Abstract
We aimed to study the effect of buspirone, an anxiolytic drug and 5-HT1A agonist on liver injury induced by carbon tetrachloride (CCl4) in rats. Rats were orally treated with CCl4 (2.8 mL/kg in olive oil) along with buspirone at 10, 20 or 30 mg/kg once daily starting with CCl4 and for one week thereafter. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) as well as alkaline phosphatase (ALP) activities were determined in the serum. Markers of oxidative stress: lipid peroxidation (malondialdehyde; MDA), reduced glutathione (GSH), nitric oxide (nitrite/nitrate) levels were measured in the liver. Moreover, paraoxonase 1 activity was determined in the liver and serum. The administration of CCl4 led to significant increases in serum ALT, AST, and ALP activities. Results showed that there were significantly increased hepatic MDA, nitrite and decreased GSH levels. PON1 activity decreased both in the liver and serum, respectively. The immunohistochemical investigations using anti-caspase-3 antibody revealed that CCl4 caused apoptosis to many hepatocytes. DNA studies showed that CCl4 caused hypoploidy in hepatocytes. Rats treated with 20-30 mg/kg buspirone showed significant decrease in serum ALT and AST by 19.5-34.3% and 24.2-31.4%, respectively. Serum ALP decreased by 21.7% after 30 mg/kg buspirone. In the liver, the higher dose of the drug resulted in decreased MDA (by 15.8%), decreased nitric oxide (17.4%) and increased GSH (by 20.1%). Significantly increased serum PON1 activity by 43.9-53.5% was observed after treatment with 20-30 mg/kg buspirone. On histopathologic examination of liver sections, there was mild protective effect for the drug at 30 mg/kg. Sections stained with anti- caspase- 3 confirmed the results obtained from histopathological examination. Moreover, buspirone given at 30 mg/kg resulted in an increase in % of cells containing normal values of DNA. These results indicate that buspirone decreases liver oxidative stress and exerts protective effect against CCl4- toxicity. The study thus indicates more beneficial effects of buspirone as an anxiolytic drug and that the drug could be used safely in patients with liver disease.
Author Contributions
Academic Editor: Wei Wu, Nanjing Medical University
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2017 Omar M.E.Abdel-Salam, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Buspirone is a partial 5-HT1A receptor agonist that is widely used in treating anxiety disorders and depression 1. 5-HT1A receptors are presynaptically located as somatodendritic receptors on the 5-HT neurons in the midbrain raphe nuclei and also postsynaptically in limbic and cortical regions. Stimulation of 5-HT1A receptors decreases the firing of 5-HT neurons and 5-HT terminal release. This results in inhibition of serotonergic neurotransmission 2. The drug in addition has a weak affinity for 5-HT2 receptors and act as an antagonist at dopamine D-2, D-3 and D-4 receptors 3, 4, 5. In animal models of pain, buspirone exerted analgesic action increasing the threshold to thermal, electrical, chemogenic, and visceral pain. The drug inhibits gastric acid secretion and exerts gastric mucosal protective and anti-inflammatory effects 6.
Serotonin has wide distribution in the brain and gut. In brain, serotonin plays an important role in mood, behavior, aggression, and sexual function 7. Serotonin is decreased in depression and thus drugs which increase brain serotonergic activity eg., the serotonin reuptake inhibitors (SSRIs) are the most common agents used nowadays in treating depressive disorders 8 including depression that occurs in the course of liver disease and/or results from antiviral therapy 9. There is also evidence involving the brain serotonergic system in the development of depression in patients with chronic liver disease 10 and in hepatic encephalopathy 11 and it is likely that changes in brain serotonin could modulate liver injury.
In the body, serotonin exists mainly in the gut, being produced by the enterochromaffin cells, whilst a small amount is present in plasma stored in platelets 12. Studies suggested an important role for serotonin derived from platelets in liver regeneration 13, 14 and also in causing hepatic injury 15, 16. 5-HT2A receptors are upregulated in activated hepatic stellate cells, the principal cells mediating liver fibrosis and 5-HT2A antagonists inhibit activation of HSCs 17. In hepatocytes, 5-HT1/2A receptors stimulate, whereas 5-HT2B receptors inhibit glycogen synthesis 18. There is also a decrease in hepatic 5- HT1A receptor function during hepatocyte regeneration and neoplasia while stimulation of 5-HT1A receptor inhibited hepatocyte DNA synthesis 19. The above data point to the important role of serotonin in modulating the integrity of the liver.
The administration of the serotonin reuptake inhibitors or serotonergic antagonists eg., trazodone and nefazodone protected against the CCl4- induced hepatic toxicity in rodents 20, 21, 22. The mechanism by which these drugs decrease the toxin-induced liver damage is not clear, but increased central serotongeric activity 20, prevention of the metabolic derangement induced by CCl422, reduced platelet serotonin or decreased liver nitric oxide 21 have been suggested.
In this study, our aim was to investigate the possible modulatory effect of buspirone on the development of hepatic injury caused by CCl4. The latter is a widely used industrial solvent which is known to cause heptotoxicity in humans and rodents. The acute administration of CCl4 causes fatty degeneration, and hepatocellular death with the mechanism largely involving free radical-mediated oxidative damage to cellular biomolecules23, 24.
Materials and Methods
Animals
The study was conducted using Sprague–Dawley rats of both sexes (130–140 g of body weight). Rats were fed with standard laboratory chow and water ad libitum. The animal experiments were done in accordance to the Institutional Ethics Committee and in accordance with the recommendations for the proper care and use of laboratory animals (NIH publication No. 85–23, revised 1985).
