Abstract
This study evaluated the effects of aluminum sulphate exposure on the histology of adrenal gland of Wistar rats. Thirty adult Wistar rats were used for this study. The Wister rats were divided into three groups; group A was the control animals and tagged C, group B animals received 10g of alum dissolved in 1000cm3 of distilled water and were tagged T1, group C animals received 50g of alum dissolved in 1000cm3of distilled water and were tagged T2, via drinking water for duration of four weeks. Twenty-four hours after the last administration, the rats were sacrificed by cervical dislocation. The adrenal gland was excised and preserved in 10 % formosaline after which it was routinely processed for hematoxylin and eosin staining (H&E).
Histological observations showed normal cell distribution in the control group but treated group revealed evidences of cellular obliteration& hemorrhagic necrosis.
The results obtained from this study suggest that aluminum sulphate has a damaging effect on the structure of the adrenal gland.
Author Contributions
Academic Editor: Yi Zhang, Beijing University of Chinese Medicine, China
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2020 Abodunrin V. K, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
There was no conflict of interest among the authors and every necessary detail was agreed upon during the preparation of the work.
Citation:
Introduction
The use of metals has been critical to the progress of human civilization and this has resulted in their ubiquity despite their suspected toxicity 1. Human exposure has risen dramatically as a result of an exponential increase of their use in several industrial, agricultural, domestic and technological applications 2. Some metals however are essential in biological processes while others have unknown biological functions; either favorable or toxic 3. It had been found that the non-essential toxicant metalsmimic essential ones thereby gaining access into the body and potentially disrupting key cellular enzymes resulting into a wide range of health conditions 4. Among these metals, Aluminum is the most widely distributed being a trivalent cation found in its ionic form in most kinds of animal tissues and natural water 5.Human are continually exposed to this metal via cooking utensils, food products, drinking water and medicines 6
A report of its detrimental effect on reproductive organ 1, the kidney 7, the brain 8 and liver 9 has been documented. Since aluminum sulphate is being used in urban water treatment despite its presence in natural water, food and vegetables, this might expose the populace to a higher concentration of aluminum. Epidemiological studies have indicated a link between aluminum in drinking water and Alzhiemer’s disease and a variety of human and animal studies have implicated learning and memory deficits after aluminum exposure. 10, 11. Evidences of its accumulation in various organs have been documented; brain, bone, liver and kidney 12, 13 and is accompanied by renal failure or associated with age 14. Aluminum has also been reported to displace essential metals from their position thereby altering normal cellular processes 15. Despite evidences linking the exposure to aluminum with endocrine disruption 16, and unsatisfactory result from water treatment (inability to reduce the turbidity of water), yet many countries still employs the use of aluminum sulphate in its water treatment. Considering the inadequacy for toxicological data in this area, this experiment was conducted to explore the risk factor by testing aluminum sulphate in the adrenal gland of an adult Wistar rat for possible alterations in structure.
Materials and Methods
Thirty male adult Wistar rats weighing between 120 - 150g were procured from a breeding house and were used for this experiment. They were obtained and housed in standard cages under standard conditions. They were fed with feed pellets (grower mash). The rats were acclimatized for two weeks before commencement of drug administration. The Wister rats were divided into three groups each contained 10 animals; group A was the control group received no addition with drinking water, group B animals received 10g of alum dissolved in 1000cm3 of distilled water and were tagged T1, group C animals received 50g of alum dissolved in 1000cm3 of distilled water and were tagged T2 via drinking water for four weeks.
Sacrifice of Animals and Histological Techniques
Twenty-four hours after the last drug administration, the rats were sacrificed by cervical dislocation and the adrenal glands were carefully excised removing the surrounding fats and kidney, they were then preserved in 10% formosaline after which they were processed using paraffin wax embedding method. Sections of 5 μm thickness were obtained from the paraffin embedded tissues and stained with haematoxylin and eosin stain to demonstrate the general histoarchitecture of the various layers of adrenal gland 17.
