Abstract
The aim of the present study was to compare mandibular neurovascular canal anatomy in human and great apes by using cone beam computed tomography (CBCT). The anatomical variability of mandibular neurovascular canals (mandibular, incisive and lingual canals) of 129 modern humans and great apes (Homo, Pan and Gorilla) were analyzed by linear measurements on CBCT images. The Kruskal-Wallis non-parametric test and Dunn’s all pairs for joint ranks were applied to compare the variability of mandibular canals among these groups.
Human, Chimpanzee and Gorilla groups showed significant differences in the dimensions of the mandibular canal, mental foramen, incisive canal, lingual canal and anterior mandibular bone width. Bifid mandibular canals and anterior loops were the anatomical variations most frequently observed in the Gorilla. Humans had a larger mental foramen and a distinctive incisive canal. The latter could not be identified in the Gorilla group.
The variability in the anatomy within mandibles of human and non-human primates, shows different forms in the neurovascular structures. In comparison to the mandible of great apes, the incisive canal is suggested to be a feature unique to the human mandible.
Author Contributions
Academic Editor: Hesham N. Mustafa, Associate Professor of Anatomy, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2018 Livia Corpas, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
The anatomy of the human mandible has been widely studied by means of advanced imaging technologies such as cone beam computed tomography (CBCT)1, 2, 3, 4, which is able to provide accurate, essentially immediate and non-invasive 3D radiographic images of teeth, soft-tissues, nerve paths and bone structures in the craniofacial region. With the growing morphology studies using 3D imaging in vivo or in vitro, in combination with increasingly sophisticated computer graphics applications, it also shows potentials of CBCT in the application of comparative anatomy, anthropology and forensic medicine for paleontologists5, 6, 7.
Mandibular anatomy has been revisited by 3D imaging with a focus on the mental foramen 8, mandibular canal9, 10, incisive canal11, 12, lingual canal6, 13, 14, 15. Although the mandibular form may reflect functional adaptation to forces experienced during mastication16, the mandibular neurovascular canal has been considered as the most stable structure guiding mandibular development17, 18. It thus may be a relevant structure to indicate nerves intra- and inter- specific patterning related to mandibular anatomy. Furthermore, it was considered that the diversities related to nerves are cranial discrete traits of the modern human skull19. These anatomy variations in the mandible canal may result from a process of adaptation to various environmental and subsistence patterns as well as random drift by population size, network and isolation, leading to the development of regional frequency patterns20, 21, 22. Dimensional variability, eg., larger canal diameter and proximity to root apices, should be considered so as to avoid, or at least anticipate as high risk factors for inferior alveolar nerve injuries during the treatment planning and oral maxillofacial surgery23.
The present study was therefore aimed to quantitatively evaluate anatomical characters of mandibular neurovascular canals of humans and great apes. The crossing of information generated by intra- and inter-specific approaches may yield useful outcomes for clinical applications, anthropology and forensic dentistry.
Materials and Methods
The study sample consisted of 129 mandibles from modern humans (Homo sapiens; n=94), chimpanzees (Pan paniscus; n=20) and gorillas (Gorilla gorilla graueri; n=15). Mandibular datasets were derived from the Oral Maxillofacial Imaging Center of the University Hospitals (KU Leuven, Leuven, Belgium), the Royal Belgian Institute of Natural Sciences (Brussels, Belgium) and the Royal Museum for Central Africa (Tervuren, Belgium). Homo sapiens specimens from Brazil, Belgium, China, Congo, Greenland, India and Indonesia, were included to cover a broad range of modern human variation. Nonhuman hominoids were allocated to sex based on data from museum records and by examining canine size and shape. Modern human skulls were sexed using museum records and standard osteological criteria. Ethical approval was obtained from the local Commission for Medical Ethics of the University Hospitals Leuven (S57587).
