Abstract
Introduction
Cognitive and physical (especially aerobic) training have been reported to enhance cognition in the elderly. The goal of this study was to compare the effectiveness of two types of training, namely combined cognitive-and-physical training and cognitive training alone, for cognition and in particular for executive function and working memory.
Material and Method
Healthy older adults (aged 65–86 years) were included in cognitive-and-physical - CAP (n=16) - or cognitive - COG (n=16) - training groups or in a passive control group – CONT (n=16). The training took place in 60-minute sessions conducted twice a week for 8 weeks. Cognitive functions were assessed before and immediately after the interventions and at a 1-month follow-up.
Results
In the short-term, the CAP and COG groups showed a transfer on updating, unlike the CONT group. In the long-term, although the gains achieved by both CAP and COG persisted, the benefit observed in the COG group was greater than that in the CAP group.
Conclusion
Our data suggest that there may be a complementarity between cognitive and physical training effects at the level of short-term transfer, given that physical training was able to boost cognitive training. Moreover, regarding transfer, physical training may help improve performance on untrained tasks. However, as far as the long-term persistence of the benefits of training is concerned, the results tend to indicate the superiority of cognitive training.
Author Contributions
Academic Editor: Ian James Martins, Edith Cowan University, Australia
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2019 Clemence Joubert, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Life expectancy is increasing systematically in western societies thanks to advances in medicine and improvements in quality of life. However, this increases not only the risk of age-related diseases but also that of normal aging-related frailty. Twenty percent of the elderly aged 70 experience difficulties in everyday life linked to a cognitive or physical decline which causes the partial or complete loss of their autonomy 1, 2. It is well established that normal aging impacts cognition 3, and in particular processing speed, working memory and executive functions 4, 5, and that this decline is correlated with changes in brain structure and function. There is as yet no efficient pharmacological treatment capable of counteracting these changes, with the result that other ways to improve or stabilize cognition with aging must be explored. The STAC-R model (Scaffolding Theory of Aging and Cognition - Revised version) 4 proposes that positive and negative factors influence brain function and structure throughout life. Most importantly, the structure and function of the brain are adapted and reorganized throughout life and the success of this seems to depend, at least in part, on one’s cognitive and physical activities or exercises. These are therefore thought to protect against normal 6 and pathological aging 3, 7. These activities and exercises may be spontaneous and be part of one’s existing lifestyle or may be proposed as supplementary training.
Generally speaking, training involves specific tasks that are intended to train specific functions (direct training) or more complex activities that are underpinned by a cognitive function of interest (indirect training). It is expected that training will have an effect on the trained tasks and, in this case, it reflects the effects of practice. Most importantly, however, transfer is expected to occur on untrained tasks involving identical (nearest transfer), close (near transfer) or different capacities (far transfer).
Cognitive Training
Cognitive training (i.e. repetitive exercises targeted at specific cognitive functions, performed individually and usually computer-based) has been widely studied in the scientific literature. The two cognitive functions which are the most frequently reported to decline with age and are thus the most frequently targeted by cognitive training are working memory and executive functions.
As far as working memory is concerned, some authors 8 showed that this improves after indirect training using video games. The authors selected three video games that targeted one specific cognitive function (i.e. auditory perception – Brain Fitness, visuomotor skills – Space Fortress, strategic reasoning – Rise of Nations). Beyond an improvement in the targeted function due to playing a specific video game, the authors reported the far transfer of visuomotor skill training toward working memory. Indeed, they observed working memory improvements only in the participants who played Brain Fitness and Space Fortress. It was found that the improvement was greater for the Space Fortress group, meaning that the training of visuomotor skills transfers more to working memory capacities than does auditory perception training. It has been shown that training cognitive strategies (SMART program) can improve working memory performance and complex abstraction 9. In addition, this improvement is correlated with an increase in cerebral blood flow in the prefrontal and middle/posterior cingulate cortex when the participants are at rest.
It has been shown that direct working memory training can lead not only to domain-specific improvement, but also to transfer to numerous untrained tasks : nearest transfer (i.e. visual working memory), near transfer (i.e. short-term memory) and far transfer (i.e. fluid intelligence and processing speed) 10. In addition, the 8-month follow-up showed that only the far-transfer effects were maintained. These results suggest that training working memory can counteract more than one aspect of cognitive decline in aging. This is probably due to the intrinsic features of working memory that involves several aspects of cognitive processing such as short-term memory, executive attention and inhibition 11. Thus, training working memory possibly involves the training of many cognitive components and can lead to the improvement of several cognitive processes. The reverse is also true and the direct training of certain other cognitive processes may have an impact on working memory. For example, some data reported that individuals with a high working memory capacity have better inhibition, i.e. a function that is considered to form part of the executive functions 12, 13. In addition, some authors have shown that working memory training leads to long-lasting improvements in executive functioning 14. Executive functions correspond to different cognitive control processes 15, such as switching, updating and inhibition 16. Following executive function training intended to improve switching, some authors observed not only a reduction in the cost of switching but also improvements in executive control (near transfer) and fluid intelligence U = U (H I ,C i ,L i) i= 1,….,n (far transfer) 17. Another study investigated the training of updating, i.e. an executive function that is particularly important H ifor working memory 18. The authors showed a significant C ishort-term, practice-related improvement in directly L itrained updating. Long-term follow-up (18 months) showed that the trained participants maintained nthe updating level they reached at post-test 1, suggesting that the benefits are strong enough to persist over time. Most importantly, the transfer of updating training to other cognitive functions (processing speed, working memory, episodic memory, verbal fluency and reasoning) was examined. No transfer of the benefits was observed, leading the authors to conclude that the generalization of the benefits of updating training to other cognitive functions is limited. Some authors
 trained participants with video games consisting of reading, arithmetic and memory exercises 19. The authors found H iimprovements not only on the trained tasks, but also on executive functions and processing speed. They concluded that this reflects the operation of near D itransfer since, in their opinion, the untrained cognitive domains (i.e. executive function and processing speed) are closely related to the trained domains. U iIt therefore seems that directly training working memory or executive functions such as inhibition or updating may have a positive impact on both the directly S itrained function and certain untrained capacities. Given that the decline in working memory and executive functions impacts everyday life 18, 20, 21 it seems
trained participants with video games consisting of reading, arithmetic and memory exercises 19. The authors found H iimprovements not only on the trained tasks, but also on executive functions and processing speed. They concluded that this reflects the operation of near D itransfer since, in their opinion, the untrained cognitive domains (i.e. executive function and processing speed) are closely related to the trained domains. U iIt therefore seems that directly training working memory or executive functions such as inhibition or updating may have a positive impact on both the directly S itrained function and certain untrained capacities. Given that the decline in working memory and executive functions impacts everyday life 18, 20, 21 it seems
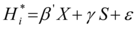 important to target these functions in cognitive training.
important to target these functions in cognitive training.
Physical Training
It has been shown that physical exercise enhances some cognitive functions such as learning, memory and executive function, and thus counteracts age-related 7, 22, 23 and disease-related cognitive decline 2, 24. This enhancement is probably due to the impact of the physical exercise on brain structure and function (e.g., increase in cortical thickness of specific regions or modification of activity in some regions 25, 26, 27, 28. Of the different types of physical exercises, aerobic exercise (i.e. cycling, running, walking, swimming) seems to have the greatest benefits for cognition due to enhanced cardiorespiratory fitness and, more specifically, to the oxygen consumption by the body during maximal physical effort. One neural efficiency hypothesis proposes that cognitive benefits due to cardiorespiratory fitness result from the combination of the mechanisms described by the oxygen hypothesis, which stipulates, namely, that cardiorespiratory fitness involves a higher cerebral blood flow, and the neurotrophic stimulation hypothesis predicting that it induces the better functioning of higher brain centers 29.