Photomicrography
Stained sections were viewed under a Leica DM750 microscope (Leica Microsystems, Heerbrugg, Switzerland) with digital camera attached (Leica ICC50) and digital photomicrographs were taken at various magnifications.
Statistical Analysis
One-way ANOVA was used to analyze data, followed by Student Newman-Keuls (SNK) test for multiple comparisons. Statistically significant difference was set at p<0.05.
Results
There was a decrease in the mean weight of rats in the treated groups when compared to the control group even at the initial stages of administration, also there was a significant decrease (p < 0.05) in the mean weight of rats in the treated groups at the final stages of the administration (Table 1).
Table 1. showing the mean and standard error of mean weights of the rats at significance level of p < 0.05 and p > 0.05.| Period | Control | T 1 ( 10g) | T 2 ( 50g) |
| Initial | 143.0 ± 4.00 | 139.0 ± 8.98** | 135.0 ± 4.78* |
| Final | 161.0 ± 6.91 | 150.0 ± 6.19** | 125.0 ± 9.29* |
Histological Findings
The adrenal gland of group A (control) showed different normal cellular layers of the adrenal gland, evenly distribution of cells with definite shapes and sizes such as columnar cells with dark nuclei, polyhedral cells with swollen lipid droplets and evenly distributed polyhedral cells were evident in the cellular layers zona glomerulosa, fasciculata and reticulosa respectively, and also normal cell arrangement together with chromaffin cells, and ganglion nerve cells accompanying the central vein were seen in the adrenal medulla (Figure 1A, Figure 2A, Figure 3A, Figure 4A).
In the group B (T1) there were evidences of cellular obliteration in the adrenal cortex; zona glomerulosa, fasciculata and reticulosa and also in the zona reticulosa were haemorrhagic necrosis sites indicated by black star, also evident in the adrenal medulla were distorted and pyknotic appearance of the chromaffin cells, obliterated ganglion nerve cells and enlarged