The CBCT images of modern humans were taken using i-CAT CBCT scanner (I-CAT®, Imaging Sciences International, Hatfield, PA, USA) with a voxel size of 0.2 mm. As for the dry mandibles of primates, images were acquired by means of 3D Accuitomo CBCT (J.Morita, Kyoto, Japan) with a voxel size of 0.125mm. All axial, sagittal and coronal images were carefully examined under the standardized viewing condition. Considering the high reliability of linear measurements on CBCT images24, 25, 26, linear measurements on the previous taken CBCT images were performed by using i-Dixel (J. Morita, Kyoto, Japan) and MVE (Dr. Jürgen Abel, Neuss, Germany) software tools. The smallest voxel size of each system was used in this study. The sections of anatomical structures were observed in coronal, sagittal and axial views through 3D imaging reconstruction.
The analyses consisted of the measurements in neurovascular canals- mandibular, incisive and lingual canals. The mandibular canal was the canal extending from the mandibular foramen on the medial surface of the ramus of the mandible to the mental foramen. Lingual canals were bony canals found at the middle anterior region of the mandible, while the lateral canals were those located to the right or left side of this middle region27. The lingual canals were further categorized as upper, middle and lower, according to their vertical position related to the genial tubercles or classified into midline and lateral according to their relation with the midline. The mandibular incisive canal was the anterior extension of the mandibular canal after passing the mental foramen.
Statistical Methods
To avoid data clustering, only one side of the mandible was chosen for statistical analyses. All data were collected and statistically analyzed using JMP 8 (SAS Institute Inc., SAS Campus Drive, Cary, North Carolina 27513, USA) for windows software version 7, at a significance level of 5%. Descriptive statistics were used to describe variability within groups. The Kruskal-Wallis Non-Parametric test and Dunn’s All Pairs for Joint Ranks were applied to identify which variables differ between groups. For categorical variables, the Contingency Analysis and Chi-Square tests were applied to define how responses distribute distinctively between groups.