Drugs and Chemicals
Carbon tertrachloride (BDH Chemicals, England) and buspirone hydrochloride (Bristol Global Napi Pharmaceutical Co. Cairo, A.R.E.) were used in the experiments. The rest of chemicals and reagents were of the analytical grade (Sigma, St Louis, MO, USA). The dose of CCl4 used in the study was based on previous observations 20, 21, 22.
Experimental Design
Hepatic injury was induced by treating rats by gavage with CCl4–olive oil (1:1, v/v) at a dose of 2.8 ml/kg through an orogastric tube. The effect of buspirone given at doses of 10, 20 or 30 mg/kg on CCl4-induced hepatic damage was then studied. Rats were divided into five equal groups (six rats each). Groups 1–4 were treated with CCl4 in olive oil along with saline (group 1), or buspirone at doses of 10, 20 or 30 mg/kg (groups 2, 3, 4) once a day orally and treatments continued for 1 week. Rats were given half the initial dose of CCl4 (0.14 ml/100 g body weight) 3 days after the first dose of CCl4 in order to maintain the hepatic damage. A 5th group of rats (n=6) received only the vehicle (olive oil) at 2.8 ml/kg and this is followed 3 days later by an additional dose of 1.4 ml/kg olive oil. Rats were allowed free access to food and tap water during the study. Rats were euthanized by decapitation 7 days after the first dose of CCl4.
Biochemical Studies
Serum Liver Enzymes
At the end of the study, retro-orbital vein plexus blood samples were obtained under light ether anaesthesia. The activities of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALP) in serum were then measured colorimetric ally using commercially available kits (BioMérieux, France).
Liver Lipid Peroxidation
The measurement of malondialdehyde (MDA) was used to determine the extent of lipid peroxidation in the liver tissue. The method used is that of Ruiz-Larrea et al. 25. In this assay, thiobarbituric acid reactive substances (TBARS) react with thiobarbituric acid to produce TBA-MDA adduct with a red color that can be determined using spectrophotometer at 532 nm.
Liver Reduced Glutathione
The method used is that of Ellman et al. 26. Ellman's reagent (DTNB; 5, 5’-dithiobis (2-nitrobenzoic acid)) is reduced by the sulfhydryl groups of GSH to produce 2-nitro-s-mercaptobenzoic acid. Absorption was measured at 412 nm using a spectrophotometer.
Liver Nitric Oxide
Nitric oxide levels were measured in tissue homogenates using the Griess reaction (Moshage et al. 27.
Paraoxonase 1 Activity
The arylesterase activity of paraoxonase was determined in liver supernatants and serum. In this assay, phenyl acetate used as a substrate is cleaved by the arylesterase/paraoxonase yielding phenol, the rate of its formation is determined by monitoring the increase in absorbance at 270 nm at a temperature of 25°C. One unit of arylesterase activity is considered equal to 1 μM of phenol formed per minute. The activity of PON-1 is expressed in kU/L (based on the extinction coefficient of phenol of 1,310 M−1 cm−1 at 270 nm, pH 8.0, and 25°C) 28.
Histopathological and Immunohistochemical Studies
The liver sections of each rat were fixed in 10% neutral-buffered formal saline for 72 hours at least and processed routinely for the microscopic examination. Serial sections (5 μm thick) were cut and stained with haematoxylin and eosin (Hx & E) and examined by light microscopy.
Caspase-3 immunohistochemical staining was performed with the use of streptavidin-biotin. In brief, deparaffinized sections (4 μm thick) were incubated for 30 min with fresh 0.3% hydrogen peroxide in methanol at room temperature, followed by incubation with anti caspase-3 antibodies (1: 100 dilution). The specimens were counter stained with H & E. In negative controls, normal mouse serum was substituted for anti caspase-3 antibodies. All sections were investigated by the light microscope. Adobe Photoshop version 8.0 was used for capturing and processing images.
DNA Ploidy Studies
For DNA analysis, we used Feulgen stained sections countered stained with Light green. Analysis was carried out using Leica Quin 500 image cytometry (Pathology Department, NRC, Cairo). For each section (100-120) cells were randomly measured 29.
Statistical Analysis
Data presented are means ± SE. For comparing values before and after CCl4, paired Student's t-test was used. For multiple group comparisons, ANOVA and Duncan test were used. P < 0.05 was considered statistically significant.
Results
Biochemical Results
Serum Liver Enzymes
Results are presented in Table 1. Rats treated with only CCl4 exhibited markedly elevated alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) activities in plasma by 118.4% (122.3 ± 8.9 vs. 56.0 ± 3.1 U/L), 170.5% (184.2 ± 10.0 vs. 68.1 U/L), and 121.7% (159.6 ± 7.2 vs. 72.0 U/L), respectively, indicating the severity of hepatic injury caused by CCl4. The administration of buspirone to CCl4-treated rats resulted in a dose-dependent decrease in serum ALT by 17.5%, 19.5% and 34.3%, respectively, compared to the respective control value. Serum AST levels decreased by 24.2% and 31.4% by buspirone given at 20 and 30 mg/kg, respectively. Meanwhile, 21.7% decrease in serum ALP activity was observed after treatment with 30 mg/kg buspirone (Table 1).
