Abstract
Introduction:
Cyclophosphamide (CPA) is an anticancer drug .Fennel (Foeniculum vulgare Mill) essential oil is a traditional medicine used against many diseases.
Aim.
The present work studied the effect of fennel oil against testicular damage and oxidative stress induced by the anticancer drug, cyclophosphamide (CPA) in albino rats.
Methods.
Animals were divided into 4 groups: group1, control, group2, orally given fennel oil, group3 treated with CPA and group4 treated with CPA and fennel oil. The testes were removed for histological and immune histochemical preparation. Blood was collected and sera were prepared for hormonal and biochemical analysis.
Results.
The results revealed that CPA caused histological alterations in the testis including decrease in diameter and germinal epithelial height of the seminiferous tubules, degeneration of germ cells, cytoplasmic vacuolation and congestion of blood vessels. Cell proliferation marker was decreased and apoptotic marker caspase-3 was decreased. Biochemical results revealed decrease in the hormones LH and testosterone. Moreover, the serum activity of the antioxidant enzymes, SOD, CAT was decreased and the lipid peroxidation marker, DMA was increased. Treating rats with CPA and fennel oil caused an improvement in the histological structure of the testis. There was an increase in LH ,testosterone,SOD and CAT, while MDA level decreased.
Conclusion.
It is concluded that administration of fennel oil exhibited protective effects against CPA-induced reproductive toxicity in male rats. The protective effect of fennel oil might be due to induction of antioxidant defense systems by one or more of its constituents.
Author Contributions
Academic Editor: Feng Ding, Wenzhou Medical Universityï
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2017 Saber A.Sakr, et al
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Cyclophosphamide (CPA) is a nitrogen mustard alkylating agent, and anti-neoplastic drug. It is used to treat Hodgkin’s disease, lymphomas, leukemia, granulomatosis, severe rheumatoid arthritis, and lupus erythematosus. It is also used in combination with other drugs to treat breast cancer, leukemia, and ovarian cancer. The drug also has immunosuppressive action when it has used in smaller doses 1. CPA acts by modifying and cross linking purine bases in DNA, thus inhibiting DNA, RNA and protein synthesis and death of rapidly dividing cells. CPA has been found to cause many toxic effects including haemorrhagic cystitis, pulmonary fibrosis, irreversible a zospermia in man, gastrointestinal bleeding and hepatotoxicity2
Cardiotoxicity is a major problem with people treated with higher dose regimens 3.In addition, CPA was found to affect male reproduction. Wetzel 4 Indicated that men exposed to CPA may develop oligo spermia or azoospermia associated with increased gonadotropin release. Turk et al., 5 reported that CPA impaired spermatogenesis and androgenesis and induced germ cells apoptosis.
Medicinal plants contain phytochemicals and numerous chemical compounds, which are used in treatment of different diseases. Fennel plants (Foeniculum vulgare) is a medicinal plant belongs to the family Apiaceae (Umbelliferae) 6. This herb is traditionally used as treatment for colic, wind, irritable bowel, kidneys, spleen, liver, lungs, suppressing appetite, breast enlargement, promoting menstruation, improving digestive system, milk flow and increasing urine flow 7. The chief component of fennel, anethole, had anticarcinogenic and anti-inflammatory effects through modulation tumor necrosis factor–induced cellular processes 8 and antimicrobial properities 9. Furthermore, fennel has a bronchodilatory effect 10 as well as immunomodulatory activities by enhancing natural killer cell functions, the effectors of the innate immune response 11. Fennel extracts was found to increase male fertility and is consider as a novel medicine for treatment of infertility 12, 13. In view of these considerations, the present work investigated the effect of fennel oil on cyclophosphamide-induced testicular toxicity in albino rats.
Materials and methods
A.Chemical and Plant used:
1-Endoxan (cyclophosphamide):
Endoxan was obtained as tablets from Baxter Oncology Halle, Germany. Each tablet contain 50mg cyclophosphamide. Endoxan was dissolved in distilled water and orally given by gastric tube at a dose level of 15 mg/kg body weight once a week for six weeks 14. 2- Fennel oil:
Fennel essential oil (FEO) was purchased from a localmarket at shebin El-Kom ,Menufyia Governement (ElMasry Everline company). The main oil components of oil were found to be trans-anethol (84.1-86.1%),fenechone (7.13 – 8.86 %), limonene (3.0–3.3%),and methyl chavicol (2.5–2.7 %). Fennel essential oil was given at a dose level of 1mg/kg body weight once a week for six weeks 15.