Results
The typical images from modern human and great apes with different anatomical features were shown in Figure 1 and Figure 2. A group of variables were categorized as: the presence of the incisive canal, the number of mandibular canal bifurcations, the potential occurrence of an anterior loop, the presence of the incisive canals, its end-point and connection of the incisive to the lingual canals (Figure 3). Interspecific variability was determined by comparing contemporary human and great ape mandibles. In this analysis, mandibles from the different geographical locations were grouped in the human group, and then compared to mandibles of chimpanzees and gorillas.
Figure 1.The classical sample images with different anatomical features. A general view of a 20-year-old chimpanzee (A), its three-dimensional CBCT view (B) and two-dimensional panoramic view (C).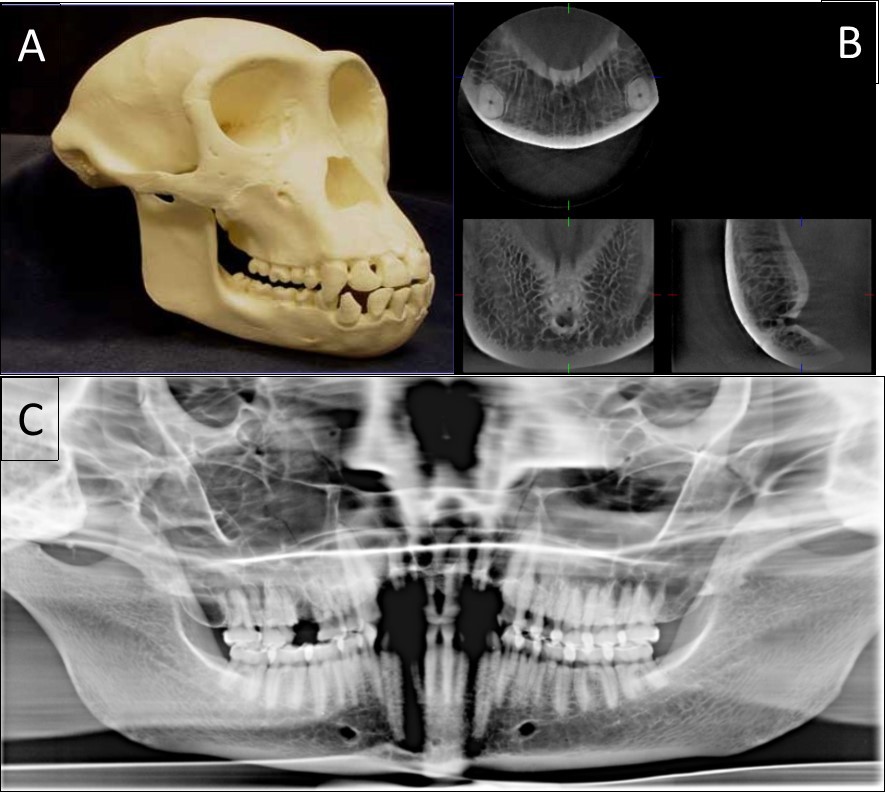
Figure 2.A general view of a gorilla mandible with a vertical double foramen (A) and a human mandible with a horizontal double foramen (B).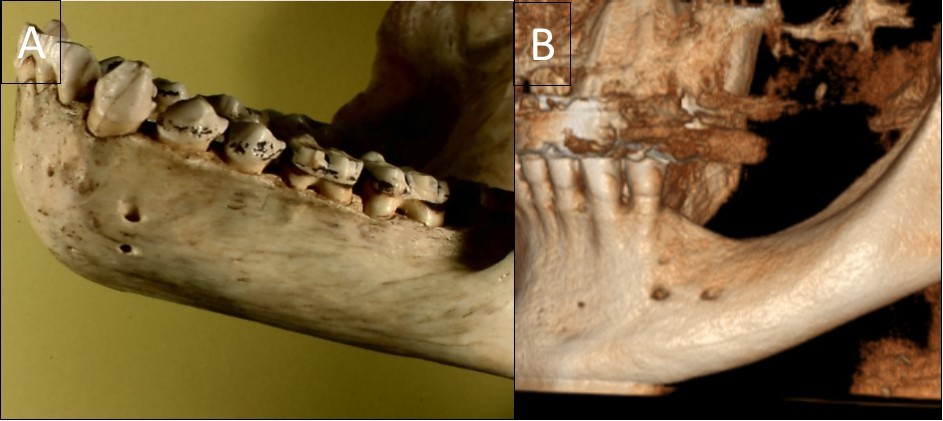
Figure 3.Cone beam CT image (A) shows the oblique reconstruction of the volume of interest at the region of premolars. In this reconstruction, the anterior loop of the mandibular canal can be clearly observed before the start of the incisive canal. The appearance of this anterior loop in a cross-sectional view is shown in image (B).
The results showed significant differences in the dimensions of neurovascular canals and tooth roots in primates. Intra- and inter-specific analyses revealed that neurovascular mandibular canals, root lengths and the distance between these structures can vary significantly amongst humans and primates.
Human, Chimpanzee and Gorilla groups showed significant differences in the dimensions of the mandibular canal, mental foramen, incisive canal, lingual canal and in the anterior mandibular bone width (Figure 4). The Gorilla group showed significant higher medians for the mandibular canal, lingual canal length and bone width than Human and Chimpanzee groups (Table 1).