central vein with haemorrhagic necrotic sites indicated with black stars (Figure 1B, Figure 2B, Figure 3B, Figure 4B). While in the group C (T2) showed normal distribution of cellular layers and also cellular shapes, sizes and distribution just as found in the control (Figure 1C, Figure 2C, Figure 3C, Figure 4C)
Figure 1, Figure 2, Figure 3, Figure 4
Figure 1.showing the cellular layer of Zona Glomerulosa of Adrenal gland group A (control), group B (T1) and group C (T2). The evenly distributed normal columnar cells with dark nuclei in A (black arrows), evenly distributed normal pyramidal cells (black arrows) with few obliterated cells (green arrows) in B and in C, the black arrows pointing to the evenly distributed normal polydhedral cells (black arrows) having long, straight cords of large cells, swollen with lipid droplets. H&E x400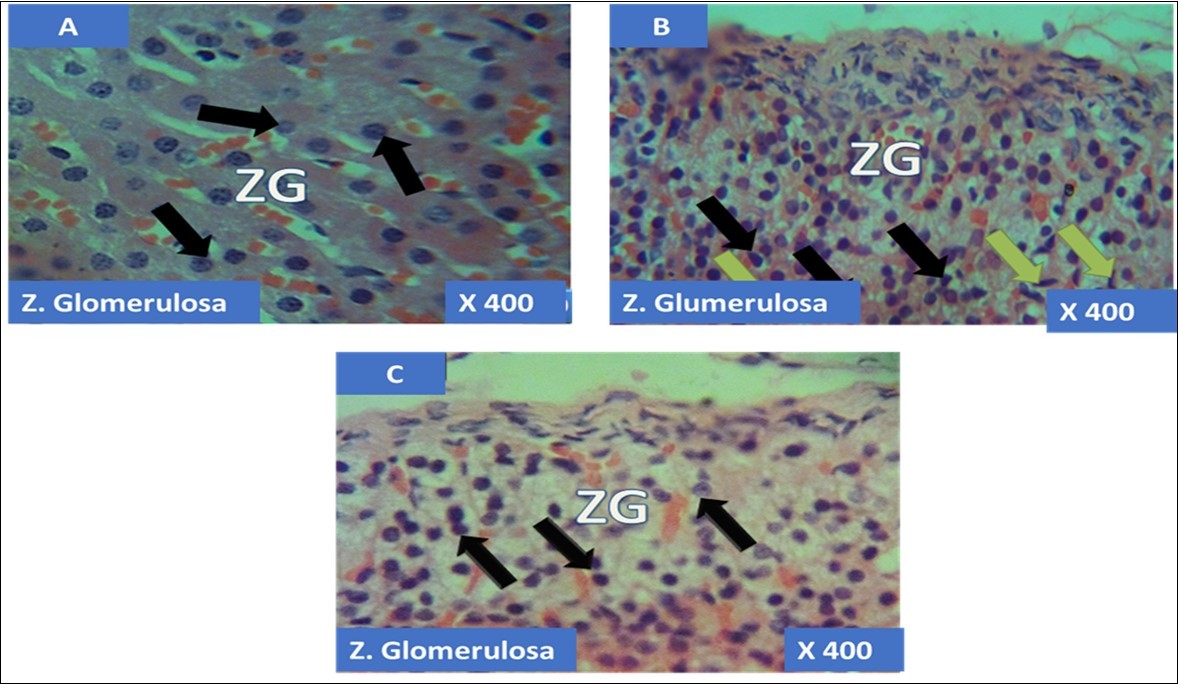
Figure 2.showing the cellular layer of Zona Fasiculata of Adrenal gland group A (control), group B (T1) and group C (T2). The evenly distributed normal large polydhedral cells having long, straight cords, swollen with lipid droplets in A (black arrows), evenly distributed normal polydhedral cells (black arrows) with few obliterated cells(green arrows) in B and in C, the black arrows pointing to the evenly distributed normal polydhedral cells (black arrows). H&E x400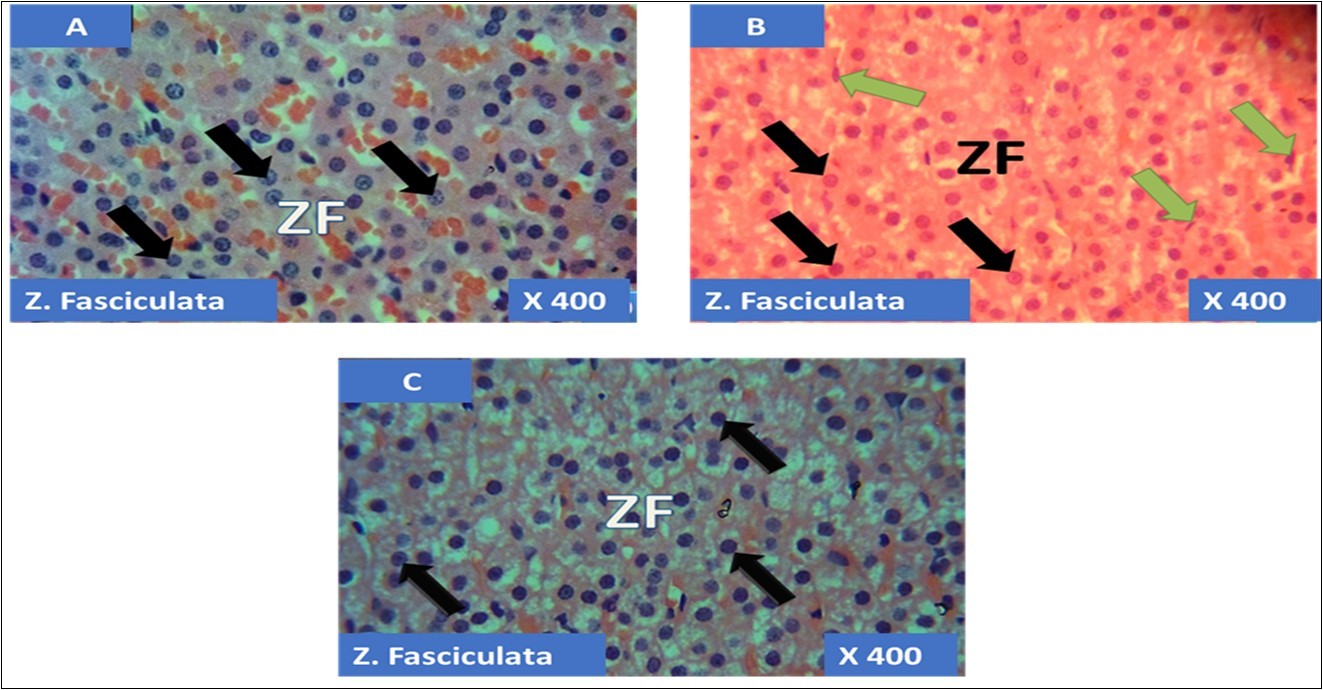
Figure 3.showing the cellular layer of Zona Reticulosa of Adrenal gland group A (control), group B (T1) and group C (T2). The evenly distributed normal polydhedral cells in A (black arrows), evenly distributed normal polydhedral cells (black arrows) with few obliterated cells (green arrows) as well as haemorrhagic necrosis sites (Black stars) in B and in C, the black arrows pointing to the evenly distributed normal polydhedral cells (black arrows). H&E x400