B. Experimental animals:
Forty eight healthy adult Wister male albino rats, three months age weighting 150±10 g were purchased from experimental rat house localized in Helwan. Animals were kept in plastic cages (each contained six animals) in the animal house for two weeks before the experimental work. Animals were kept at 25 ± 2 ْC with relative humidity of 50-60% and on 12h light/ 12h dark cycle. They received a standard diet composed of 50% barley, 20% yellow corn, 20% dry milk, 10% different vegetables and tap water. The study and all procedures were approved by the Animal Care and Bioethics Committee, Menoufia University, Egypt (Approval No. MNSH173). Animals were divided into four groups
Group 1 (Control group): Animals of this group (12 rats) were served as control group and were kept without any treatment and were given standard diet and tap water.
Group 2 (Fennel oil group): Animals of this group (12 rats) were orally given fennel oil at a dose level of 1 mg/kg body weight once a week for six weeks.
Group 3 (CPA group): Animals of this group (12 rats) were orally treated with endoxan at a dose of 15mg/kg body weight once a week for six weeks.
Group 4 (CPA+Fennel oil group): Animals were given endoxan and then after two hours they were given fennel oil, with the same doses of group 2 and 3.
C. Histological studies:
For histological study, testes were immediately removed after 3 and 6 weeks, and fixed in 10% formalin for 24 hours. Specimens were dehydrated in ascending series of ethyl alcohol, cleared in two changes of xylene, infiltrated in three changes of molten paraffin (melting point 58-60 °C) and then embedded in molten paraffin blocks. Paraffin sections (5 micron thickness) were sectioned using a rotary microtome and mounted on clean glass slides. Sections were stained with Ehrlich’s hematoxylin and counter stained with eosin for histological examination 16.
E) Immuno histochemical studies:
For Immunostaining methods of kip67 and Caspase-3, slides were deparaffinized in xylene and rehydrated in a series of graded alcohol concentrations. then rinsed in phosphate-buffered saline (PBS)containing 0.1% tween-20. Antigen retrival was performed by placing slides in sodium citrate solution (PH 6.0) at 90°C. Avidin (0.001% in PBS) and biotin (0.001% in PBS) were blocked in each section by using Avidin/biotin blocking solutions, where sections were incubated and rinsed with PBS between steps. Sections were incubated with monoclonal primary rat antibodies (Neo Markers, Cat.#Ms-113-P, Fremont, CA,USA), at appropriate dilution (1:200) in antibody diluent, directed against rat Ki-67 or Caspase (each antibody was used separately to react on different slides) at room temperature. Slides were washed in PBS-Tween 20. Sections were incubated in peroxidase blocking solution (3%H2O2 in PBS) at room temperature. Slides were washed in PBS-Tween 20. Sections were incubated in biotinylated secondary antibody in PBS at room temperature. Slides were washed in PBS-Tween 20. For detection, sections were incubated in horse radish peroxidase (HRP)-streptavidin solution at room temperature. Slides were washed in PBS-Tween 20. Sections were incubated in peroxidase substrate solution “3,3-diaminobenzidine tetra hydro chloride (DAP)” until adequate color was developed. Slides were washed in PBS-Tween 20. Sections were counterstained with hematoxylin, dehydrated through garded alcohol series, clear in xylene and mounted with DPX 17.
F- Biochemical analysis:
For biochemical analysis, blood samples were collected in clean centrifuge tubes. Blood samples left to clot in room temperature and then serum separated by centrifugation at 3000 rpm for 20 minutes. The collected serum stored at -18 -20 °C until analysis. The activity ofsuperoxide dismutase (SOD) was estimated according to 18, while the activity of Catalase (CAT) was estimated according to 19. Glutathione (GSH) was determined according to 20. Malondialdehyde (MDA) was assayedaccording to 21. Testosterone and Luteinizing hormone LH were estimating according to the method of 22.
G- Statistical Analysis
Data were expressed as mean ± standard deviation (SD). The significance of differences means was evaluated by using independent sample t test. All statistical analysis was performed using SPSS statistical version 16 software package.
Results
Histological observations
The testis of control animal is formed of a number of seminiferous tubules each contain the different stages of spermatogenesis (spermatogonia,1ry,2nd spermatocytes and spermatozoa). Between the seminiferous tubules, the interstitial region, there are blood vessels and the interstatial cells (Leydige cells) (Figure 1. A). Testes of animals treated once a week with fennel oil (1ml/kg) for three and six weeks showed nearly normal histological structure (Figure 1.B).