The impact of physical exercise on cognition is usually studied by (1) comparing physically non-active participants who undertake physical training with non-active and non-trained participants 2, 7, 30 or (2) comparing physically active individuals who spontaneously practice regular physical activity with physically non-active participants 7, 24, 31, 32, 33, 34, 35. It has been shown that after physical training 30, participants in a physically active group 35 exhibit better executive function performance, especially in inhibition. Some authors have shown that after aerobic exercise, not only did the recall and recognition memory of older adults improve, but also that this improvement was due to an increase in hippocampal perfusion, indicating fitness-related vascular plasticity 27. Some authors have also found improved memory performance after physical training (aerobic), which was correlated with increased cerebral blood flow in the hippocampus measured during the resting state 9. Some authors found that physically active individuals have better working memory updating performance and executive functions than physically non-active participants 32. Other studies have shown that physically active individuals have better spatial memory 31, episodic memory 33, and short-term memory 34. Some authors showed that after following physical training, participants achieved the same pattern of results as physically active participants and exhibited better resistance to interference 7. This study suggests that to promote cognitive fitness, it is important to encourage regular physical activity as early as possible and that it is also important to propose physical training to older adults in order to improve cognition.
Combined Cognitive-and-Physical Training
The question of the potential value of combining cognitive and physical training within one and the same training intervention has recently been raised. Physical and cognitive training may play different but complementary roles in brain plasticity 9, 36, 37, 38. It has been suggested that physical training might enhance brain metabolism and plasticity, whereas cognitive training, by increasing mental demands, might use and reinforce the enhanced brain metabolism and guide brain plasticity 39. If the mechanisms underlying improvements in cognitive function due to these two types of training are different, there is reason to assume that combining both in one intervention would increase the benefits as compared to a single training mode. However, to our knowledge, only four studies 39, 40, 41, 42 have directly compared combined training with physical or cognitive training on their own. Some authors compared cognitive training alone with a combined cognitive-and-physical training 39. Both types of training consisted of four elements, three of which were identical for both types of training and were performed in groups: (a) board games that targeted memory, attention and executive functions, (b) psychoeducation and (c) computerized training that targeted memory, attention and executive function. The fourth element consisted of individual cognitive training (paper-and-pencil tasks) for the cognitive training group and physical activity (strength, flexibility, coordination/balance, walking and aerobic exercises) for the combined cognitive-and-physical training group. The authors found that combining cognitive and physical training in one intervention as compared to cognitive training on its own led to a greater improvement in attention at 1-year follow-up, while the gains were similar for both types of training from pre- to post-test. Similar post-test and follow-up benefits for the two types of training were observed for general cognitive state, immediate and delayed verbal memory, and letter fluency. The authors concluded that there is no clear evidence that combined training is superior to cognitive training on its own in improving cognition. Another study also failed to show any advantage of combined physical (aerobic, strength, flexibility) and cognitive training (e.g., memory, attention, eye-hand coordination) compared to cognitive training alone 43. On the contrary, and surprisingly, the combined training appeared to be less efficient even though the total training time was doubled. To the same conclusions arrived another study, although the authors suggested that physical, cognitive and combined training show different rather than equal benefits 41.
However, some authors found that as compared to both cognitive (visual-based Insight Program and auditory-based Brain Fitness Program) and physical training (walking and strength) administered on their own or to a passive control group, only a cognitive-physical training group exhibited an improvement in verbal long-delayed episodic memory recall after 16 weeks of training 40. In addition, this improvement was associated with a significant increase in glucose intake in the left sensorimotor cortex and a tendential increase in the left frontal cortex. Some authors showed that combined simultaneous training (verbal working memory and walking on a treadmill) elicited better performance in a paired-associates task and motor-cognitive dual task (gate walking plus counting backwards in steps of seven) than cognitive training on its own 42. A similar transfer of benefits after the two types of training was observed for executive control, but there was no significant improvement in performance in selective attention, reasoning and memory span tasks as a result of the two training modes. A study used electroencephalographic recordings (EEG) to investigate the link between neurophysiological synchronization patterns and cognitive performance 44. They demonstrated a positive impact of combined training consisting of cognitive exercises (auditory-related sensory information processing, memory, attention and learning) and physical exercises (aerobic, flexibility and strength) on neurophysiological synchronization. This reflects a coherent interaction of distant brain regions which, in turn, reflects the level of cognitive performance in the trained domains (i.e. auditory information processing). The implications of this study lie in the fact that it shows that cognitive and physical training can have a real impact on neural synchronization, at least in the short term. However, this study did not directly compare the combined training with cognitive training alone and only an active control group was included in the study.
Objective of the Present Study
Given the scarce and rather inconsistent data, it is necessary to further examine the relevance of combining cognitive and physical training in one intervention. Thus, the main objective of the present study was to compare the effectiveness of two types of training, namely combined cognitive-and-physical training and cognitive training alone, on cognition, and in particular on executive function and working memory. In line with the suggestion that cognitive and physical training act differently but complementarily 39, we expected to observe greater benefits on cognition after the combined cognitive-and-physical training than after cognitive training alone.
Method
Study Design
Participants were controlled for age, sex, and education. Then, they were pseudo-randomly assigned to the cognitive training group (COG, n = 16), the cognitive-and-physical training group (CAP, n = 16) or the no-contact control group (CONT, n = 16). The study was not double-blinded as participants and the examiner who administered all outcome measures knew to which training group the participants were assigned. The physical training was supervised by the experimenter present during training or online via internet connection for cognitive training. The initial duration of each training session and its contents were respected throughout the eight sessions.
At the end of the protocol participants from CONT group received internet access for 10 weeks to the same cognitive exercises as those performed by COG group during training sessions.
Study Population and Eligibility Criteria
Forty-seven older adults participated in the study after giving their informed consent. Exclusion criteria were significant cognitive dysfunction (Montreal Cognitive Assessment (MoCA) score <24) functional impairment (assistance in activities of daily living (ADLs), stroke within the last 12 months, current chemotherapy, poor vision or hearing. Five individuals did not complete the study because of time constraints and illness, which left us with a total of forty-two participants (mean age = 69.58, SD = 3.36, male = 18, female = 30). Participants were recruited through advertisements in a local newspaper. The participants did not receive any payment or refund of their transport costs. They received general and personalized feedback at the end of the study. At the beginning of the study, the participants were screened for cognitive health and medical antecedents. The demographic characteristics and general cognitive and mental state of the participants are presented in Table 1. There were no significant differences in cognitive status (apart from on the RAVLT test), baseline scores on experimental tasks or demographic data. The CONSORT (Consolidated Standards of Reporting Trials) flowchart (Figure 1) shows the number of participants in the protocol at each stage of the study.
Table 1. Demographic characteristics and general cognitive and mental state of the participants included in the study.| Variable | Cognitive training only (n = 16)M (SD) | Combined cognitive-and-physical training (n = 16)M (SD) | No-contact control group (n = 16)M (SD) | F | p |
| Age | 69.5 (3.74) | 69.44 (3.12) | 69.8 (3.21) | F (2, 42) = 0.04 | .96 |
| Education (in years) | 15.06 (2.77) | 14.38 (3.3) | 14.13 (2.86) | F (2, 45) = 0.43 | .66 |
| Gender: Male/Female | 6/10 | 6/10 | 6/10 | ||
| MoCA | 27.63 (1.63) | 27.25 (1.69) | 27.06 (2.35) | F (2, 45) = 0.36 | .70 |
| IADL:Male/Female | 5/8 | 5/8 | 5/8 | F (2, 45) = 0.38 | .96 |
| PSQI | 5 (2.99) | 5 (2.25) | 5.63 (2.31) | F (2, 45) = 0.32 | .73 |
| RAVLT (Total recall) | 49.25 (10.14) | 56.94 (5.43) | F (1, 30) = 7.14 | .01* | |
| Forward Digit Span | 8.8 (1.82) | 8.94 (1.39) | 8.5 (2.28) | F (2, 44) = 0.46 | .63 |
| Backward Digit Span | 8.31 (2) | 7.63 (1.75) | 6.85 (1.8) | F (2, 44) = 2.12 | .13 |
| Sequencing Digit Span | 8.31 (1.7) | 8.69 (2.21) | 7.57 (1.7) | F (2, 44) = 1.63 | .2 |
| Victoria Stroop (Interference score) | 0.88 (0.76) | 0.42 (1.08) | 0.27 (0.41) | F (2, 41) = 2.11 | .2 |
Figure 1.The CONSORT (Consolidated Standards of Reporting Trials) flowchart.