Figure 4.Cross-sectional views show the lingual canal. Images (A), (B) and (C) show the measurement of bone width (BW, the longest distance in the anterior mandibles under the lingual canal), canal length (CL) and lingual canal diameter (buccal and lingual), respectively.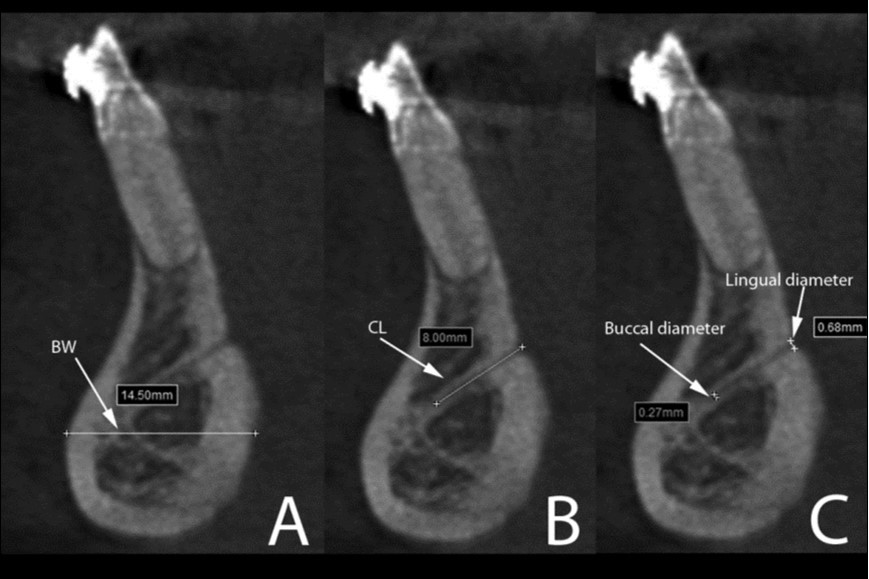
| Mandibular canaldiameter (mm) | Lingual canallength (mm) | Anterior bonewidth (mm) | |||||||
| Group | Median | 25% | 75% | Median | 25% | 75% | Median | 25% | 75% |
| Human (n=94) | 2.6 c | 2.2 | 3.0 | 3.9 c | 3.0 | 5.3 | 12.5 b | 11.0 | 14.0 |
| Chimpanzee (n=20) | 2.8 | 2.4 | 3.8 | 3.0 bc | 1.9 | 3.6 | 9.6 b | 8.7 | 11.1 |
| Gorilla (n=15) | 3.5 c | 3.5 | 4.0 | 5.6 b | 3.6 | 8.6 | 18.4 b | 16.1 | 24.3 |
On the other hand, human mandibles showed significant larger range of mental foramen (3.3mm/2.7-4.0mm) compared to Chimpanzee (2.1mm/1.7-2.7mm) and Gorilla (2.1mm/1.9-2.9mm). No incisive canal could be observed in the Gorilla group, while very few were presented in Chimpanzee. The incisive canal diameter was significantly larger in Human (IC/start: 1.9mm/1.6-2.6mm) than in Chimpanzee (IC/start: 0.9mm/0.8-1.1mm). For the root lengths, the longest third molar was observed in the Gorilla group (13mm/10.1-14.8mm), whereas the Human group presented the longest second premolar (14.5mm/13.4-16.4mm) and Chimpanzee showed the longest canine (18.1mm/15.4-21.9mm).
No statistically significant results were found for the categorical variables, although interesting findings could be observed in our sample. Bifid mandibular canals were anatomical variations that most frequently observed in the Gorilla (Table 2). Neither a bifid canal nor an anterior loop could be observed in Chimpanzee (Table 2).
Table 2. Overview of sample size and frequency of bifid mandibular canal and anterior loop in the study sample.| Group | Sample size(n) | Bifid mandibular canal (n) | Anterior loop(n) |
| Human | 94 (60M, 34F) | 7 | 7 |
| Chimpanzee | 20 (9M, 11F) | 0 | 0 |
| Gorilla | 15 (8M, 7F) | 6 | 0 |
Different distributions were observed in the lingual canal, related to its vertical and horizontal position. Regarding to the horizontal position, this canal was mostly found at the midline in Human, whereas it was frequently found in the midline and left position in Gorilla and Chimpanzee. Vertically, it was more frequently observed above the superior genial tubercles in humans, in contrast to a more middle position in Chimpanzee and Gorilla groups. A higher number of extra lingual canals were also observed in Chimpanzee group. Multivariate analysis showed variable redundancies, patterning in data sample group differentiation between Gorilla, Chimpanzee and contemporary Human mandibles, without overlapping in their distributions (Figure 5).