Testes of animals examined after three weeks of treatment with cyclophosphamide exhibited a distinct histological change when compared with control group. The spermatogenic layers were degenerated and appeared with less compact spermatogenic cells. The basement membrane appeared in some tubules irregular and others were separated from the underline. Within the intertubular connective tissue, haemorrhage (Figure 1.C) and congested blood vessels were seen (Figure 1.D). Testis of animals treated with cyclophosphamide for six weeks showed exfoliated germ cells to the center of the tubules (Figure 2-A), Marked degeneration of the spermatogenic cells and appearance of vacuoles were observed (Figure 2-B). The interstitial connective tissue was degenerated and the number of germ cells was reduced (Figure 2-C). Testes of rats treated with cyclophosphamide and fennel oil for 3 weeks showed an improvement in the histological appearance of the testicular tissue, but intertubular haemorrhage was rarely seen. After 6 weeks of treatment with cyclophosphamide and fennel oil, the histological picture of the testes appeared better than testes of animals treated only with cyclophosphmide. There was an increase in number of spermatogenic cells (Figure 2-D).
Figure 1.A) A photomicrograph obtained from testis of a control rat showing normal seminiferous tubules, different stages of spermatogenic cells, spermatozoa (Sp) and interstitial tissue (IT), (H&E). B) A photomicrograph obtained from testis of a rat treated with fennel oil for six weeks showing normal structure of seminiferous tubules, (H&E). C) A Photomicrograph obtained from testis of a rat treated with CPA for three weeks showing interstitial haemorrhage (h), and separation of germ layers from underline basement membrane (arrow), (H&E). D) A Photomicrograph obtained from testis of a rat treated with CPA for three weeks showing congested and enlarged blood vessel, (H&E).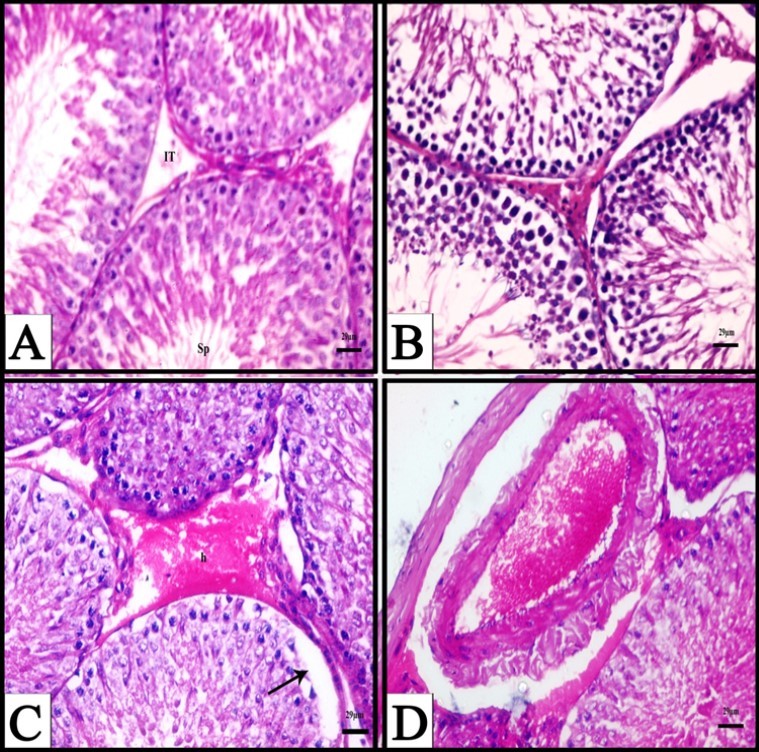
Figure 2.A) A Photomicrograph obtained from testis of a rat treated with CPA for six weeks showing exfoliated cells (arrows), (H&E). B) A Photomicrograph obtained from testis of a rat treated with CPA for six weeks showing appearance of vacuoles (arrows) and degenerated spermatogenic cells, (H&E). C) A Photomicrograph obtained from testis of a rat treated with CPA for six weeks showing degenerative interstitial tissue and reduced and n degenerated spermatogenic cells, (H&E). D) A Photomicrograph obtained from testis of a rat treated with CPA followed by fennel oil for six weeks showing advanced degree of improvement of seminiferous tubules and increase of spermatogenic layers, (H&E).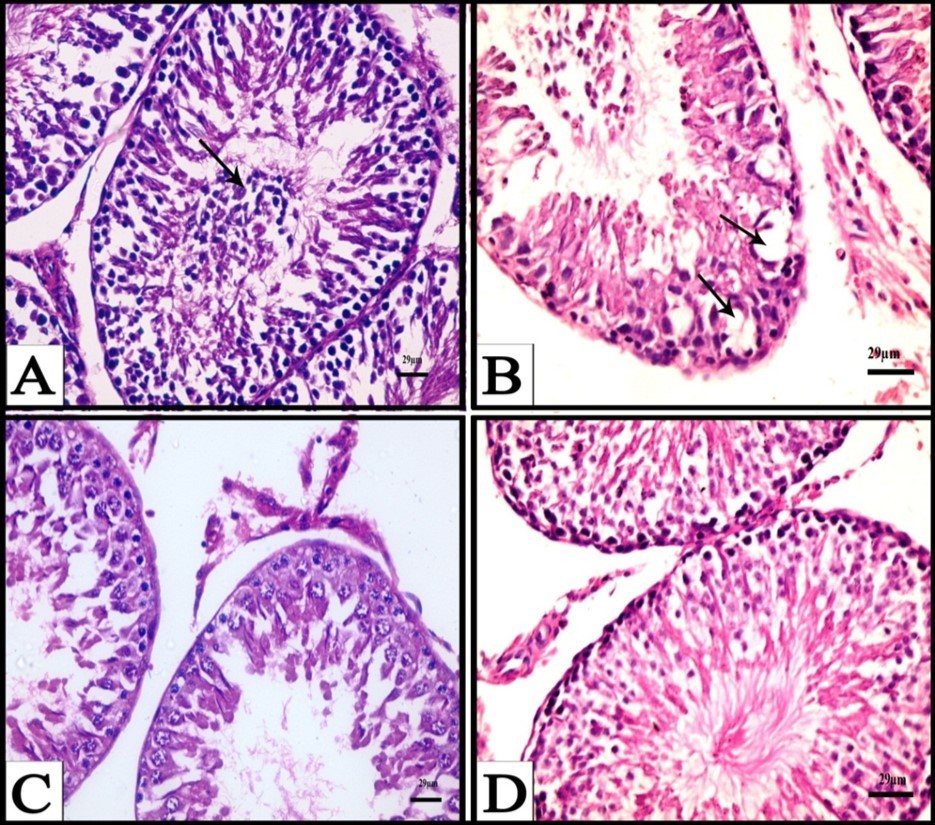