Material
Neuropsychological Assessment
Tests
The neuropsychological assessment included 7 paper-and-pencil tests. We assessed global cognition with MoCA (Montreal Cognitive Assessment) 45, memory with the Rey Auditory Verbal Learning test - French version (RAVLT) 46, switching with TMT A/B (Trail Making Test 47, verbal fluency 48, short-term and working memory with the digit span subtest of the WAIS (Wechsler Adult Intelligence Scale) 49, and visual inhibition with the Victoria Stroop test 50. The control group undertook a shorter version of the test battery (see details in Table 1 and Table 2).
Table 2. Effect of training on neuropsychological tests at T0 (pre-test evaluation) and T2 (post-test evaluaion).| Variable | Test | Cognitive training only (n = 16)M (SD) | Combined cognitive-and-physical training (n = 16)M (SD) | No-contact control group (n = 16)M (SD) | F | p |
| GDS | Pre-Test | 1.56 (2.31) | 1.68 (3.09) | 3.25 (3.40) | F (2, 45) = 1.61 | .21 |
| Post-Test | 1.57 (2.07) | 1.17 (1.67) | 2.92 (4.03) | F (2, 36) = 1.37 | .27 | |
| McNair | Pre-Test | 14.31 (6.16) | 11 (4.50) | 7.38 (4.04) | F (2, 45) = 8.25 | .0009* |
| Post-Test | 18.67 (6.33) | 10.92 (5.71) | 9.08 (4.72) | F (2, 37) = 11.37 | .0001* | |
| SF-12 Mental | Pre-Test | 52.18 (7.19) | 52.24 (6.81) | 51.43 (8.34) | F (2, 41) = 0.05 | .95 |
| Post-Test | 49.9 (7.19) | 53.22 (3.49) | 52.26 (9.17) | F (2, 41) = 1,37 | .37 | |
| SF-12 Physical | Pre-Test | 54.4 (5.38) | 53.29 (6.56) | 50.42 (7.37) | F (2, 35) = 1 | .26 |
| Post-Test | 55.08 (5.28) | 52.44 (7.22) | 51.61 (9.28) | F (2, 35) = 0.83 | .44 | |
| Verbal fluency (Lexical, Z score) | Pre-Test | 0.74 (1) | 0.75 (0.88) | 0.27 (0.76) | F (2, 44) = 1.05 | .36 |
| Post-Test | 0.86 (0.87) | 0.88 (1.29) | 0.70 (0.73) | F (2, 39) = 0.79 | .46 | |
| Verbal fluency(Categorial, Z score) | Pre-Test | -0.42 (0.79) | 0.32 (1.01) | 0.17 (0.71) | F (2, 44) = 3.64 | .04* |
| Post-Test | 0.21 (0.83) | 1.19 (1.32) | 0.39 (0.92) | F (2, 39) = .15 | .86 | |
| TMT (B-A, Time, Z score) | Pre-Test | 0.61 (0.58) | 0.44 (0.56) | 0.60 (0.33) | F (2, 43) = .55 | .58 |
| Post-Test | 0.62 (0.49) | 0.34 (0.56) | 0.49 (0.59) | F (2, 39) = 1.32 | .28 |
Questionnaires
Autonomy was assessed using the IADL (Instrumental Activities of Daily Living) 51, memory disorders with McNair -15 items 52, mood with GDS (Geriatric Depression Scale) 53, sleep with PSQI (Pittsburgh Sleep Quality Index) 54, quality of life with SF-12 55 (see details in Table 1 and Table 2).
The participants also filled in sociodemographic and sociocultural questionnaires to allow us to collect data about medication, housing and cultural, social and physical activities.
Executive Function and Working Memory Tasks : Primary Outcomes Measures
Four computer-based tasks were constructed to measure flexibility and switching, visual attention and inhibition, updating, and maintenance. The tasks were programmed using E-prime 2.0 professional (Psychology Software Tools, Pittsburgh, PA). Participants undertook the primary outcomes measures at short-term follow-up (i.e. 1 week after the end of the training program) and at long-term follow-up (i.e. 1-month after the end of the training program).
Flexibility and switching were measured using the Plus Minus task 56. This task comprised three lists of 20 two-digit numbers. For the first list, the participants were instructed to add 7 to each number. For the second list, they were instructed to subtract 7 from each number. For the third list, they were instructed to switch between addition and subtraction. The numbers were randomized, and the lists were counterbalanced. This task allowed us to calculate the flexibility cost for correct answers and the reaction times, which were obtained by subtracting the mean performance in the two first conditions from the mean performance in the switching condition.
Visual attention and inhibition were measured using the Flanker task 7, 57. This task consisted of three lists of 50 sequences of five arrows. The first list constituted the "Congruent" condition, in which the five arrows pointed in the same direction. The second list represented the "Incongruent" condition, which measured inhibition and in which the arrow placed in the middle of the screen pointed in the opposite direction to the other four. The third condition was the "Neutral" condition in which there was only one arrow. The sequences of arrows were randomized and the participants were instructed to decide whether the arrow in the center of the screen pointed right or left.
Updating was measured using the Updated Span task 58. This task consisted of sequences of numbers that were presented sequentially, for 1000 ms each, in the center of the computer screen. The quantity of numbers presented per sequence (3, 4, 5, 6, 7, 8, or 10) varied randomly across trials. A total of 23 trials were presented: 2 three-digit sequence trials, 2 four-digit sequence trials, 3 five-digit sequence trials, 3 six-digit sequence trials, 3 seven-digit sequence trials, 3 eight-digit sequence trials, 7 ten-digit sequence trials. The participants were instructed to recall the last three numbers presented.
Maintenance was measured using the Complex Span task 59. This task consisted of series of numbers and letters that were presented successively on the computer screen for 1000 ms each. The participants were instructed to decide whether each number was even or odd by pressing a corresponding key on the keyboard and to remember the letters. At the end of each sequence, the participants were asked to perform free recall of the letters. Each sequence consisted of 4 numbers and 5 letters. A total of 10 sequences were presented.
Physical Assessment
After obtaining a doctor's certificate indicating that the participants were able to perform cardiovascular training, physical performance was measured using physical measures. Participants had to walk four times 400 meters as quick as possible. They had 1 minute break between the different sessions. The heart rate and time of completion were measured after the last 400 meters walked, at pre and post-test (Table 3).
Table 3. Physical assessment of participants that undertook physical training| Cognitive-and-Physical training group | |||
| Pre-Test (T0) (n = 16)(Mean, SD) | Post-test (T2) (n = 15)(Mean, SD) | F, p | |
| Heart Rate | 124 (24) | 122 (22) | F (1, 14) = 0.03, p = .9 |
| Time (min, sec) | 4.9 (2.3) | 3.6 (0.9) | F (1, 14) = 7.5, p = 0.02 |
Training
Cognitive Training
The cognitive training consisted of a computer-based program (HAPPY neuron Professional, SBT product https://www.happyneuronpro.com), including exercises that trained executive functions and working memory and which was accessible via the online platform. For each participant, we programmed a series of exercises that trained both executive function and working memory. All the participants started with the lowest-level training for each exercise. The duration of training with each exercise and the time at which they moved on to the next level depended on each individual's individual progress. Thus, the exercise level was adaptive and task difficulty increased or decreased depending on each participant's performance. In consequence, all the programmed exercises were not necessarily performed during one and the same training session. However, in each session, each participant trained with at least one executive function exercise and one working memory exercise. Each training session lasted 1 hour. The starting level for training in each session was determined by the level the participant had reached at the end of the previous session.