Figure 4.showing the cellular layer of medulla of Adrenal gland group A (control), group B (T1) and group C (T2). The evenly distributed normal chromaffin cells (black arrows) and the ganglion nerve cells (green arrows) accompanying the central vein (CV) in A. Evenly distributed distorted and pyknotically appearing chromaffin cells (black arrows) and the obliterated ganglion nerve cells (green arrows) with enlareged central vein (CV) having haemorrhagic necrosis sites(black stars) in B. normal chromaffin cells (black arrows) and the ganglion nerve cells (green arrows) with central vein (CV) in C. H&E x400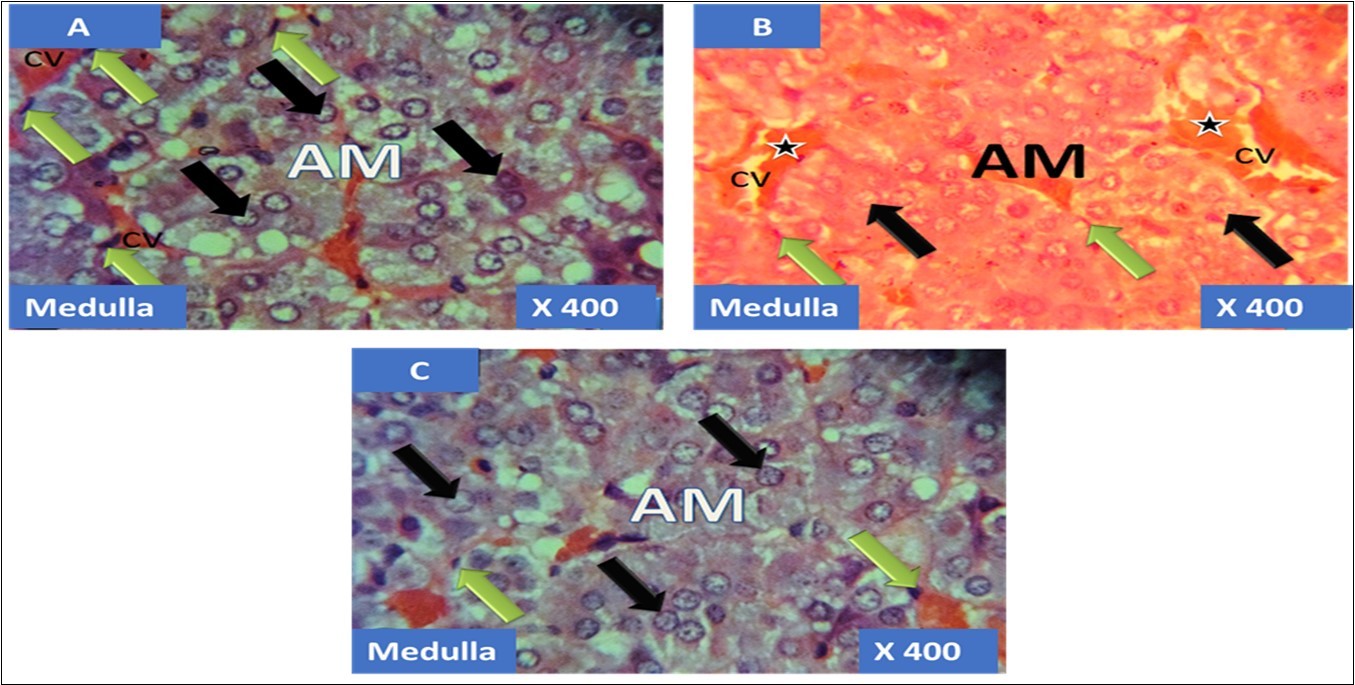
Discussion
Analysis of body mass provides important information on the general toxicity of a compound and its implications on health 6. According to existing literature, there seems to be apparently conclusive evidence that aluminum intoxication is associated with a decrease in the body weight of exposed subjects. The significant decrease in body weight associated with aluminum administration that was recorded is similar with the findings of Johri et al., 17, 18. Physically, the rats were observed to manifest signs of weakness during the period of administration. This lethargy was observed to be accompanied by loss of appetite, as a large amount of the feed were untouched during this period. The significant decrease in the body weight that was observed during this period may be attributed to a decline in food consumption since weight change depends on a balance between energy expenditure and food consumption.
The main mechanism of aluminium toxicity involves the disruption of the homeostasis of metals, such as magnesium (Mg), calcium (Ca), and iron (Fe) 15. The physical and chemical properties of aluminium allow it to effectively mimic these metals in their respective biological functions and trigger biochemical abnormalities. Aluminium has been shown to replace magnesium and bind to phosphate groups present in cell membrane, DNA and ATP 19. However, the effect of aluminium on iron homeostasis is the pivotal factor that renders this metal