Morpho Metrical Results:
Figure 3 & Figure 4 showed that the diameter and epithelial height of seminiferous tubules of CPA-treated animals for 3 weeks were nearly similar to control animals. A significant decrease was seen in diameter and epithelial height of seminiferous tubules of the CPA treated groups for 6 weeks. Animals treated with CPA followed by fennel oil for 6 weeks showed a significant increase in diameter and epithelial height when compared with CPA groups.
Figure 3.Change in diameter (μm±S.D) of seminiferous tubules in different experimental groups. (*): significant at P < 0.05 compared with control group.(**): significant compared with CPA group.
Figure 4.Change in germinal epithelial height (μm±S.D) of seminiferous tubules in different experimental groups. (*): significant at P < 0.05 compared with control group.(**): significant compared with CPA group.
Immuno histochemical results:
1- The expression of Ki-67
In control group, Ki-67 was expressed in the nuclei of the spermatogonia as brown color. On the other hand, the negative nuclei of the spermatocytes are stained blue with hematoxylin (Figure 5-A). Animals given fennel oil for six weeks showed expression of Ki-67 in the nuclei of the spermatogonia which is nearly similar to control group (Figure 5-B). A decrease expression of Ki-67 immunore activity was observed in most of the nuclei of the spermatogonia after six weeks of treatment with CPA (Figure 5-C). Testis sections obtained from rats treated with CPA followed by fennel oil for six weeks, showed an increase in expression of Ki-67 immunore activity in the nuclei of spermatogonia (Figure 5-D). The results in (Figure 6) showed the percentage area of Ki-67 positive nuclei in the spermatogonia in the different experimental animals after six weeks. The percentage area of Ki-67 positive nuclei of the spermatogonia showed a significant decrease (P<0.05) in rats treated with CPA for six weeks when compared with control groups. Treatment of rats with CPA followed by fennel oil for six weeks, resulted in a significant increase of Ki-67 positive nuclei of the spermatogonia when compared with CPA groups.