The training progression was evaluated by two scores: accuracy of responses and the reached level of exercise difficulty. In the end of each session the mean accuracy for each exercise was calculated and the reached level of exercise difficulty recorded. To examine progression of training we computed for each session the composite scores for accuracy and reached level of exercises difficulty, separately for executive function and working memory.
Executive Function Training
Executive Function Training Three Exercises.
Ballons de basket (Basketball ball) intended to train reasoning and planning. In this exercise, the first screen section contained a figure representing balls in three basketball hoops. The configuration in the first screen section provided a model. In the second screen section, there were again balls in basketball hoops but the configuration was different. The participants were instructed to decide, without performing any manipulation, how many manipulations they would need to change the configuration in the second screen section to be the same as that in the first section.
Tour de Hanoi (Tower of Hanoi) also intended to train planning. There were three poles and a large number of rings of different sizes. In the first screen section, the rings were placed in a specific configuration which represented the model to be reproduced. In the second screen section, participant had to represent the configuration presented in the first section while making as few moves as possible. The participants had to follow three rules: only one ring can be moved at a time; each move must consist of taking the topmost ring from a pole and placing it on another one; no ring can be placed on top of a smaller one.
Vive l’alternance (Long live alternation) intended to train switching. The goal is to permanently switch between alphabetical and numerical classification. Series of numbers and letters were presented and the participants had to select the items in alphabetical and numerical order. The aim of the exercise was to systematically switch between a letter and a number.
Working Memory Training
Working Memory Training Included Four Exercises.
Chants D’oiseaux (Birdsongs)- the goal was to memorize different birdsongs associated with the names of the birds in question, and then to find the birdsong that corresponded to any given bird name. The participants had to keep the information in memory while comparing it to other information.
Sous-Ensembles (Subsets) - the goal was to memorize successively the location of different geometric shapes on a grid. The grid was rotated, with the result that the view of the elements and their location changed. The participants had to maintain the information in memory while memorizing new elements.
Garçon SVP! (Waiter please!) - the goal was to memorize the menus ordered by the clients in a restaurant. The participants had to memorize several menus at a time together with the orders placed by the various clients. Depending on the level of difficulty, the participants had to memorize the menus of different tables at the same time. What is more, the customers could move during the exercise, meaning that the participants not only had to retain the table number associated with the order, but also the names of the clients.
Jeux de Blasons (Game of Heraldry) - the goal was to memorize a coat of arms and the elements that constitute it. For this, it was necessary to pay attention to the shapes, colors and their arrangement. This coat of arms had to be reproduced with all its components. An intermediate task could interfere with memorization. The participants had to perform this secondary task while keeping the coat of arms in memory.
Physical Training
The physical training took the form of walking exercise. The participants walked at the speed they chose after having been asked to walk as quickly as possible on a treadmill for 1 hour, including a warm-up period. After 30 minutes, participants were authorized to take 2 minutes of break. In the first and last sessions, participants only undertook the physical assessment. At the beginning of each training session, the participants walked on the treadmill for a few minutes in order to determine their optimum walking speed and to get used to using the equipment. The exercise level was adaptive and the participants were instructed to increase their physical effort from session to session. The progress of training across training sessions was determined by the walked distance and the physical assessment.
Procedure
The participants were evaluated at the beginning of the study, before the training (pre-test – T0), in the middle of the training after four weeks (middle-test – T1), and 1 week after the completion of the training - at short-term follow-up (post-test – T2). The COG and CAP groups undertook a long-term follow-up four weeks after the post-test to investigate the persistence over time of any benefits (follow-up – T3). Participants were asked to maintain their usual way of life, and not to undertake new activities. The test sessions lasted between one and two hours. The participants received oral or computerized instructions and could ask questions if they did not understand. At pre-test, the entire neuropsychological assessment was performed together with the executive function and working memory tasks. Only the participants in the combined cognitive-and-physical training group undertook the physical assessment. At middle-test, only executive function and working memory were assessed. At post-test, the participants performed the executive function and working memory tasks, as well as the McNair, GDS, SF-12, TMT A/B, and verbal fluency tests. At follow-up, only the executive function and working memory tasks were performed.
The participants in the two training groups attended 16 training sessions (2 sessions per week for 8 weeks) and three test sessions. Regarding training sessions, the COG group followed 16 hours of cognitive training, whereas the CAP group followed 8 hours of cognitive training and 8 hours of physical training. The participants in the COG group visited the university once a week to follow the one-hour computerized training program. They also performed the program once a week at home for one hour on their personal computers. The participants in the CAP group visited the university once a week to walk on the treadmill for one hour, and also performed the computerized cognitive training program at home for one hour once a week. The participants in the control group were seen only for test sessions and were asked to maintain their usual behavior.
We hypothesized that the combined cognitive-and-physical training would lead to (1) a higher improvement of cognition, and (2) a stronger persistence of benefits through time, as compared to the cognitive training alone.
Statistical Analysis
We examined the normality of the distribution for each composite score of the training progress (accuracy and reached level of exercises difficulty) and each primary outcome measure with Shapiro-Wilk test. For the measures of training progress, the distribution was normal so we performed the mixed ANOVA. For the primary outcomes measures, the distribution was normal only for updating. Thus, we decided to perform the MANOVA including all the executive function and memory measures as dependent variables. The statistics were performed with SPSS software.
Results
The results are presented in 4 sections: (1) baseline characteristics, (2) trained tasks, and (3) executive function and working memory tasks to investigate transfer of abilities to untrained tasks, and (4) follow-up.
Baseline Characteristics
We used a one-way Kruskal-Wallis ANOVA to compare the age and education level of the different groups as well as for the tests included in the neuropsychological assessment.
The participants in the three groups did not differ significantly either in age (all p > .05) or in education (all p > .05) (See Table 1).
Neuropsychological Assessment
At baseline, the participants in the three groups differ significantly on RAVLT (see Table 1), McNair, and category fluency (See Table 2) (all p < .05). Planned comparisons showed that the CAP group performed better at baseline on RAVLT than the COG group, F (1, 30) = 7.14, p = .01. Both the CAP and COG groups scored higher on the McNair questionnaire than the CONT group, respectively t(30) = 2.56, p = .02, t (30) = 3.93, p = .0005. The COG group had a lower Z score (i.e. performed worse) for category fluency than the CAP group, t (30) = -2.3, p =.03, or the CONT group t (30) = 0.70, p = .02. There was no other significant difference between groups concerning neuropsychological tests.
Regarding the post-training assessment, the between-group differences were significant only for the McNair questionnaire, with the COG group scoring higher than the CAP or CONT group, respectively t (25) = 3.38, p = .001, t (25) = 4.49, p = .0001. There was no significant difference between the CAP and CONT group (p > .1).
Training Progress
The distribution was normal for the composite scores (mean accuracy and mean level of exercises difficulty) for executive function tasks (Ballons de Basket, Vivel’alterance, Tour de Hanoï) and working memory training (Chants d’Oiseaux, Sous-ensembles, Garçon SVP !, Jeux de blasons), so we performed ANOVAs on these scores. The ANOVAs included the between-subject factor Group with two modalities (COG, CAP) and the within-subject factor Training Week with eight-modalities (W1 – week 1, W2, W3, W4, W5, W6, W7 and W8).
Cognitive Training
Executive Function Training
A significant effect of Training Week on the composite score for correct answers was observed, F (7, 189) = 4.6, p = .00009. Post-hoc Bonferroni comparisons showed that the number of correct answers produced by the participants increased significantly at W3 (M = 92%, SD = 9.31), W5 (M = 92%, SD = 7.37), W6 (M = 93%, SD = 8.85), W7 (M = 92%, SD = 11.87) as compared to W1 (M = 80%, SD = 12.43), respectively p = .0009, p = .002, p = .0009 and p = .0009 (see Figure 2a). There were no other significant differences. There was no significant effect of Group, F (1, 27) = .17, p > .05, and no interaction between Group and Training Week, F (7, 189) = 0.82, p = .9.