toxic 20, 21. This interaction generates labile iron from iron-containing enzymes and proteins. The intracellular pool of free iron therefore increases; a situation conducive to the formation of reactive oxygen species. Indeed, elevated levels of reactive oxygen species have been documented in various systems exposed to aluminium 22, 23, 24. In the present study, the histology of the adrenal gland of the control group (plate 1-6) shows apparently healthy histoarchitecture with well define-cellular layers and boundaries. Cells at each layer are well defined and identifiable. Whereas, observed in the treated groups were obliterated cells, haemorrhagic necrosis and enlarged central vein. A disruption in cellular integrity is a pointer to a disturbed functionality. Since the adrenal gland plays a vital role in the production of hormones that regulates the storage of end products of metabolism and also secretes dopamine (adrenal medulla), therefore, the aforementioned process might have been impaired. To the best of our knowledge, little or no literature exists on the effect of aluminum sulphate on the adrenal gland.
Conclusion
Aluminum sulphate has a detrimental effect on the body weight and structure and by extension, on function of the adrenal gland. Therefore, its use in the treatment of water and other industrial application should be done with caution. We recommend further studies to elucidate the mechanism via which aluminum causes its toxicity and the dosage of aluminum that would be considered safe.
References
- 1.Pandey G, G C Jain. (2013) A Review on toxic effects of aluminium exposure on male reproductive system and probable mechanisms of toxicity. , International Journal of Toxicology and Applied Pharmacology 3(3), 48-57.
- 2.Bradl H. (2002) Heavy Metals in the Environment: Origin, Interaction and Remediation. , London: 6.
- 3.Mathur N, Pandey G, G C Jain.Male reproductive toxicity of some selected metals: A Review.J Bio sci.2010;. 10, 396-404.