Table 1. The effect of buspirone on serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) activities in CCl4-treated rats| Treatment group | ALT (U/l) | AST (U/l) | ALP (U/l) |
|---|---|---|---|
| Vehicle | 56.0 ± 3.1 | 68.1 ± 4.2 | 72.0 ± 5.8 |
| CCl 4 (control) | 122.3 ± 8.9* | 184.2 ± 10.0* | 159.6 ± 7.2* |
| CCl 4 + buspirone (10 mg/kg) | 100.9 ± 6.3*, +(-17.5%) | 182.2 ± 5.7* | 164.52 ± 10.6* |
| CCl 4 + buspirone (20 mg/kg) | 98.4 ± 4.7+(-19.5%) | 139.6 ± 8.9+(-24.2%) | 165.8 ± 12.0* |
| CCl 4 + buspirone (30 mg/kg) | 80.3 ± 3.2+(-34.3%) | 126.4 ± 7.2+(-31.4%) | 124.98 ± 8.7+(-21.7%) |
Liver Oxidative Stress
The administration of only CCl4 significantly increased liver MDA and nitric oxide by 99.2 % (71.7 ± 4.1 vs 36 ± 2.2 nmol/g.tissue) and 128.6 % (63.1 ± 3.4 vs 27.6 ± 1.40 μmol/g. tissue), respectively, compared to the corresponding control value. Meanwhile, GSH decreased by 53.5% (2.93 ± 0.10 vs 6.3 ± 0.38 μmol/g. tissue) compared with the vehicle treated group. In CCl4-treated rats, buspirone given at 10 or 20 mg/kg had no significant effect on hepatic MDA, nitric oxide, or GSH. A significant decrease in liver MDA by 15.8% was, however, observed after treatment with 30 mg/kg of buspirone which also resulted in a significant decrease in nitric oxide level by 17.4% compared with the corresponding control value. Moreover, buspirone at 30 mg/kg elicited a significant increase in GSH by 20% compared with the CCl4 control group (Table 2).
Table 2. The effect of buspirone on liver tissue levels of malondialdehyde (MDA), nitric oxide (NO), and reduced glutathione (GSH) in CCl4-treated rats| Treatment group | MDA (nmol/ g.tissue ) | NO (mmol/ g.tissue ) | GSH (mmol/ g.tissue ) |
| Vehicle | 36.0 ± 2.2 | 27.6 ± 1.4 | 6.3 ± 0.38 |
| CCl 4 (control) | 71.7 ± 4.1* | 63.1 ± 3.4* | 2.93 ± 0.10* |
| CCl 4 + buspirone (10 mg/kg) | 68.56 ± 3.9* | 62.1 ± 3.7* | 2.99 ± 0.13* |
| CCl 4 + buspirone (20 mg/kg) | 67.57 ± 2.8* | 58.47 ± 2.0* | 2.94 ± 0.14* |
| CCl 4 + buspirone (30 mg/kg) | 60.38 ± 4.3+(-15.8%) | 52.1 ± 1.9+(-17.4%) | 3.52 ± 0.22+(20.1%) |
Serum and Liver Paraoxonase-1 Activity
In rats treated with CCl4, the activity of PON1 in the liver and serum was significantly depressed by 38.7% and 60.6%, respectively (Table 3). Treatment with buspirone had no significant effect on liver tissue PON1 activity. However, significantly increased serum PON1 activity by 43.9% and 53.5% was observed after treatment with 20 and 30 mg/kg buspirone, respectively (Table 3).
Table 3. The effect of buspirone on liver tissue and serum paraoxonase 1 activity (PON1) in CCl4-treated rats| Treatment group | Vehicle | CCl 4 (control) | CCl 4 + buspirone (10 mg/kg) | CCl 4 + buspirone (20 mg/kg) | CCl 4 + buspirone (30 mg/kg) |
| Liver PON1 | 78.31 ± 4.1 | 47.96 ± 3.9* | 58.68 ± 1.14* | 48.72 ± 2.18 | 47.32 ± 4.5* |
| Serum PON1 | 231.0 ± 8.2 | 90.93 ± 3.9* | 99.6 ± 5.2* | 130.84 ± 8.1*, +(43.9%) | 139.56 ± 6.0*, +(53.4%) |
Histopathological Results
Sections from CCl4 only-treated rats stained with Hx & E revealed severe damage of hepatocytes and liver tissue in the form of marked vacuolar degeneration and/or acidification of many cells, congestion of blood sinusoids and cellular infiltration(Figure 1A). Buspirone given at 10 mg/kg showed no protection against CCl4-induced liver damage as vacuolar degeneration of hepatocytes, cellular infiltration and nuclear changes were still observed (Figure 1B) whereas a low ameliorating effect on the degree of liver damage was observed at buspirone dose of 20 mg/kg