Discussion
In the present study, results revealed that treating rats with CPA induced many histological alteration in the testis and these alterations were more prominent in animals treated for six weeks and include degeneration of sperm cells, hemorrhage and congestion of blood vessels. Moreover, the diameter and germinal epithelial height of the seminiferous tubules were decreased. These results are in agreement with many studies which reported that a period of 3 to 6 weeks of CPA treatment leads to testicular toxicity. It was reported that the administration of CPA once a week for 5 weeks caused oligospermia, azoospermia, testicular damage 23 and germ cell toxicity in mice 24. Sakr et al. 25 indicated that CPA-treated mice showed many histological changes including the appearance of irregular seminiferous tubules, reduction in the number of spermatogenic cells, degeneration of Leydig cells and appearance of intertubular hemorrhage. El- Seedy et al. 26 reported that a marked increase in sperm abnormality induced by CPA in mice proved the ability of this drug to interfere with different stages of spermatogenic cells. They concluded that these abnormalities may be resulted directly from DNA damage or at specific levels of differentiation of spermatozoa. Reduction in diameter of seminiferous tubules and germinal epithelial height was recorded in testes of rats given CPA. The recorded decrease in the height of germinal epithelium is attributed to the inhibition of spermatogenesis by CPA. Morphometrical parameters such as diameters of the seminiferous tubules and tubular lumen can give information about the testicular damage degree as a consequence of germ cell death 27.
Immunohistochemical observations revealed decrease in expression of Ki-67 and increase of caspase-3. Antigen KI-67 is a nuclear protein that is associated with cellular proliferation. Furthermore, it is associated with ribosomal RNA transcription 28. Inactivation of antigen KI-67 leads to inhibition of ribosomal RNA synthesis 29.Caspase-3 is a marker of the early phase of apoptosis 30, and is essential for certain processes associated with the formation of apoptotic bodies 31.Thus,CPA inhibited spermatogenesis via decrease of cell proliferation and increase of apoptosiswere reduced in rats treated with CPA. These results are in agreement with results of some investigators 23, 24.The reduction in these hormones is due to the effect of active metabolites such as acrolein which break DNA, affect RNA and protein synthesis 32. Degeneration of interstitial tissue was observed in the present work. Interstitial tissue plays an important role in the testis as it contained the Leydig cells which are the main testosterone producer. When the testosterone levels are diminished, the process of spermatogenesis is affected.
Examination of sera of animals treated with CPA in the present study revealed a significant increase in the lipid peroxidation marker, MDA.
It has been reported that CPA treatment resulted in elevated MDA levels because of the excessive generation of free radicals 33, 34, 35 and generally, it is accepted that the increased lipid peroxidation is one of the toxic manifestations of CPA administration in testis. 36.
A reduction in the activities of the antioxidant enzymes (super oxide dismutase, catalase). Similarly, Manda and Bhatia, 37 reported that fifteen days oral administration of CPA induced depletion in the levels of glutathione peroxidase, catalase and super oxide dismutase. Reduction of these enzymes was also recorded in various tissues as a result of CPA treatment 38, 39, 40. CPA toxic effects on testis were mainly attributed to oxidative stress on seminiferous tubules and Sertoli cells, impairing spermatogenis and androgenesis, and inducing germinal cells apoptosis 5.
The current study, for the first time, showed that treating rats with CPA followed by fennel oil revealed nearly normal appearance of testicular tissue and increased the number of germ cells. In addition, animals treated with CPA and fennel oil caused an increase in expression of Ki-67 and decrease in expression of casepase-3. Sakr et al. 41 obtained the same results in liver of rats treated with CPA and fennel oil. They added that this result is due to antiproliferative and antioxidant effect of fennel oil. Fennel oil,in the present work, caused a decrease in caspase 3, the marker of apoptosis. Similarly,
Ibrahim 12 reported that fennel oil resulted in amelioration in testicular tissue lesions and decrease of apoptosis in rats exposed to tobacco smoke. Fennel treatment was found to improve sperm quality, and spermatogenic cells apoptosis in obese rats 13. The level of testosterone and LH increased. Ibrahim 42 showed that there were significant increases over the control in testosterone level in the serum of rats treated with fennel oil. The author added that the improvement effect of fennel oil on testicular function as indicated by the testosterone may be attributed to the powerful active components of the fennel oil.