Regarding the level of difficulty, a significant level of Training Week was observed, F (7, 189) = 148, p < .0000001. The group effect was also significant, F (1, 27) = 12.08, p = .002. The Training Week x Group interaction was significant, F (7, 189) = 5.75, p = .000005. The post-hoc Bonferroni comparisons showed that in W4 and W8, the COG group progressed significantly more (respectively, M = 2.83, SD = 0.78; M = 5.19, SD = 0,41) than the CAP group (respectively, M = 2.01, SD = 0.66; M = 3.76, SD = 0.84), all p <.001. As far as the week-to-week progress in training is concerned, the first significant progression observed for the COG group occurred between W1 and W3 (p < .0001) and the subsequent significant progressions were as follows: from W3 to W5 (p < .0001), from W5 to W6 (p < .03) and from W7 to W8 (p < .002). For the CAP group, the first significant progression was observed from W1 to W4 (p < .004), and then from W4 to W6 (p < .009) and from W6 to W8 (p < .004).
Working Memory Training
A significant effect of Training Week was observed on the composite score for correct answers, F (7, 196) = 3,61, p = .001. Post-hoc Bonferroni comparisons showed that the participants gave significantly more correct answers at W8 (M = 92.82%, SD = 9.23) than at W1 (M = 80.19%, SD = 19.41), p = .029 or W3 (M = 79.91%, SD = 20.58), and also at W3 than at W7 (M = 92.32%, SD = 14.8) (see Figure 2a). There was no significant effect of Group F (1, 28) = 0.29, p > .05, and no significant Group* Training Week interaction F (7, 196) = 1.89, p = 0.9.
As far as the level of difficulty is concerned, a significant effect of Training Week was observed F (7, 196) = 85.86, p < .0000001. The Group effect was also significant, F (1, 28) = 10.2, p = .003. The Training Week x Group interaction was significant, F (7, 196) = 5.73, p = .000005. The post-hoc comparisons showed that in W7 and W8, the reached level of difficulty of the COG group was significantly higher (respectively, M = 4.39, SD = 1.9; M = 5.19, SD = 2.25) than that of the CAP group (respectively, M = 2.79, SD = 0.7; M = 3.42, SD = 0.93), all p <.001. As far as the week-by-week progress in the reached level of exercise difficulty is concerned, the first significant progression in the COG group was observed from W1 to W3 (p < .02) and the subsequent significant progressions were as follows: from W3 to W5 (p < .003), from W4 to W6 (p < .0002), from W5 to W7 (p < .0001), and from W6 to W8 (p < .0001). For the CAP group, the first significant progression was observed from W1 to W5 (p < .01), and then from W3 to W6 (p < .04), from W4 to W7 (p < .004), from W5 to W8 (p < .0001) and from W6 to W8 (p < .004) (Figure 2a) (Figure 2b)
Figure 2.a) Executive functions and working memory training progress, based on composite scores for correct answers, for all groups (CAP and COG) depending on Training Week (W1, W2, W3 , W4, W5, W6, W7, W8) ;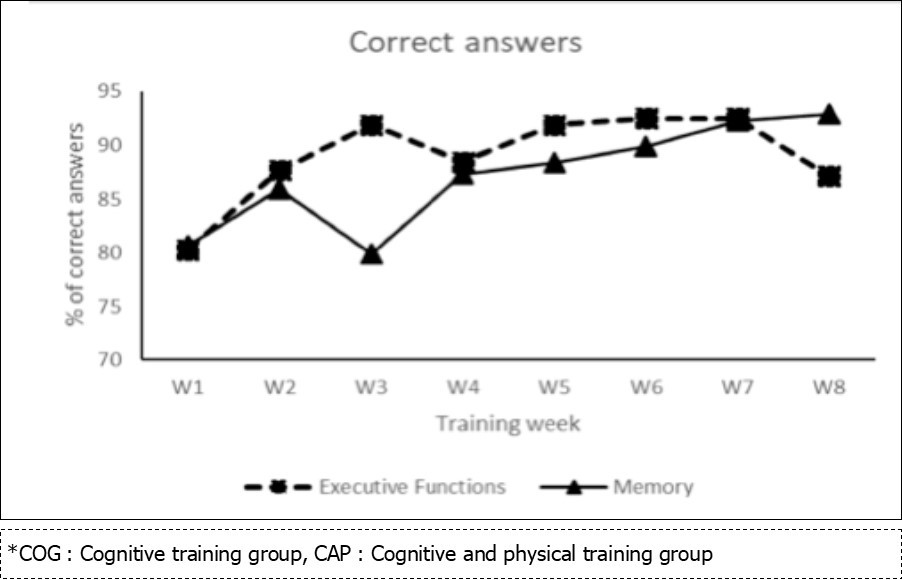
Figure 2.b) Executive functions and working memory training progress, based on composite scores for level of difficulty, depending on Group (CAP, COG) and Training Week (W1, W2, W3 , W4, W5, W6, W7, W8).
Physical Training
Physical assessment showed difference in time need to walk 400 m between pre- and post-training. Indeed, participants walked faster at week 8 than at week 1 (See Table 3). However, no heart rate difference was found between pre-test and post-test. Regarding the walked distance across the training sessions, participants showed significant general improvement 7, F(1, 15) = 10.6, p < .001. Post Hoc comparisons showed that participants improved their walked distance between Week 2 and Weeks 4, 5, 6 and 7 (all p < .003); between Week 3 and Weeks 6 (p = .02) and 7 (p < .001) ; and between Week 4 and 7 (p = .03). As participants were authorized to take 2 minutes of break after 30 minutes of walking, we reported in Table 4 the mean distance walked in 30 minutes for each training session (Table 4).
Table 4. Distance walked by participants that undertook physical training| Training Week | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 |
| Meters / 30 minutes (Mean, SD) | 2201 (686) | 2415(714) | 2551(559) | 2605(557) | 2714(602) | 2834(591) |
Transfer to Executive Function and Working Memory Tasks : Primary Outcomes Measures
Descriptive data for executive function and working memory measures for all groups are given in Table 5and Table 6.
Table 5. Descriptive data for correct responses at Pre-test (T0), Middle-test (T1), Post-test (T2) and Follow-up test (T3).| Group/Task | Pre-test(T0)(Mean, SD) | Middle-test (T1)(Mean, SD) | Post-test (T2)(Mean, SD) | Follow-up (T3)(Mean, SD) |
| COG | ||||
|---|---|---|---|---|
| Flexibility cost | -2,42 (6,26) | -0,71 (1,47) | -0,42 (2,6) | -1,04 (2) |
| Visual attention and inhibition | 148,6 (1,6) | 149 (1) | 148,6 (1,7) | 145,5 (12,9) |
| Maintenance | 4 (2,7) | 4,25 (2,3) | 5,42 (2,5) | 5,71 (2,4) |
| Updating | 20,25 (4,3) | 22,08 (2,8) | 23,08 (1,1) | 23,29 (2,2) |
| CAP | ||||
| Flexibility cost | 0,63 (1,8) | -1,08 (2,5) | -1,21 (2,7) | -0,3 (1,8) |
| Visual attention and inhibition | 144,3 (11,9) | 146,5 (7,7) | 147,1 (7,4) | 147,87 (6,6) |
| Maintenance | 2,33 (1,5) | 3,83 (2,6) | 4,08 (2,2) | 4,67 (2,5) |
| Updating | 17,42 (4) | 19,08 (3,4) | 20,75 (3,5) | 21,73 (3,7) |
| CONT | ||||
| Flexibility cost | 0,38 (0,9) | 0,69 (2,3) | 0,04 (1,3) | N/A |
| Visual attention and inhibition | 143,08 (14,1) | 145,5 (13,1) | 145,2 (13,9) | N/A |
| Maintenance | 3,92 (2,7) | 4,54 (2,4) | 4,85 (2,3) | N/A |
| Updating | 15,31 (7) | 16,23 (7,8) | 17 (6,9) | N/A |
| Group/Task | Pre-test(T0)(Mean, SD) | Middle-test (T1)(Mean, SD) | Post-test (T2)(Mean, SD) | Follow-up (T3)(Mean, SD) |
| COG | ||||
|---|---|---|---|---|
| Flexibility cost | 425 (599) | 468 (401) | 466 (908) | 393 (362) |
| Visual attention and inhibition | 665 (211) | 621 (205) | 532 (90) | 545 (192) |
| Maintenance | 9889 (3594) | 7872 (2461) | 6718 (2172) | 6877 (2180) |
| Updating | 4253 (1094) | 3789 (1094) | 4124 (1124) | 4072 (1470) |
| CAP | ||||
| Flexibility cost | 1014 (614) | 422 (548) | 538 (778) | 373 (478) |
| Visual attention and inhibition | 709 (227) | 604 (133) | 599 (113) | 528 (100) |
| Maintenance | 100129 (2661) | 8542 (2427) | 8366 (2674) | 7300 (1252) |
| Updating | 5093 (1997) | 4564 (1600) | 4270 (1645) | 4058 (1112) |
| CONT | ||||
| Flexibility cost | 720 (828) | 448 (629) | 364 (641) | N/A |
| Visual attention and inhibition | 864 (329) | 715 (261) | 601 (130) | N/A |
| Maintenance | 11730 (4560) | 9624 (3983) | 5299 (2452) | N/A |
| Updating | 5299 (985) | 4648 (1178) | 4465 (859) | N/A |
Baseline Comparison
To test for significant differences at pre-test between groups we used paired t-tests. COG and CAP groups did not differ at pre-test for any of the specific executive functions and working memory tasks, all p > .05. However, COG group showed significantly better scores, t(30) = 2,19, p = .04, and shorter reaction times, t(30) = -2,2, p = .03, than CONT group for updating. CAP group showed at pre-test shorter reaction times for visual attention and inhibition than CONT group, t(30) = -2,11, p = .04.