Figure 5.Linear discriminant analyses from the canonical plots show interspecific results of neurovascular canal features between Gorilla (*), Chimpanzee (Í) and contemporary Human mandibles (+), with distinctive, non-overlapping distributions in (A) and (B). RL 38: root length of #38 tooth; DA38: distance canal to root apex of #38 tooth; φMC38: the diameter of mandibular canal at molar #38; φMC35: the diameter of mandibular canal at premolar #35; φMF: the diameter of mandible mental foramen.
Discussion
This study evaluated anatomical variability of mandibular neurovascular canals between humans and non-human primates, including Chimpanzee and Gorilla groups.
With the obtained image quality, large differences were observed in the inter-specific analysis. The largest bone width at anterior regions of Gorilla group was followed by the longest lingual canal and highest prevalence of lateral canals. More interestingly, no gorillas and just a few chimpanzees presented an incisive canal, whereas almost all modern humans showed the canal, which is in agreement with a previous study27. It is suggested that the highest prevalence of lateral canals in great apes, as well as the highest prevalence of incisive canal in modern humans might be related to some morphological and functional characteristics of those two different taxa, e.g. the superior transverse torus or simian shelf in great apes and a protruding osseum mentum or chin in anatomically modern humans.
In fact, the simian shelf, which is a lingual protuberance responsible to provide a more robust mandible, characterizes great apes’ mandibles28. On the other hand, the chin is a feature unique to modern humans that was speculatively related to human speech ability, although others advanced hypotheses that it is decided by the functional and biomechanical significance of the mental protuberance28, 29, 30. In this way, the incisive canal might be related to the emergence of the mental protuberance in humans. Furthermore, morphology and function must be likewise responsible for divergences in the lingual canal position since its foramina are close to muscle attachments. Those muscles are intimately involved in the function and support of the tongue and its associated soft tissues31.
The mental foramen and incisive canal in modern humans were larger than in great apes. So far as we know, no similar report on the mental foramen has been found in the literature. In spite of being constantly addressed as a feature descriptor for fossil mandibles and extant animals16, 32, 33. Instead of evaluating of the differences of mental foramen position34, we further compared the dimensional changes of the mandibular canal, mental foramen, incisive canal, lingual canal and anterior mandibular bone width in the present research.
According to another study35, modern humans, chimpanzees, orang-utans, and many other primates share the morphology of mandibular ramus which differs from that of gorillas, so that the gorilla anatomy must represent a unique condition with its appearance from an independently derived morphology. Indeed, gorillas did not share the same dimensions for tooth root and mandibular canals neither with humans, nor with chimpanzees. Furthermore, humans did not share the same dimensions for tooth roots and mandibular canals with chimpanzees.
It has to be noted, though, the comparative anatomy studies using 3D imaging modality largely depends on the voxel size applied. The higher the resolution of the scanners, the finer the anatomical details. In this study, we could reach a resolution about 0.125mm-0.2mm with a big field of view to cover the whole region of interest, which was capable to distinguish mandibular neurovascular canal anatomy in human and great apes. Whereas micro-CT used to be experimental with even dedicated voxel size, varying between 1µm and 50µm, the micro-CT scanning is generally limited by the smaller size of the specimen, which is not applicable for our samples. Another possible limitation for this study was that only one side of the mandible from each sample was chosen for statistical analyses, considering the number of sample from each group distributed unequally.
The current research may yield useful anatomical information to researchers, anthropologists, surgeons, radiologists and forensic specialists. Furthermore, this study highlights the importance of using CBCT images when conducting interspecific comparisons of morphological features in a nondestructive way.