Figure 1.Hx & E stained liver tissue from (A) control rat receiving CCl4 showing severe degenerative changes in liver tissue in the form of marked vacuolar degeneration of many hepatocytes (black arrow), acidified hepatocytes (green arrowhead), congestion of blood sinusoids (green arrow) and cellular infiltration either diffuse in the center of the lobule or in blood sinusoids (black arrowhead). The lower right part of the figure shows focal aggregation of cellular infiltrates. (B) CCl4 and 10 mg/kg buspirone showing no protective effect against the damaging effect of CCl4 as acidified cells (arrow), vacuolar degeneration of most hepatocytes and cellular infiltration are still observed. (C) CCl4 and 20 mg/kg buspirone showing minimal protection against the damaging effect of CCl4 as some cells appear with normal nuclei (arrow) and others are with pyknotic nuclei (arrowhead), although many cells show vacuolar degeneration, cellular infiltration in the center of the lobule is still present and architecture of liver tissue is markedly deformed. (D) CCl4 and 30 mg/kg buspirone showing mild protection of the drug against the damaging effect of CCl4 . Cellular infiltration is localized at focal areas beside central vein (arrowhead) with restriction of vacuolar degeneration to the area around central vein (arrow).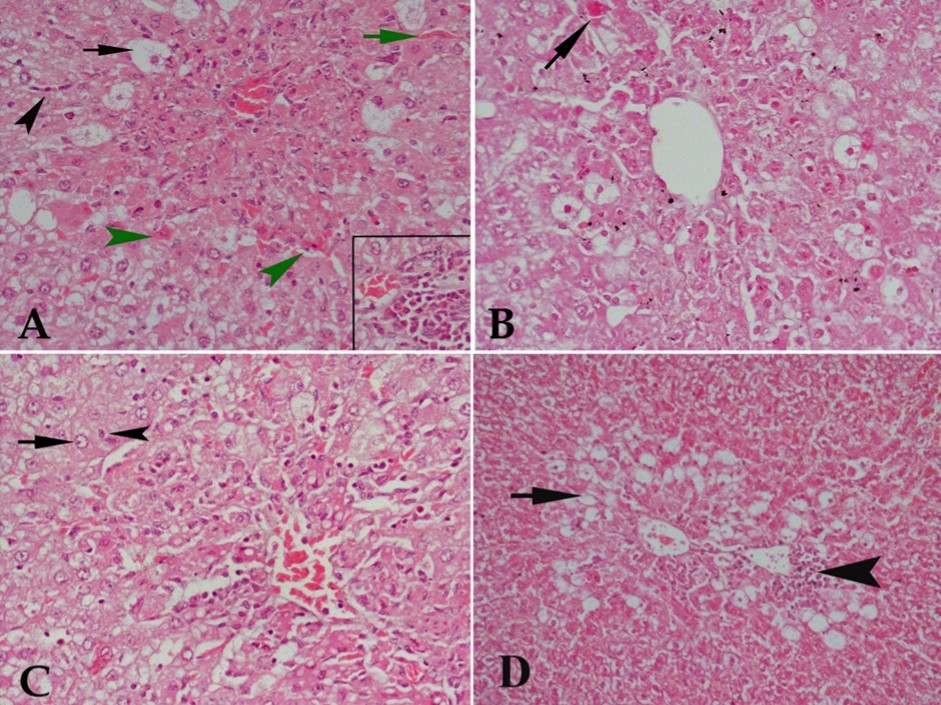
Caspase-3 Immunostaining
The immunohistochemical investigations using anti-caspase-3 antibody revealed that CCl4 caused apoptosis to many hepatocytes (Figure 2, A). Buspirone had a very weak protecting effect against the damaging effect of CCl4 at doses of 10 and 20 mg/kg as many positively stained hepatocytes were still observed (Figure 2B & C). The highest dose of buspirone (30 mg) showed slight reduction of positively stained cells denoting mild protecting effect against CCl4 induced apoptosis (Figure 2D).
Figure 2.A photomicrograph of a section of liver tissue stained with anti-caspase-3 antibody with streptavidin-biotin from (A) control rat receiving CCl4 showing many hepatocytes with positive immune- reaction to the stain (arrow). (B) CCl4 and 10 mg/kg buspirone showing a result close to that of the previous group. (C) CCl4 and 20 mg/kg buspirone showing minimal reduction of the positively stained cells. (D) CCl4 and 30 mg/kg buspirone showing mild reduction of the positively stained cells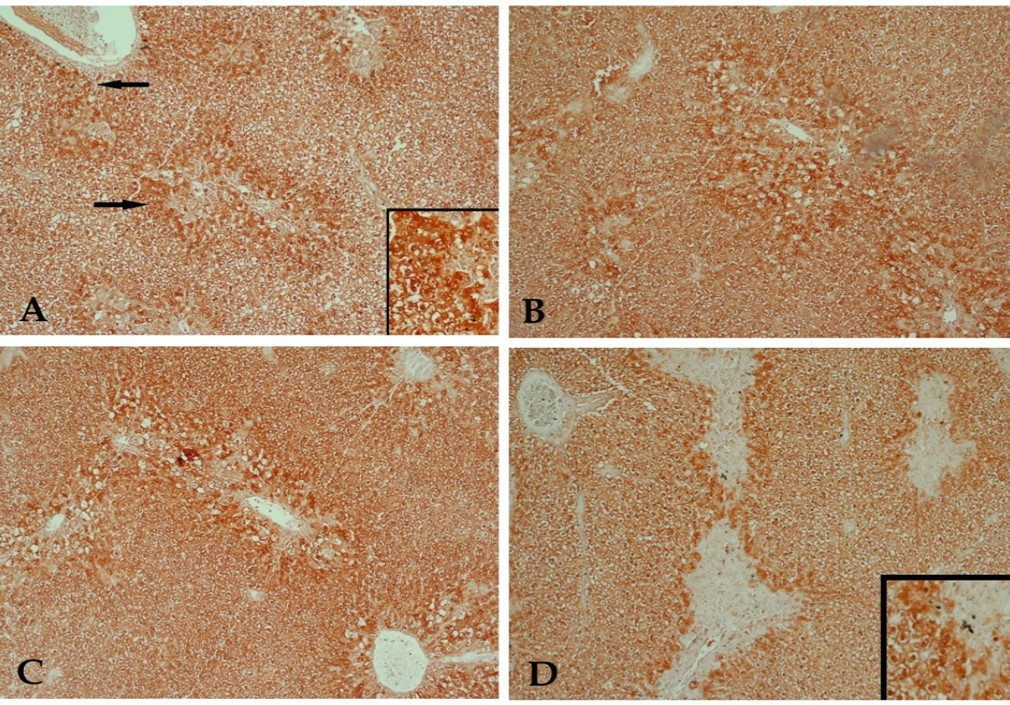
DNA Ploidy Results
In the present study, the Qwine 500 image analyzer was used to evaluate the DNA content of examined cells. The image analysis system automatically expresses the DNA content of each individual cell measured then gives the percentage of each cell out of the total number of cells examined. Also, it classifies the cells into four groups; diploid (2C), proliferating cells (3C), tetraploid (4C) and aneuploid cells (>5C). The proliferating cells are further subclassified into; (<10%) low proliferation index, (10-20%) medium proliferation index and (>20%) high proliferation index 29.