Decrease in lipid peroxidation marker,MDA and increase in antioxidant enzymes, SOD, CAT and GSH was recorded in sera of animals treated with CPA and fennel oil. Several reports indicated the antioxidant effect of fennel oil. Pretreatments with fennel oil significantly inhibited the frequencies of aberrant metaphases, chromosomal abreactions, micronuclei formation, and cytotoxicity in mouse bone marrow cells induced by CPA and antagonized the reduction of CPA-induced SOD, CAT, and GSH activities and inhibited increased MDA content in the liver of mice 43. Sheweita et al. 44 reported that fennel oil restored changes in activities of antioxidant enzymes SOD, CAT, GR, GST, and GPx caused by CPA to their normal levels compared to control mice. Mohamad et al., 45 have demonstrated that fennel oil acts like antioxidants due to its ability to inhibit lipid peroxidation. Moreover, it has been revealed that oil extracted from this fennel herb has a protective tetrachloride in rat liver 46. Fennel essential oil has physiologic antioxidant activities including the radical scavenging effect, inhibition of hydrogen peroxides H2O2 and Fe chelating activities where it can minimize free radical which initiate the chain reactions of lipid peroxidation 47.
48. Studies have shown that antioxidants have a widespread effect on reproduction. These protect spermatozoa from ROS producing abnormal spermatozoa, prevent DNA fragmentation, improve semen quality in smokers, reduce cryodamage to spermatozoa, block premature sperm maturation, and stimulate spermatogenesis 49. It is concluded that administration of fennel oil exhibited protective effects against CPA-induced reproductive toxicity in male rats. The protective effect of fennel oil might be due to induction of antioxidant defense systems by one or more of its constituents.
References
- 1.Avendano C, Menendez J.. C (2008):Medicina Chemistry of Anticancer Drugs. 1st Ed , Hungary 139-149.
- 2.A G Gilman, T W Rall. (1999) Pharmacokinetics and side effects of Cyclophosphamide. Goodman Gillman Pharmacological basis of therapeutics 9.
- 3.J D Floyd, D T Nguyen, R L Lobins, Bashir Q, D C et al. (2005) Cardiotoxicity of cancer therapy. , Journal of Clinical Oncology 23(30), 7685-96.
- 4.Wetzels J F M. (2004) Cyclophosphamide-induced gonadal toxcicity: a treatment dilemma in patients with lupus nephritis? The Netherl. , J. Med 62, 347-352.
- 5.Turk G, A O Ceribasi, Sakin F, Sonmez M, Atessahin A. (2010) Antiperoxidative and anti-apoptotic effects of lycopene and ellagic acid on cyclophosphamide induced testicular lipid peroxidation and apoptosis. , Reproduction, Fertility and Development 22(4), 587-596.
- 6.Rather A, Bilal A Dar, Shahnawaz Sofi, N Bilal A Bhat, Mushtaq A et al. (2012) Foeniculum vulgare: a comprehensive review of its traditional use, phytochemistry, pharmacology, and safety. http://dx.doi.org/10.1016/j.arabjc.2012.04.011
- 7.Delaram M, Kheiri S, M R Hodjati. (2011) Comparing the effects of echinop hora-platyloba, fennel and placebo on pre-menstrual syndrome. , J. Reprod. Infertil 12(3), 221-226.
- 8.B, A B Kunnumakkara, K B Harikumar, S T Tharakan, Sung B et al. (2008) Potential of spice-derived phytochemicals for cancer prevention. , Planta Med 74(13), 1560-9.
- 9.E M Soylu, Soylu S, Kurt S. (2006) Antimicrobial activation of the essential oils of various plants against tomato late blight disease agent phytophthora infestans. , Mycopathalogic 161(2), 119-28.
- 10.M H Boskabady, Khatami A, Nazari A. (2004) Possible mechanism (s) of relaxant effects of Foeniculum vulgare on guinea pig tracheal chains. , Pharmazie 59(7), 561-4.
- 11.Ibrahim A A E. (2007) The immunomodulatory effect of fennel oil in mainstream cigarette smoke exposed rats. , Egyptian Journal of Medical Science 28(1), 163-186.
- 12.A. (2008) Correlation between fennel- or anise-oil administration and damage to the testis of adult rats. , Egyptian J. Biol 10, 62-76.
- 13.R, Farrokhi S, Yazdinezhad A Rostamkhani S Mahmazi S, Kazemi A, Shokri M et al. (2016) Ameliorating effects of fennel and cumin extracts on sperm quality and spermatogenic cells apoptosis by inducing weight loss and reducing leptin concentration in diet- induced obese rats. Andrologia. 1-11.
- 14.A O Ceribasi, Turk G, Sonmez M, Sakin F, Atessahin A. (2010) Toxic effect of cyclophosphamide on sperm morphology, testicular histology and blood oxidantantioxidant balance, and protective roles of lycopene and ellagic acid. Basic and Clinical Pharmacology and Toxicology. 107(3), 730-736.
- 15.M Rabeh Naeem, Alaa O.Aboraya (2014): Hepatoprotective effect of Dill (Anethum graveolens L.) and fennel (Foeniculum Vulgare) oil on Hepatotoxic Rats. , Pak.J.Nut 13(6), 303-309.