Immediate Transfer Effects (pre – T0 vs Middle-test – T1 vs Post-test – T2)
In order to analyze the immediate transfer effect of training on executive function and working memory, we performed two multivariates analysis of variance (MANOVAs), one with correct responses and one with reaction times as dependent variables, with Group (COG, CAP, CONT) as the between-subjects factor and Time (T0, T1, T2) as the within subject factor. We reported Pillai’s Trace statistics, assuming that it yields the most robust outcome.
We first present the MANOVA for correct responses, and then for reaction times. For significant MANOVA, we further analyzed each specific executive function and working memory measure separately with univariate tests (we report Greenhouse-Geisser test as far as the sphericity was not respected for some measures), and we also performed pairwise comparisons for significant univariate effects.
Correct Responses
The MANOVA for accuracy with all the executive function and memory measures as dependent variables showed significant effect of Group, F (8, 64) = 2,9, p = .008, ƞp2 = .268. Specifically, univariate tests showed a significant effect of Group for flexibility cost (plus-minus test), F (2, 34) = 3,6, p = .04, ƞp2= .175. Pairwise comparisons showed an increased flexibility cost for COG as compared to CONT group, p = .01. Effect of Group was also significant for updating (updating span task), F (2, 34) = 5,79, p = .007, ƞp2 = .254.
The MANOVA also showed significant effect of Time, F (8, 27) = 3,16, p = .01, ƞp2 = .48. Specifically, univariate tests showed a significant effect of Time for maintenance (complex span task), F (2, 68) = 6,14, p = .004, ƞp2 = .153. Pairwise comparisons showed that participants had significantly higher scores at T2 as compared to T0, p = .001. Participants also presented tendency to higher scores at T2 as compared to T1, p = .06. Effect of Time was also significant for updating, F (2,68) = 4,9, p = .01, ƞp2 = .126.
Although the MANOVA showed no significant Time x Group interaction, F (16, 56) = 0,6, p = .9, ƞp2 = .146, the effects of Time and Group were significant for updating, and as we had specific predictions regarding this interaction, we performed planned comparisons (see Figure 3). The results showed that the COG, t (29) = -2,45, p = .02, and CAP groups, t (29) = -2,42, p = .02 had higher scores at T2 than at T0, whereas no such difference was observed in the CONT group, p = .5.
COG : Cognitive training group, CAP : Cognitive and physical training group, CONT : Control group ; T0 : Pre-test, T1 : Middle-test, T2 : Post-test
Figure 3.Mean correct answers and standart deviation for updating accuracy, based on Updating span task, depending on the group (COG, CAP, CONT) and on time (T0, T1, T2).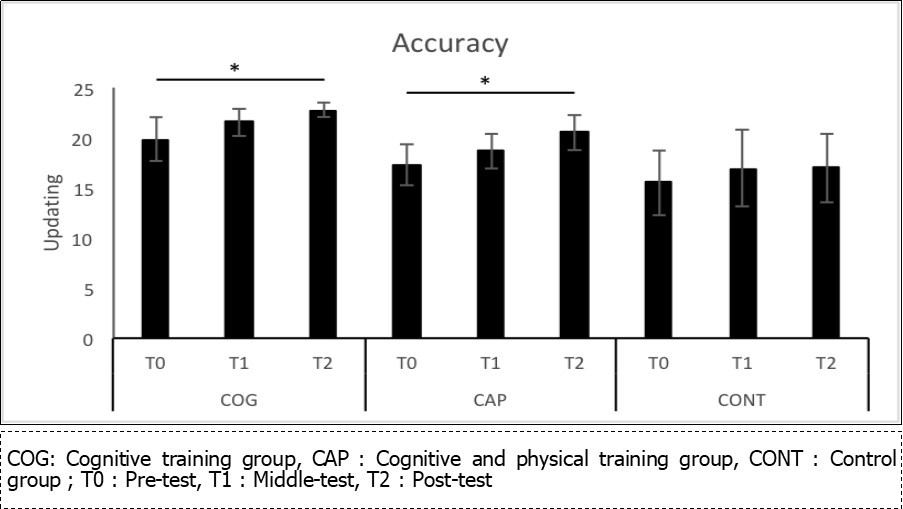
Reaction Times
The MANOVA for reaction times with all the executive function and memory measures as dependent variables showed significant effect of Time, F (8, 28) = 7,7, p > .0001, ƞp2 = .69. Specifically, univariate tests showed a significant effect of Time for attention and inhibition (flanker task), F (2, 70) = 18,4, p < .000001, ƞp2= .345. Pairwise comparisons showed that participants had shorter reaction times at T2 as compared to T1, p = .004, and as compared to T0, p < .0001. The reaction times were also shorter at T1 than at T0, p = .001. Significant effect of Time was also found for maintenance, F (2, 70) = 11.55, p < .0001, ƞp2= .248. Pairwise comparisons showed that participants had shorter reaction times at T2 as compared to T0, p < .0001. The reaction times were also shorter at T1 than at T0, p = .005. However, there was no significant difference between T1 and T2, p = 0.1. Significant effect of Time was also observed for updating, F (2, 70) = 11,96, p < .0001, ƞp2= .255. Pairwise comparisons showed that reaction times were shorter at T2 as compared to T0, p < .0001. Reaction times were also shorter at T1 as compared with T0, p < .0001. However, there was no significant difference between T1 and T2, p = 0.74. The MANOVA was not significant for Group, F (8, 66) = 1,05, p = .4, ƞp2 = .11 and interaction Time x Group F (16, 58) = 1,5, p = .15, ƞp2 = .28.
Follow-Up (post-test – T2 vs follow-up – T3)
To analyze the follow-up data, we performed two multivariates analysis of variance (MANOVAs), one with correct responses and one with reaction times as dependent variables, with Group (COG, CAP) as the between-subjects factor and Time (T2, T3) as the within subject factor. We first present the MANOVA for correct responses and then for reaction times. For significant MANOVA, we further analyzed each specific executive function and working memory measure separately with univariate tests (we report Greenhouse-Geisser test as far as the sphericity was not respected for some measures), and we also performed pairwise comparisons for significant univariate effects.