Normal distribution of DNA content in the liver cells of the control group (G. 1) showed that 13.2% of the examined cells contained DNA (<1.5C), 75.47% contained diploid DNA value (2C), 11.32% contained (3C) DNA value (medium proliferation index) and 0.0% of the examined cells at (4C) area (Figure 3 & Table 4).
Figure 3.(A) A chart showing the distribution of DNA content in normal hepatic cells. Notice that most of cells contain the normal content of DNA (2C). (B) Shows abnormal mitosis in Feulgen-stained sections of liver tissue.
| DNA index (total) | < 1.5 C | DNA Index | 1.5 – 2.5 C | DNA Index | 2.5 – 3.5 C | DNA Index | 3.5 – 4.5 C | DNA Index | >4.5 C | DNA Index | |
| C - ve | 1.00 | 13.208± 0.201% | 0.603 | 75.472± 0.271% | 1.009 | 11.321± 0.261% | 1.404 | 0.0% | - | 0.0% | - |
| C + ve | 0.604 | 89.744± 0.189%* | 0.576 | 10.256± 0.247%* | 0.844 | 0.0%* | - | 0.0% | - | 0.0% | - |
| G 3 | 0.637 | 80.392± 0.198%* | 0.576 | 19.608± 0.258%* | 0.891 | 0.0%* | - | 0.0% | - | 0.0% | - |
| G 4 | 1.743 | 0.0%*,# | - | 11.0± 0.129%*,# | 0.998 | 42.0± 0.269*,+,# | 1.472 | 30.0± 0.270%*,+,# | 1.936 | 17.0± 0.273%*,+,# | 2.550 |
| G 5 | 1.088 | 24.771± 0.099%*,+,# | 0.589 | 46.789± 0.284%*,+,# | 0.998 | 20.183%± 0.260%*,+,# | 1.497 | 6.422± 0.453%*,+,# | 1.962 | 1.835± 0.275%*,+,# | 2.558 |
Examination of cells from G. 2 (control +ve group) treated with CCl4 showed that the cells contained DNA (<1.5C) were 89.74%, while 10.25% only contained DNA value (2C), which means decrease in DNA content (hypoploidy) compared to the control group. 0.0% of examined cells contained DNA value (3C) and (4C) (Figure 4 A & Table 4).
Figure 4.(A) A chart of DNA content in liver cells of a rat treated with CCl4 shows deviation to the left (<2C). (B) A chart of DNA content in liver cells of a rat treated with CCl4 and 10 mg/kg buspirone shows the same result as the previous group. (C) A chart of DNA content in liver cells of a rat treated with CCl4 and 20mg/kg buspirone shows deviation to the right (> 2C). (D) A chart of DNA content in liver cells of a rat treated with CCl4 and 30 mg/kg buspirone shows increase in percentage of cells containing (2C value or less) and decrease in percentage of cells containing (>2C).
Examination of cells from the group treated with CCl4 and buspirone at a dose of 10 mg/kg (G. 3) showed hypoploidy where cells contained DNA (<1.5C) were 80.39%, cells contained DNA value (2C) were 19.60% . Cells contained (3C) and (4C) values were 0.0%(Figure 4 B & Table 4).
On the other hand, 0.0% of the examined cells from the group treated with CCl4 and 20 mg/kg of buspirone (G. 4) contained DNA value (<1.5 C), 11.0% of cells contained DNA value (2C), while 42.0% of the examined cells contained (3C) DNA value (high proliferating index) and 30.0% of the examined cells contained (4C) DNA value (Figure 4 C & Table 4).
Examination of cells from the group treated with CCl4 and 30 mg/kg of buspirone (G. 5) showed that 24.77% of examined cells contained (< 1.5 C), 46.78% of examined cells contained (2 C) value of DNA. Cells contained (3C) value were 20.18%, while 6.42% of examined cells contained (4C) value of DNA (Figure 4 D & Table 4).
From the above results it was clear that CCl4 caused hypoploidy in the examined cells as percentage of cells containing DNA value less than the normal was increased markedly, while percentage of cells containing the normal value of DNA was greatly reduced. Nearly the same results were obtained after treatment with the lowest dose of buspirone, whereas buspirone given at 20 mg/kg to CCl4-treated rats caused hyperploidy as the percentage of cells containing DNA value higher than normal was increased at the expense of cells containing normal value of DNA. The highest dose of the drug showed mild amelioration of DNA values in hepatic cells as the percentage of cells containing normal values of DNA was increased, although the percentage of cells containing less or more DNA values was still higher than normal.
Discussion
The results of the present study indicate that treatment with buspirone was associated with a decrease in CCl4-induced hepatotoxicity. Buspirone treatment at 20-30 mg/kg resulted in a significant decrease in the activities of the hepatocellular enzymes ALT and AST in serum. It also reduced the leakage of ALP into the plasma. The release of these intracellular enzymes into the circulation is an important marker for detecting hepatocyte injury 30. This enzyme is present at the hepatocyte’s canalicular membrane and increased enzyme activity occurs whenever there is a stagnation of bile flow 31. It is therefore clear that the decrease in the activity of these enzymes is a function of a decreased extent of liver damage and this notion was supported by histopathological examination of the liver and by the DNA studies. The administration of CCl4 caused marked vacuolar degeneration and/or acidification of many cells. Study of the DNA content of hepatic tissue indicated that CCl4 caused marked decrease in DNA content of hepatocytes (hypoploidy). Buspirone decreased hepatic vacuolar degeneration, the expression of the apoptotic marker caspase-3 and the CCl4-induced changes in DNA values in liver cells. The above findings collectively indicate a beneficial effect for the drug upon CCl4 hepatotoxicity.