Conclusion
This study described the significant variability of neurovascular canals and tooth roots in modern humans and great apes, with the former often having larger-diameter canals in the anterior mandible. Tooth root, mental foramen and incisive canal presented a high variability for mandibles from different periods of time, geographical origins and species. It suggests that incisive canal may be a feature unique to the human mandible. Further researches with larger samples would be helpful to verify and confirm this morphologic links.
Acknowledgements
Fellowship support from Research Foundation Flanders (FWO) from the Belgian government.
References
- 1.Oliveira-Santos C, Souza P H, Azambuja Berti-Couto S De, Stinkens L, Moyaert K et al. (2011) Characterisation of additional mental foramina through cone beam computed tomography. , Journal of Oral Rehabilitation 38, 595-600.
- 2.W De Vos, Casselman J, Swennen G R. (2009) Cone-beam computerized tomography (CBCT) imaging of the oral and maxillofacial region: a systematic review of the literature. , International Journal of Oral Maxillofacial Surgery 38, 609-625.
- 3.Greenstein G, Cavallaro J, Tarnow. (2008) Practical application of anatomy for the dental implant surgeon. , Journal of Periodontology 79, 1833-1846.
- 4.Spoor F, Jeffery N, Zonneveld F. (2008) Using diagnostic radiology in human evolutionary studies. , Journal of Anatomy 197, 61-76.
- 5.A R El-Beialy, M S Fayed, A M El-Bialy, Y A Mostafa. (2011) Accuracy and reliability of cone-beam computed tomography measurements: influence of head orientation. , Am J Orthod Dentofac Orthop 140(2), 157-165.
- 6.Liang X, Jacobs R, Lambrichts I, Vandewalle G. (2007) Lingual foramina on the mandibular midline revisited: a macroanatomical study. Clinical Anatomy. 20, 246-251.
- 7.Beaini T L, Duailibi-Neto E F, Chilvarquer I, Melani R F.Human identification through frontal sinus 3D superimposition:. , Pilot study with Cone Beam Computer Tomography. Epub2015Sep11. J Forensic Leg Med 2015, 63-9.
- 8.Greenstein G, Tarnow D. (2006) The mental foramen and nerve: clinical and anatomical factors related to dental implant placement: a literature review. , Journal of Periodontology 77, 1933-1943.
- 9.Kim S T, Hu K S, Song W C, Kang M K, Park H D et al. (2009) Location of the mandibular canal and the topography of its neurovascular structures. , Journal of Craniofacial Surgery 20, 936-939.
- 10.Levine M H, Goddard A L, Dodson T B. (2007) Inferior alveolar nerve canal position: a clinical and radiographic study. , Journal of Oral Maxillofacial Surgery 65, 470-474.
- 11.Jacobs R, Mraiwa N, D van Steenberghe, Gijbels F, Quirynen M. (2002) Appearance, location, course, and morphology of the mandibular incisive canal: an assessment on spiral CT scan. Dentomaxillofacial Radiology. 31, 322-327.
- 12.Romanos G E, Greenstein G. (2009) The incisive canal. Considerations during implant placement: case report and literature review. , International Journal of Oral and Maxillofacial Implants 24, 740-745.
- 13.Liang X, Jacobs R, Lambrichts I. (2006) An assessment on spiral CT scan of the superior and inferior genial spinal foramina and canals. Surgical and Radiological Anatomy. 28, 98-104.
- 14.Katakami K, Mishima A, Kuribayashi A, Shimoda S, Hamada Y et al. (2009) Anatomical characteristics of the mandibular lingual foramina observed on limited cone-beam CT images. Clinical Oral Implants Research. 20, 386-390.
- 15.Mowafey B, Vande Casteele E, Yousset J M, Zaher A R, Omar H. (2015) Can mandibular lingual canals be used as a forensic fingerprint?. , J Forensic Odontostomatol 33, 26-35.
- 16.Plavcan J M, Daegling D J. (2006) Interspecific and intraspecific relationships between tooth size and jaw size in primates. , Journal of Human Evolution 51, 171-184.