- 16.R D Lillie, H M Fullmer. (1976) . Histopathological techniques and practical histochemistry. 4th ed., MC. Graw , New York .
- 17.S M Hsu, Raine L, Franger H. (1981) Use of Avidin-biotin peroxidase complex (ABC) in immunoperoxidase techniques: a comparison of ABC and unlabeled antibody (PAP) procedure. , J. Histochem. Cytochem 29(4), 577-580.
- 18.Minami M, Yoshikawa H. (1979) A simplified assay method of super oxide dismutase activity for clinical use. , Clin Chim Acta 92(3), 337-42.
- 19.Cowell D C 1, A, R J Lewis, Pirzad R, S D Watkins. (1994) The rapid potentiometric detection of catalase positive microorganisms. , Biosens Bioelectron 9(2), 131-8.
- 20.Tietze F. (1969) Enzymic method for quantitative determination of nano gram amounts of total and oxidative glutathione: applications to mammalian blood and other tissues. , Anal Biochem 27(3), 502-22.
- 21.H Cheeseman Esterbauer, H K. (1990) Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 186, 407-21.
- 22.Maruyama Y, Aoki N, Suzuki Y, Ohno Y, Imamura M et al. (1987) Sex-steroid-binding plasma protein (SBP), testosterone, oestradiol and dehydroepiandrosterone (DHEA) in prepuberty and puberty. , Acta Endocrinol 114(1), 60-67.
- 23.Elangovan N, T J Chiou, W F Tzeng, S T Chu. (2006) Cyclophosphamide treatment causes impairment of sperm and its fertilizing ability in mice. , Toxicology 222(1), 60-70.
- 24.D N Tripathi, G B Jena. (2008) Astaxanthin inhibits cytotoxic and genotoxic effects of cyclophosphamide in micegermcells. , Toxicology 248(2), 96-103.
- 25.S A, H A Mahran, S M Abo-El-Yazid. (2011) Effect of fenugreek seeds extract on cyclophosphamide-induced histomorphometrical, ultrastructural and biochemical changes in testes of albino mice. , Toxicol Ind Health 28(3), 276-288.
- 26.A S El-Seedy, T A, M A El-Seehy, A. (2005) Ultrastructure sperm defects in male mice during carcinogenicity of urethane and indoxan. , Arab Journal of Biotechnology 9(1), 27-40.
- 27.Bustos-Obregon E, Carvallo M, Hartley-Belmar R, Sarabia L, Ponce C. (2007) Histopathological and Histometrical Assessment of Boron Exposure Effects on Mouse Spermatogenesis. , Int. J. Morphol 25(4), 919-925.
- 28.Bullwinkel J, Baron-Lühr B, Lüdemann A, Wohlenberg C, Gerdes J et al. (2006) Ki-67 protein is associated with ribosomal RNA transcription in quiescent and proliferating cells. , J. Cell. Physiol 206(3), 624-35.
- 29.Rahmanzadeh R, Hüttmann G, Gerdes J, Scholzen T. (2007) Chromophore-assisted light inactivation of pKi-67 leads to inhibition of ribosomal RNA synthesis. , Cell Prolif 40(3), 422-30.
- 30.Chrysomali E, Nikitakis N G, Tosios K, Sauk J J, Papanicolaou S I. (2003) Immunohistochemical evaluation of cell proliferation antigen Ki-67 and apoptosis-related proteins Bcl-2 and caspase-3 in oral granular cell tumor. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 96, 566-72.
- 32.A M Codrington, B F Hales, Robaire B. (2007) Chronic cyclophosphamide exposure alters the profile of rat sperm nuclear matrix proteins. , Biol Reprod 77(2), 303-11.
- 33.Ghosh D, U B Das, Misro M. (2002) Protective role of a-tocopherol-succinate (provitamin-E) in cyclophosphamide induced testicular gametogenic and steroidogenic disorders: a correlative approach to oxidative stress. Free Radic Res. 36, 1209-18.
- 34.Selvakumar E, Prahalathan C, Mythili Y, Varalakshmi P. (2005) Beneficial effects of DL-a-lipoic acid on cyclophosphamide-induced oxidative stress in mitochondrial fractions of rat testis. , Chem Biol Interact 152, 59-66.
- 35.Selvakumar E, Prahalathan C, P T Sudharsan, Varalakshmi P. (2006) Chemoprotective effect of lipoic acid against cyclophosphamideinduced changes in the rat sperm. , Toxicology 217, 71-8.