The drug exerted an antioxidant action against the liver oxidative stress caused by CCl4. The latter is a well known hepatic toxin in humans and in experimental animals. This is largely due to its metabolic activation by cytochrome (CYP)2E1, CYP2B1 or CYP2B2, and the formation of the trichloromethyl radical, CCl3, capable of causing lipid peroxidation, protein and DNA damage 32. In this study, the administration of CCl4 caused a marked increase in liver content of the lipid peroxidation product malondialdehyde. This occurred along with depletion of the antioxidant reduced glutathione. In the cell, glutathione (L-g-glutamyl-L-cysteinyl-glycine) is the most abundant thiol in the cystosol, present mainly in its reduced form. Glutathione is a direct free radical scavenger and is also a co-factor for glutathione peroxidase and glutathione reductase enzymes 33. Glutathione is essential for hepatoceullar integrity. This is because cellular glutathione depletion resulted in hepatic steatosis, inflammation and cell death in mice 34. On the other hand, the glutathione donor N-acetyl-cysteine was able to correct the biochemical and the pathological changes in the liver of glutathione deficient mice 35. In this study, the increase in liver lipid peroxidation and the depletion of the liver tissue content of reduced glutathione was significantly decreased by the administration of 30 mg/kg of buspirone, possibly due to lowered level of oxidative stress by the drug.
Our results also indicated markedly increased hepatic nitric oxide content following CCl4 administration. In the liver, nitric oxide that is constitutively formed by the action of endothelial isoform of nitric oxide synthase (eNOS) maintains hepatic microcirculation and the integrity of endothelium. In contrast, the increased generation of nitric oxide by the inducible form of NOS (iNOS) due to the action of inflammatory cytokines contributes to hepatocyte apoptosis, liver tissue damage and fibrosis under such conditions as ischaemic-reperfusion injury and also after exposure to CCl436, 37. Moreover nitric oxide synthase inhibitors were shown to prevent hepatic necrosis and to decrease the expression of tumour necrosis factor alpha (TNF-α) and cycloozygenase-2 in liver tissue of CCl4 treated rats 38. In this study, the administration of buspirone at 30 mg/kg was associated with a significant decrease in liver nitric oxide in CCl4 intoxicated rats. The finding in the present study of the decrease in lipid peroxidation and nitric oxide as well as the increase in reduced glutathione after treatment with CCl4 and buspirone might therefore indicate improved cell-redox state by the drug and/or a lower degree of tissue damage due to other mechanism of buspirone.
The present study also demonstrated markedly reduced paraoxonase 1 (PON1) activity in the liver tissue and serum from CCl4-treated rats. This enzyme is synthesized in the liver and is found in plasma in association with high-density lipoproteins to prevent their oxidation. Paraoxonase 1 is also endued with xenobiotic metabolizing and antioxidant activities 39. Paraoxonase-1 exerts an antioxidant and anti-inflammatory actions in the liver and is considered a biomarker of liver diseases 40, 41, 42. Mice deficient in paraoxonase 1 fed high fat and cholesterol diet exhibited increased extent of fatty degeneration as well as increased lipid peroxides and oxidative stress markers relative to their wild-type counterparts on the same diet 43. It is conceivable that the serum level of PON1 depends on the ability of the liver to synthesize the enzyme 44. The decrease in PON1 activity observed in the current study might thus reflects decreased synthesis by the intoxicated liver cells and the recovery in PON1 could be the result of reducing oxidative stress and improving the condition of hepatocytes by buspirone treatment.
In the peripheral tissues, synthesis of serotonin is carried out by the enterochromaffin cells of the gut. Serotonin is then released into circulation and most of this circulating serotonin is actively taken up, sequestrated within vesicles in platelets and released upon their stimulation 7, 45. This platelet-derived serotonin has been implicated in both liver protection 46, 47 and regeneration 14, 15 but also in the development of hepatic injury 16, 17. Thus, in the aged rat stimulation of 5-hydroxytryptamine receptor 2 resulted in improving sinusoidal perfusion and in restoring the deficit in liver regeneration via vascular endothelial growth factor 46. In mice, absence of peripheral serotonin caused increased acetaminophen-induced liver damage 47. Moreover, mice lacking tryptophan hydroxylase 1 which is the rate-limiting enzyme for synthesizing serotonin in periphery showed blunted liver regeneration 48.
Serotonin administration increases hepatic glycogen synthesis and concentration 49. This would provide a substrate for glycolysis and cellular ATP synthesis. On the other hand, in a murine model of non-cytopathic lymphocytic choriomeningitis viral infection, platelets recruited to the liver, and their activation resulted in reduced sinusoidal microcirculation, and delayed virus elimination while increasing liver damage. Fluoxetine, a SSRI resulted in a reduction of hepatocyte damage 15. Platelet serotonin increases neutrophil recruitment to the sites of inflammation and this could be decreased by fluoxetine 16.