Correct Responses
The MANOVA for correct responses with all the executive function and memory measures as dependent variables was not significant neither for Group, F (4, 24) = 1,3, p = .29, ƞp2 = .181, nor for Time, F (4, 24) = 1,78, p = .16, ƞp2 = .23. The interaction Time x Group was not significant, F (4, 24) = 1,34, p = .29, ƞp2 = .182. However, the univariate tests showed the significant effect of Group for updating, F (1, 27) = 4.9, p = .04, ƞp2 =.154. Pairwise comparisons showed that the COG group had significantly higher scores than the CAP group. There was a tendency to a significant effect of Time for maintenance, F (1, 27) = 3,95, p = .06, ƞp2 = .128. Pairwise comparisons showed that participants had higher scores at T3 as compared to T2. The effect of Time was also significant for updating, F (1, 27) = 4.47, p = .04, ƞp2 = .128. Pairwise comparisons showed that participants tended to have higher scores at T3 than at T2.
Reaction Times
The MANOVA for reaction times with all the executive function and memory measures as dependent variables were not significant neither for Group, F (4, 24) = 0,292, p = .8, ƞp2 = .046, nor for Time, F (4, 24) = 0,231, p = .9, ƞp2 = .037. The Group x Time interaction was not significant, F (4, 24) = 0,665, p = .6, ƞp2 = .1.
Discussion
The main objective of this study was to determine whether combined cognitive-and-physical training is better than cognitive training alone for improving older adults’ cognition. Based on previous studies, we hypothesized that combined training would lead to a greater improvement on untrained cognitive tasks involving executive functions and working memory. In the following section, we will first discuss the effects of practice (training progress), transfer to working memory and executive function after both types of training, and long-term persistence of the effects of training (1-month follow-up).
Neuropsychological Assessment
Globally the three groups were equivalent at baseline for the basic neuropsychological assessment (see Table 1and Table 2). Surprisingly, we observed that the memory performance of the CAP group was significantly better than that of the COG group on RAVLT. However, these two groups did not significantly differ in terms of subjective memory impairment as measured by the McNair test, although both seemed to judge their memory as more impaired than the CONT group.
At post training, the only intergroup difference revealed by the neuropsychological assessment was on the McNair questionnaire. The participants in the COG group judged their memory more impaired than those in the CAP and CONT groups. These results might be due to the baseline difference, given that the participants in the COG group judged their memory more impaired before commencing the training than those in the other groups. However, at baseline, the CAP group also judged their memory more impaired than the CONT group and this difference was no longer significant after training. It is therefore possible that the difference continued to be significant for the COG group because this group had to perform more challenging cognitive exercises than the CAP group and might therefore have been more frequently placed in situations in which they had the impression of memory failure.
Training Progress
In the present study, the cognitive training was conducted using Happyneuron (SBT Product Professional) and involved working memory and executive function exercises. Independently of the level of difficulty, the number of correct answers increased among the participants in both trained groups. Indeed, the scores increased globally between weeks 3 and 7 for executive functions, and between weeks 1 and 8 for working memory. Moreover, the participants progressed in terms of the reached level of task difficulty. These two results suggest that older adults can present practice (learning) effects on repeatedly performed tasks. In the present study, the tasks involved executive functions and working memory. These results are in line with several previous studies. In the IMPACT study (Improvements in Memory with Plasticity-based Adaptive Cognitive Training) 60, for example, the authors showed task-specific improvements during the training of auditory information processing, which were reflected through improvements in accuracy and speed. In the ACTIVE study (Advanced Cognitive Training for Independent and Vital Elderly) 61, the authors demonstrated an improvement on trained tasks at the post-training evaluation as compared to the pre-training evaluation in three training groups: Memory, Reasoning, Processing speed. Another study was conducted, in which they trained participants in a wide variety of cognitive functions, including working memory, executive functions and processing speed 62. The authors showed a general improvement on all the trained tasks. Overall, our results confirm previous results and suggest that the capacity to learn new cognitive tasks and abilities is preserved in older people.
Nonetheless, it is interesting that, globally, the CAP group did not reach the same level of difficulty as the COG group in either the executive function or the working memory tasks. The main difference between these two groups was that the CAP group had 8 hours of cognitive training (the remaining 8 hours being used for physical training), whereas the COG group had 16 hours of cognitive training. Even though both training groups had the same total hours of training, the COG group spent more time on trained tasks (had more practice). Thus, it seems that physical training did not compensate for the less hours of cognitive training. These data therefore suggest that the respective mechanisms of cognitive and physical training are probably not the same and that the training modes may not be interchangeable, at least when an impact is expected on a specific trained task.
Transfer to Working Memory and Executive Function Tasks
With regard to transfer to untrained working memory and executive function tasks, we observed little evidence supporting our hypothesis about the superiority of combined cognitive-and-physical training over cognitive training alone. Even more surprisingly, our results provide little evidence in support of the idea of transfer to untrained tasks.
We observed a significant effect of Time on reaction times, with reaction times being shorter for attention and inhibition, maintenance and updating tasks at T2 than at T0. However, as this improvement was independent of training group (no significant interaction was observed) and there was no effect of Group, we cannot attribute the better performance at T2 (post-training) to either cognitive or combined cognitive-and-physical training. One explication for these results may be learning effects, given that at T0, T1 and T2, the same tasks (different versions) were used to evaluate the transfer of the effects of training to untrained tasks. It is therefore possible that the performances of the participants in the CONT group improved simply because the participants performed the tasks three times. Furthermore, reaction times are generally not taken into consideration in studies of cognitive and physical training because they do not lead to stable and consistent results.
Concerning correct responses, a significant effect of Group was observed on flexibility cost. Indeed, the COG group showed higher flexibility cost as compared to CONT group, meaning a less effective realization of the switching condition in Plus Minus task. However, there was not significant interaction between Group and Time, and more importantly, the means reported in Table 2show rather puzzling performances, making the interpretation of the Group effect difficult. A significant effect of Time on maintenance was also observed. Participants had higher scores at T2 as compared to T0. However, in the absence of interaction, we cannot determine if these improvements are due to training condition or just to learning of the task. The significant effects of both Group and Time were observed only in the updating task. The participants in the COG and CAP groups were more accurate than those in the CONT group. Irrespective of group, the participants were more accurate at T2 than T0. Further analysis showed that only the COG and CAP groups performed better at T2 than T0. However, the fact that there was no significant interaction between Group and Time means that this analysis should be interpreted with caution. The results of the present study are consistent with a certain body of literature showing that training benefits are transferred to untrained tasks. Indeed, the present study shows a near transfer to updating. Another study also showed a near transfer to executive functions and processing speed following video game-based training of reading, arithmetic and memory 19. In addition, some authors showed a near transfer to short-term memory following working memory training 10. However, our results only allow us to draw conclusions regarding near transfer. They also investigated far transfer following training. They showed that training working memory resulted in transfer to fluid intelligence and processing speed, while another study showed a similar transfer after training in switching 17. Nonetheless, our results concerning the transfer of training benefits to untrained functions are not consistent with another study who did not find any transfer of benefits following updating training 18.
It is interesting to note that in the present study, the COG and CAP groups showed similar improvements on the updating task, even though the COG group progressed better in training on the trained tasks involving working memory. These data suggest that as far as transfer is concerned, physical training may help improve performance on untrained tasks. The question of the transfer of cognitive and physical training to cognitive abilities (i.e. untrained tasks) has also been investigated by testing the impact of training on attention 39. These authors also compared a group that received only cognitive training with a combined cognitive-and-physical training group. They showed the same pattern of results as in the present study, since both training groups improved to a similar extent on untrained tasks involving attention. Thus, these two studies suggest that there is some transfer of benefits from physical training to cognition. Moreover, another study showed that the benefit of combined cognitive-and-physical training on untrained functions was greater than that of cognitive training alone 42. This finding supports the idea that physical training contributes to cognitive improvements. However, some author did not find any enhanced effect on an untrained task after combined training as compared to cognitive training 43. Interestingly, this latter study compared cognitive training on its own, combined cognitive-and-physical training and physical training on its own. The improvement on untrained tasks was shown only for the participants who performed the cognitive training (both cognitive training alone and combined cognitive-and-physical training). Thus, in the Shatil study, there was no improvement in cognitive performance after physical training, leading the author to the conclusion that it is only the cognitive training component that drives cognitive enhancement 43. Indeed, cognitive training may have a greater impact on cognitive and neuropsychological measures because of its specificity 63. In other words, the cognitive training programs are thought to be related to cognitive outcomes and neuropsychological measures, and this is why some studies have found benefits due to cognitive training.
