration of cartilage. doi: 10.1117/1.3614565.16(8). , Journal of Biomedical Optics.080902(August2011).Vol.16(8)
- 22.Iryanov Yu M, Dyuryagina O V, Nakoskin A N.Iryanova T.Yu. Support of the device for transosseous osteosynthesis. Patent 87900 RF. (Russian Ilizarov Scientific Center “Restorative Traumatology and Orthopaedics”) October27,2009;Bul,30(2009)1.[Article in Russian] .
- 23.Iryanov Yu M, Dyuryagina O V, Nakoskin A N.Iryanova T.Yu., Naumov EA. Spoke for osteosynthesis Patent 87899 RF; (Russian Ilizarov Scientific Center “Restorative Traumatology and Orthopaedics”) October27,2009;Bul,30(2009)2.[Article. in Russian
- 24.Iryanov Yu M, Petrovskaya N V, Iryanova T Yu, Naumov E A, O V Dyuryagina.Device for bone perforation. Patent 87894 RF; (Russian Ilizarov Scientific Center “Restorative Traumatology and Orthopaedics”) October27,2009;Bul,30(2009)3.[Article. in Russian
- 25.Iryanov Y M, Naumov E A, Iryanova T Y.Device for osteosynthesis of small bones Patent № 113651. RF (Russian Ilizarov Scientific Center “Restorative Traumatology and Orthopaedics”) February27,2012;Bul,6(2012)1.[Article. in Russian
- 26.Ilizarov G A, Iryanov Y M. (1991) Features of osteogenesis in tension conditions. [Article in Russian] , Bull Exp Biol 2, 194-196.
- 27.Iryanov Y M, VShevtsov I. (1995) Osteogenesis and angiogenesis in distraction osteosynthesis. [Article in Russian] , Bull Exp Biol 7, 95-99.
- 28.Iryanov Y M, Dyuryagina O V. (2014) Effect of the local granulation tissue focus formed in the medullary cavity on reparative osteogenesis. Bull Exp Biol. 1, 121-125.
- 29.Iryanov Y M, Popkov A V, Antonov N I. (2014) Morphological details of reparative osteogenesis under transosseous osteosynthesis and intramedullary insertion of wires with hydroxyapatite coating. , Morfologiia.[Article in Russian] 4, 53-57.
- 30.Iryanov Y M, Sazonova N V, Kiryanov N A. (2015) The Effect of Ultra-High Frequency Electromagnetic Radiation on Reparative Osteogenesis and Angiogenesis under Transosseous Osteosynthesis. , J Orthop Res Physiother 1, 009.
- 31.Iryanov Y M, Dyuryagina O V. (2014) Effect of the local chamber of granulation tissue formed in the medullary cavity on reparative osteogenesis. , Bull Exp Biol 1, 121-125.