Studies have shown that the SSRIs inhibitors fluoxetine, sertraline, citalopram, fluvoxamine or the serotonergic antagonists trazodone and nefazodone were able to protect against the hepatotoxic effect of CCl420, 21, 22. These agents inhibit the serotonin transporter, thereby inhibiting the uptake of serotonin into platelets and impairing platelet aggregation 50. The same mechanism could possibly be also involved in their hepatic protective effects described earlier by increasing the plasma serotonin and therefore serotonin availability for liver cells. Buspirone, however, does not affect the reuptake of serotonin into platelets and in healthy subjects causes an increase in plasma levels of free serotonin without affecting platelet serotonin or platelet aggregation 51.
In summary, the present study has demonstrated that the 5-HT1A agonist buspirone reduced experimental liver injury induced by CCl4 in the rat. Buspirone displayed antioxidant action, reduced apoptosis and improved the CCl4-induced changes in DNA values in hepatic cells. These data suggest that the drug is safe in patients with liver disease.
Acknowledgements
This work is was not supported by research grants.
References
- 2.Barnes N M, Sharp T. (1999) A review of central 5-HT receptors and their function. Neuropharmacology. 38(8), 1083-152.
- 3.Bergman J, Roof R A, Furman C A.(1013) Modification of cocaine self-administration by buspirone (buspar (R)): potential involvement of D-3 and D-4 dopamine receptors. , Int J Neuropsychopharmacol; 16, 445-458.
- 4.Kim S W, Fowler J S, Skolnick P. (2014) Therapeutic doses of buspirone block D3 receptors in the living primate brain. , Int J Neuropsychopharmacol 17(8), 1257-67.
- 5.Sagarduy A, Llorente J, Miguelez C. (2016) Buspirone requires the intact nigrostriatal pathway to reduce the activity of the subthalamic nucleus via 5-HT1A receptors. , Exp Neurol; 277, 35-45.
- 6.Abdel Salam OME, Baiuomy A R. (2008) Effect of buspirone on inflammation, pain and gastric injury in mice:. , The Internet Journal of Pharmacology 6(1).
- 7.Mohammad-Zadeh L F, Moses L, Gwaltney-Brant S M. (2008) Serotonin: a review. , J Vet Pharmacol Ther 31(3), 187-99.
- 8.Shelton R C. (2009) Serotonin norepinephrine reuptake inhibitors: similarities and differences. Primary Psychiatry 16,5(Suppl4): 25-35.
- 9.Scalori A, Pozzi M, Bellia V, Apale P. (2005) Interferon-induced depression: prevalence and management. Dig Liver Dis. 37(2), 102-7.
- 10.Cai W, Khaoustov V L, Xie Q. (2005) Interferon-alpha-induced modulation of glucocorticoid and serotonin receptors as a mechanism of depression. , J Hepatol 42, 880-887.
- 11.Chojnacki C, Walecka-Kapica E, Stepien A. (2013) Serum and ascitic fluid serotonin levels and 5-hydroxyindoleacetic acid urine excretion in the liver of cirrhotic patients with encephalopathy. Advances in Medical Sciences 58(2), 251-256.
- 12.Lesurtel M, Soll C, Humar B. (2012) Serotonin: a double-edged sword for the liver? Surgeon. 10(2), 107-13.
- 13.Papadimas G K, Tzirogiannis K N, Mykoniatis M G. (2012) The emerging role of serotonin in liver regeneration. Swiss Med Wkly. 142-13548.
- 14.Naito K, Moteki H, Kimura M. (2016) Serotonin 5-HT2B receptor-stimulated dna synthesis and proliferation are mediated by autocrine secretion of transforming growth factor-α in primary cultures of adult rat hepatocytes. Biol Pharm Bull. 39(4), 570-7.
- 15.Lang P A. (2008) Contaldo C, Georgiev P et al. Aggravation of viral hepatitis by platelet-derived serotonin. , Nat Med 14(7), 756-61.
- 16.Duerschmied D, Suidan G L, Demers M. (2013) Platelet serotonin promotes the recruitment of neutrophils to sites of acute inflammation in mice. Blood. 121(6), 1008-15.
- 17.Kim D C, Jun D W, Kwon Y I. (2013) 5-HT2A receptor antagonists inhibit hepatic stellate cell activation and facilitate apoptosis. Liver Int. 33(4), 535-43.
- 18.Tudhope S J, Wang C C, Petrie J L. (2012) A novel mechanism for regulating hepatic glycogen synthesis involving serotonin and cyclin-dependent kinase-5. Diabetes. 61(1), 49-60.
- 19.Pyroja S, Joseph B, Paulose C S. (2007) Increased 5-HT2C receptor binding in the brain stem and cerebral cortex during liver regeneration and hepatic neoplasia in rats. , J Neurol Sci; 254, 3-8.
- 20.Abdel Salam OME, Sleem A A, Shaffie N M. (2006) Effect of the selective serotonin reuptake inhibitor fluoxetine on carbon tetrachloride induced hepatic damage in rats. , J Pharmacol Toxicol 1, 395-406.
- 21.Abdel Salam OME, Sleem A A, Shafee N. (2010) Effect of trazodone and nefazodone on hepatic injury induced by carbon tetrachloride. Drug Discoveries & Therapeutics 4(4), 285-297.
- 22.Abdel Salam OME, Sleem A A, Shafee N. (2012) The effect of serotonin reuptake inhibitors on hepatic injury induced by carbon tetrachloride. Comp Clin Pathol. 21(1), 77-89.
- 23.Ma J Q, Li Z, Xie W R. (2015) Quercetin protects mouse liver against CCl-induced inflammation by the TLR2/4 and MAPK/NF-κB pathway. Int Immunopharmacol. 28(1), 531-9.
- 24.Xin Y, Wei J, Chunhua M. (2016) Protective effects of Ginsenoside Rg1 against carbon tetrachloride-induced liver injury in mice through suppression of inflammation. Phytomedicine. 23(6), 583-8.