- 17.Captier G, Lethuilier J, Oussaid M, Canovas F, Bonnel F. (2006) Neural symmetry and functional asymmetry of the mandible. Surgical and Radiological Anatomy. 28, 379-386.
- 18.Curien R, Braun M, Perez M, Bravetti P, Coqueugniot H. (2011) Discriminant study of the development of the mandibular units in a neural reference system. , Surgical and Radiological Anatomy 33, 191-196.
- 19.Hanihara T, Ishida H. (2001) Frequency variations of discrete cranial traits in major human populations. I. Supernumerary ossicle variations. , Journal of Anatomy2001; 198, 689-706.
- 20.Hanihara T, Ishida H. (2001) Frequency variations of discrete cranial traits in major human populations. II. Hypostotic variations. , Journal of Anatomy2001; 198, 707-725.
- 21.Hanihara T, Ishida H. (2001) Frequency variations of discrete cranial traits in major human populations. III. Hyperostotic variations. , Journal of Anatomy2001; 99, 251-272.
- 22.Hanihara T, Ishida H. (2001) Frequency variations of discrete cranial traits in major human populations. IV. Vessel and nerve related variations. , Journal of Anatomy2001; 199, 273-287.
- 23.Kovisto T, Ahmad M, Bowles W R. (2011) Proximity of the mandibular canal to the tooth apex. , Journal of Endodontics 37, 311-315.
- 24.Liang X, Lambrichts I, Sun Y, Denis K, Hassan B et al. (2010) A comparative evaluation of Cone Beam Computed Tomography (CBCT) and Multi-Slice CT (MSCT). Part II: On 3D model accuracy. , European Journal of Radiology 75, 270-274.
- 25.Loubele M, Guerrero M E, Jacobs R, Suetens P, D van Steenberghe. (2007) A comparison of jaw dimensional and quality assessments of bone characteristics with cone-beam CT, spiral tomography, and multi-slice spiral CT. , International Journal of Oral and Maxillofacial Implants 22, 446-454.
- 26.Loubele M, N Van Assche, Carpentier K, Maes F, Jacobs R et al. (2008) Comparative localized linear accuracy of small-field cone-beam CT and multislice CT for alveolar bone measurements. Oral Surgery Oral Medicine Oral Pathology Oral Radiology and Endodontics. 105, 512-518.
- 27.Jacobs R, Lambrichts I, Liang X, Martens W, Mraiwa N et al. (2007) Neurovascularization of the anterior jaw bones revisited using high-resolution magnetic resonance imaging. Oral Surgery Oral Medicine Oral Pathology Oral Radiology and Endodontics. 103, 683-693.
- 28.Gröning F, Liu J, Fagan M J, O’Higgins P. (2011) Why do humans have chins? Testing the mechanical significance of modern human symphyseal morphology with finite element analysis. , American Journal of Physical Anthropology 144, 593-606.
- 29.Ackermann R R, Cheverud J M. (2004) Detecting genetic drift versus selection in human evolution. , Proceedings of the National Academy of Sciences of the United States of America 101, 17946-17951.
- 30.Schwartz J H, Tattersall I. (2000) The human chin revisited: what is it and who has it?. , Journal of Human Evolution 38, 367-409.
- 31.Silverstein K, Costello B J, Giannakpoulos H, Hendler B. (2000) Genioglossus muscle attachments: an anatomic analysis and the implications for genioglossus advancement. Oral Surgery Oral Medicine Oral Pathology Oral Radiology and Endodontics. 90, 686-688.
- 32.Royer D F, Lockwood C A, Scott J E, Grine F E. (2009) Size variation in early human mandibles and molars from Klasies River, South Africa: comparison with other middle and late Pleistocene assemblages and with modern humans. , American Journal of Physical Anthropology 140, 312-323.
- 33.Humphrey L T, Dean M C, Stringer C B. (1999) Morphological variation in great ape and modern human mandibles. , Journal of Anatomy 195, 491-513.