- 36.Y O Ilbey, Ozbeck E, Simsek A, Otunctemur A, Cekmen M et al. (2009) Potential chemoprotective effect of melatonin in cyclophosphamide- and cisplatin-induced testicular damage in rats. Fertility and Sterility. 92(3), 1124-32.
- 37.Manda K, A L Bhatia. (2003) Prophylactic action of melatonin against cyclophosphamide-induced oxidative stress in mice. , Cellular Biology Toxicology 19(6), 367-720.
- 38.Haque R, Bin-Hafeez B, Parvez S, Pandey S, Sayeed I et al. (2003) Aqueous extract of walnut (Jglans regia L.) protects mice against cyclophosphamide induced biochemical toxicity. , Hum. Exp. Toxicol 22, 473-480.
- 39.S G Lopez, Luderer U. (2004) Effects of cyclophosphamide and buthionine sulfoximine on ovarian glutathione and apoptosis. Free Radic. , Biol. Med 36, 1366-1377.
- 40.T S Shanmugarajan, Arunsundar M, Somasundaram I, Krishnakumar E, Sivaraman D et al. (2008) Cardoprotective effects of Ficas hispida linn. On cyclophosphamide provoked oxidative myocardial injury in a rat model. , Int. J. Pharmacol 4(2), 78-87.
- 41.Sakr S, Shalaby S, Beder R. (2017) Ameliorative effect of fennel oil on cyclophosphamide induced hepatotoxicity in albino rats. , British Journal of Pharmaceutical Research 17(2), 1-12.
- 42.Ibrahim A A E. (2007) Does fennel oil have the potentials against apoptosis in testes of rats exposed to mainstream tobacco smoke?. , Egyptian Journal of Medical Science 28(2), 767-783.
- 43.Tripathi P, R K Patel, Tripathi R, N R Kanzariya. (2013) Investigation of antigenotoxic potential of Syzygium cumini extract (SCE) on cyclophosphamide-induced genotoxicity and oxidative stress in mice. , Drug Chem Toxicol 36(4), 396-402.
- 44. (2016) . Sheweita, S.A.1.; Mashaly, S.1.; Newairy, A.A.2.; Abdou, H.M.3. and Eweda, S.M.2. (2016): Changes in Oxidative Stress and Antioxidant Enzyme Activities in Streptozotocin-Induced Diabetes Mellitus in Rats: Role of Alhagi maurorum Extracts. Oxid Med Cell Longev 10-1155.
- 45.R H Mohamad, A M El-Bastawesy, M G Abdel-. (2011) and anticarcinogenic effects of methanolic extract and volatile oil of fennel seeds (Foeniculum vulgare). , J Med Food 14(9), 986-1001.
- 46.Ozbek H, Uğraş S, Dülger H, Bayram I, Tuncer I et al. (2003) Hepatoprotective effect of Foeniculum vulgare essential oil. , Fitoterapia 74, 317-319.
- 47.Singh G, Maurya S, M P de-Lampasona, Catalan C. (2006) Chemical constituents, antifungal and antioxidative potential of Foeniculum vulgare volatile oil and its acetone extract. , Food Control 17(9), 745-752.
Cited by (3)
- 1.Pourjafari Fahimeh, Haghpanah Tahereh, Nematollahi-Mahani Seyed Noreddin, Pourjafari Fariba, Ezzatabadipour Massood, 2020, Hydroalcoholic extract and seed of Foeniculum vulgare improve folliculogenesis and total antioxidant capacity level in F1 female mice offspring, BMC Complementary Medicine and Therapies, 20(1), 10.1186/s12906-020-03083-3
- 2.Ghafouri-Fard Soudeh, Shoorei Hamed, Abak Atefe, Seify Mohammad, Mohaqiq Mahdi, et al, 2021, Effects of chemotherapeutic agents on male germ cells and possible ameliorating impact of antioxidants, Biomedicine & Pharmacotherapy, 142(), 112040, 10.1016/j.biopha.2021.112040
- 3.COŞKUN ÇETİN Nurdan, ERDEM İpek, DURUSOY Ömer Faruk, ALAŞAHAN Sema, ÇİMRİN Tülay, 2022, The Effect of Supplementation of Fennel (Foeniculum Vulgare Mill.) to the Feed on Egg Production, Slaughter and Carcass Characteristics, Formation of Parasites in the Intestine and Spermatological Quality in Japanese Quail During the Laying Period, Dicle Üniversitesi Veteriner Fakültesi Dergisi, 15(2), 85, 10.47027/duvetfd.1159507