- 4.M A Veado, I A Arante, A H Oliveira, M R Almeida, R A Miguel et al. (2006) Metal pollution in the environment of minas gerais state-Brazil. , Environ. Monit. Assess 117, 157-172.
- 5.Kutlubay R, E O Oguz, Can B, M C Guven, Sinik Z et al. (2007) Vitamin E protection from testicular damage caused by intraperitoneal aluminium. , Int. J. Toxicol 26, 297-306.
- 6.Sharma P, Mishra K. (2006) Amelioration of fumonisin B1 hepatotoxicity in mice by depletion of T cells with anti-Thy-1.2. Reprod. Toxicol 21, 313-321.
- 7.Shi-liang X, Miao L, Bing S, Chong-sheng B, Ji-hong Z et al. (2013) Effects of sub-chronic aluminum exposure on renal pathologic structure in rats. , Journal of Northeast Agricultural University (English Edition) 20(1), 49-52.
- 8.Buraimoh A A, Ojo S A, Hambolu J O, Adebisi S S. (2011) . , Effects of Oral Administration of Aluminium Chloride on the Histology of the Hippocampus of WistaRats.Current Research Journal of Biological Sciences 3(5), 509-515.
- 9.S U Rani, Chitra M, &Jainu M. (2014) Hepatoprotective effect of wattkakavolubilis extract on aluminium sulphate induced liver toxicity. , International Journal of Pharmaceutical Sciences and Drug Research 6(2), 169-173.
- 10.C A Exley.The aluminium-amyloid cascade 25. Subcell.The use of the Morris watermazeinthe Biochem.2005;38: 225-234.
- 12.M R Wills, C D Hewitt, B C Sturgill, Savory J, M. (1993) Long-termoral or intravenous aluminium administration in rabbits.I. Renal and hepatic changes.Ann.Clin. Lab Sci.1993;23:. 1-16.
- 13.Sahin G, Varol I, &Temizer A. (1994) Determination of aluminium levels in the kidney,liver, and brain of mice treated with aluminium hydroxide.Biol. , Trace. Element Res.1994; 41, 129-135.
- 14.Gómez M, D J Sánchez, J M Llobet, Corbella J, J L Domingo. (1997) The effect ofage on aluminium retention in rats.Toxicol.116:. 1-8.
- 15.Kawahara M, Kato-Negishi M. (2011) Link between aluminum and the pathogenesis of Alzheimer's disease: the integration of the aluminum and Amyloid cascade hypothesis. Int J Alzheimer's Dis. 276393.
- 16.Kandaraki E, Christakou C, &Diamanti-Kandarakis E. (2009) Metabolic syndrome and polycystic ovary syndrome... and vice versa. , ArquivosBrasileiros de Endocrinologia&Metabologia 53(2), 227-237.
- 17.Hegazy R, Hegazy A. (2015) Hegazy’ Simplified Method of Tissue Processing (Consuming Less Time and Chemicals. Annals of International Medical and Dental Research 1(2), 57-61.
- 18.P K Johri, Tripathi R, Johri R. (2011) Effect of chronic oral administration of aluminum hydroxide on the fertility of male rabbit. , Journal of Experimental Zoology 14(2), 453-455.
- 19.Khattab H A M, Abdallaha I Z A, G M Kamel. (2010) Grape seed extract alleviate reproductive toxicity caused by aluminium chloride in male rats. , Journal of American Sciences 6(12), 1200-1209.
- 20.Tomljenovic. (2011) L.Aluminum and Alzheimer's disease: after a century of controversy, is there a plausible link? J Alzheimers Dis. 23, 567-98.
- 21.Peto M. (2010) V.Aluminum and iron in humans: bioaccumulation, pathology and removal. Rejuvenation Res. 13, 589-98.
- 22.Wu Z, Du Y, Xue H, Wu Y, Zhou B. (2012) Aluminum induces neurodegeneration and its toxicity arises from increased iron accumulation and reactive oxygen species (ROS) production. Neurobiol Aging. 33-199.