- 25.Ruiz-Larrea M B, Leal A M, Liza M. (1994) Antioxidant effects of estradiol and 2-hydroxyestradiol on iron-induced lipid peroxidation of rat liver microsomes. Steroids. 59(6), 383-8.
- 27.Moshage H, Kok B, Huizenga J R. (1995) Nitrite and nitrate determinations in plasma: a critical evaluation. Clin Chem;41(6Pt1):. 892-6.
- 28.Higashino K, Takahashi Y, Yamamura Y. (1972) Release of phenyl acetate esterase from liver microsomes by carbon tetrachloride. Clin Chim Acta. 41, 313-320.
- 29.Danque P D, Chen H B, Patil J. (1993) Image analysis versus flow cytometry for DNA ploidy quantitation for tumors: A comparison of six methods of sample preparation. Mod Pathol. 6, 270-275.
- 30.Limdi J K, Hyde G M. (2003) Evaluation of abnormal liver function tests. , Postgrad Med J 79, 307-312.
- 31.Dancygier H. (2010) Basic laboratory parameters. In “Clinical Hepatology Principles and Practice of Hepatobiliary Diseases” (Dancygier H Ed.),Springer-Verlag,Berlin , Heidelberg 1, 319-441.
- 32.Weber L W, Boll M, Stampfl A. (2003) Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol. 33(2), 105-136.
- 33.Lushchak.Glutathione homeostasis and functions: potential targets for medical interventions. , J Amino Acids 202, 736837.
- 34.Chen Y, Yang Y, Miller M L. (2007) Hepatocyte-specific Gclc deletion leads to rapid onset of steatosis with mitochondrial injury and liver failure. Hepatology. 45(5), 1118-28.
- 35.Chen Y, Dong H, Thompson D C. (2013) Glutathione defense mechanism in liver injury: insights from animal models. Food Chem Toxicol. 60, 38-44.
- 36.Iwakiri Y. (2015) Nitric oxide in liver fibrosis: The role of inducible nitric oxide synthase. Clin Mol Hepatol. 21(4), 319-25.
- 37.Liu H, Li Q, Wang Y. (2016) Elevated nitric oxide levels associated with hepatic cell apoptosis during liver injury. Hepatol Res doi: 10.1111/hepr.12783. [Epub ahead of print].
- 38.Tipoe G L, Leung T M, Liong E. (2006) Inhibitors of inducible nitric oxide (NO) synthase are more effective than an NO donor in reducing carbon-tetrachloride induced acute liver injury. Histol Histopathol. 21(11), 1157-65.
- 39.Kulka M. (2016) A review of paraoxonase 1 properties and diagnostic applications. , Polish Journal of Veterinary Sciences 19(1), 225-232.
- 40.Desai S, Baker S S, Liu W. (2014) Paraoxonase 1 and oxidative stress in paediatric non-alcoholic steatohepatitis. Liver Int. 34(1), 110-7.
- 41.Ceron J J, Tecles F, Tvarijonaviciute A. (2014) Serum paraoxonase 1 (PON1) measurement: an update. , BMC Vet Res 10-74.
- 42.Wang B, Yang R N, Zhu Y R. (2017) Involvement of xanthine oxidase and paraoxonase 1 in the process of oxidative stress in nonalcoholic fatty liver disease. Mol Med Rep. 15(1), 387-395.
- 43.García-Heredia A, Kensicki E, Mohney R P. (2013) Paraoxonase-1 deficiency is associated with severe liver steatosis in mice fed a high-fat high-cholesterol diet: a metabolomic approach. , J Proteome Res 12(4), 1946-55.
- 45.Lesurtel M, Soll C, Graf R. (2008) Role of serotonin in the hepato-gastroIntestinal tract: an old molecule for new perspectives. Cell Mol Life Sci. 65(6), 940-52.
- 46.Furrer K, Rickenbacher A, Tian Y. (2011) Serotonin reverts age-related capillarization and failure of regeneration in the liver through a VEGF-dependent pathway. Proc Natl Acad Sci USA 108(7), 2945-50.
- 47.Zhang J, Song S, Pang Q. (2014) Serotonin deficiency exacerbates acetaminophen-induced liver toxicity in mice. Scientific Reports. 5(1), 8098.
- 48.Lesurtel M, Graf R, Aleil B. (2007) Platelet-derived serotonin mediates liver regeneration. Science. 312(5770), 104-107.
- 49.Watanabe H, Akasaka D, Ogasawara H. (2010) Peripheral serotonin enhances lipid metabolism by accelerating bile acid turnover. Endocrinology. 151(10), 4776-86.
Cited by (3)
- 1.Rashidian Amir, Mohammadi Sina, Hamaneh Amirabbas Mohammadi, Chaboki Alireza, Shayan Maryam, et al, 2022, Buspirone Ameliorates Colon Inflammation in TNBS-Induced Rat Acute Colitis: The Involvement of TLR4/NF-kB Pathway, Drug Research, 72(08), 449, 10.1055/a-1855-1491
- 2.Stasi Cristina, Milani Stefano, Galli Andrea, 2021, , , (), 129, 10.1016/B978-0-12-821927-0.00005-X
- 3.Sepehrinezhad Ali, Shahbazi Ali, Sahab Negah Sajad, Stolze Larsen Fin, 2023, New Insight Into Mechanisms of Hepatic Encephalopathy: An Integrative Analysis Approach to Identify Molecular Markers and Therapeutic Targets, Bioinformatics and Biology Insights, 17(), 117793222311550, 10.1177/11779322231155068