Follow Up
One of the objectives of training in the elderly is to obtain long-lasting benefits. The results of the follow-up for the present study must be taken with caution since this was undertaken only by the COG and CAP groups. The 1-month follow-up showed that the improvement persisted after training regarding visual attention and inhibition (flanker task), maintenance (complex span task) and updating (updating task) in the sense that the performance did not significantly decrease at follow-up as compared to T2.
Concerning maintenance, results showed that the gains observed at post-test persisted at follow-up. Moreover, both COG and CAP groups showed a tendency to improvement at follow-up as compared to post-test. Then, as far as updating is concerned, both the COG and CAP groups exhibited a significant improvement in accuracy at follow-up. In addition, the COG group scored higher than the CAP group at follow-up. These results suggest that even if the benefits of cognitive training and combined training are equivalent in the short term, updating seems to be more responsive to cognitive training alone in the long term. Similarly, another study showed equivalent benefits of cognitive and physical training alone, and combined training on concentration in the short term. In the long-term (i.e. 3-months follow-up), only physical training alone led to improvements of concentration 41. Though, combined cognitive-and-physical training showed improved cognitive speed in short- and long-term whereas, cognitive training alone led to improvement of cognitive speed only in the long term. Then, it has been shown at 1-year follow-up that the gains improved only in the combined cognitive-and-physical training group 39. It is possible that the interval between the end of the training and the follow-up, which was much longer in the Rahe et al. study, and the fact that different cognitive processes are involved in these two studies may explain the contradiction 39. Finally, the lack of data concerning the CONT group in our study makes it difficult to conclude that the long-term improvement we observed is due to a persistent impact of training over time.
Limitations of the Study
The main limitation of the present study is that our samples are rather small. Another limitation is the absence of a group that received only physical training. It would be interesting to directly compare training groups that receive only cognitive or only physical training in order to evaluate the contribution of each type of training to cognition and to test Shatil’s suggestion that, in combined training, it is cognitive training that drives cognitive enhancement 43. And finally the limitation of this study lies in the long-term follow-up. Indeed, in the present study, we used a 1-month follow-up, which is probably too short a period to predict the long-term persistence of benefits in elderly people (see 39 for a longer interval).
Conclusion
The goal of this study was to determine whether combined cognitive-and-physical training is better than cognitive training alone in improving older adults’ cognition. We found that the effect of practice in the COG group was better than in the CAP group, thus confirming previous studies showing that older adults can learn new cognitive abilities, and that the amount of training is important for learning success. In both groups, some transfer effects to untrained tasks were observed. In fact, despite the greater practice effect in the COG group, the CAP group performed as well as the COG group in transfer tasks immediately after the end of training. These results suggest that if physical training does not compensate for the effect of practice during cognitive training, it nevertheless in some way helps to transfer and improve certain cognitive abilities. Interestingly, the cognitive training seemed to be more efficient than combined cognitive-and-physical training for long-term transfer to updating. Overall, our results suggest that training benefits have a small effect on cognition, that cognitive and physical training complement one another with regard to short-term outcomes, and that cognitive training is more beneficial with regard to long-term outcomes.
Acknowledgement
We thank Tim Pownall, native English speaker, for English editing. We also thank Annie Pellardy, Manon Tchoulfayan, Cassandre Talbotier, Caroline Pierre and Chirsmy Sergent for support in data acquisition for control group.
Funding
This work was supported by the LABEX CORTEX (Grant ANR-11-LABX-0042) of Université de Lyon, within the program "Investissements d'Avenir" (Grant ANR-11-IDEX-0007) operated by the French National Research Agency (ANR).
References
- 1.K G Manton, K C Land. (2000) Active life expectancy estimates for the U.S. elderly population: A multidimensional continuous-mixture model of functional change applied to completed Cohorts,1982–1996.Demography.https://doi.org/10.2307/2648040. 37(3), 253-265.
- 2.Penninx B W J H, W J Rejeski, Pandya J, M E, M Di Bari et al. (2002) . Exercise and Depressive SymptomsA Comparison of Aerobic and Resistance Exercise Effects on Emotional and Physical Function in Older Persons With High and Low Depressive Symptomatology. The Journals of Gerontology: Series B 57(2), 124-132.
- 3.A F Kramer, K I Erickson. (2007) Capitalizing on cortical plasticity: influence of physical activity on cognition and brain function. Trends in Cognitive Sciences,https://doi.org/10.1016/j.tics.2007.06.009 11(8), 342-348.
- 4.P A Reuter-Lorenz, D C Park. (2014) How Does it STAC Up? Revisiting the Scaffolding Theory of Aging and Cognition. Neuropsychology Review.https://doi.org/10.1007/s11065-014-9270-9. 24(3):. 355-370.
- 5.Ska B, Joanette Y. (2006) . Vieillissement normal et cognition. M/S médecine sciences.https://doi.org/10.7202/012783 22(3), 284-287.
- 6.Rabipour S, Davidson P S R. (2015) Do you believe in brain training? A questionnaire about expectations of computerised cognitive training. , Behavioural Brain Research.https://doi.org/10.1016/j.bbr.2015.01.002 295, 64-70.
- 7.S J Colcombe, A F Kramer, K I Erickson, Scalf P, McAuley E et al. (2004) Cardiovascular fitness, cortical plasticity, and aging. Proceedings of the National Academy of Sciences of the United States of America.https://doi.org/10.1073/pnas.0400266101.101(9): 3316-3321.
- 8.Strenziok M, Parasuraman R, Clarke E, D S Cisler, J C Thompson et al. (2014) Neurocognitive enhancement in older adults: Comparison of three cognitive training tasks to test a hypothesis of training transfer in brain connectivity. , NeuroImage.https://doi.org/10.1016/j.neuroimage.2013.07.069 85(3), 1027-1039.
- 9.S B Chapman, Aslan S, J S Spence, M W Keebler, L F DeFina et al. (2016) Distinct Brain and Behavioral Benefits from Cognitive vs. Physical Training: A Randomized Trial in Aging Adults.https://doi.org/10.3389/fnhum.2016.00338.Frontiers in Human Neuroscience10.
- 10.Borella E, Carretti B, Riboldi F, R De Beni. (2010) Working memory training in older adults: evidence of transfer and maintenance effects. Psychology and Aging.https://doi.org/10.1037/a0020683.25(4):. 767-778.
- 11.Melby-Lervåg M, Hulme C. (2013) Is working memory training effective? A meta-analytic review. Developmental Psychology.https://doi.org/10.1037/a0028228 49(2), 270-291.
- 12.M J Kane, M K Bleckley, A R Conway, R W Engle. (2001) A controlled-attention view of working-memory capacity. , Journal of Experimental Psychology. General 130(2), 169-183.
- 13.M J Kane, R W Engle. (2003) Working-memory capacity and the control of attention: the contributions of goal neglect, response competition, and task set to Stroop interference. , Journal of Experimental Psychology. General 132(1), 47-70.
- 14.Weicker J, Villringer A, Thöne-Otto A. (2016) Can impaired working memory functioning be improved by training? A meta-analysis with a special focus on brain injured patients. , Neuropsychology.https://doi.org/10.1037/neu0000227 30(2), 190-212.
- 15.E L Schmidt, Burge W, K M Visscher, L A Ross. (2016) Cortical thickness in frontoparietal and cingulo-opercular networks predicts executive function performance in older adults., Cortical Thickness in Fronto-parietal and Cingulo-opercular Networks Predicts Executive Function Performance in Older Adults. , Neuropsychology, Neuropsychology 30(33), 322-331.
- 16.Miyake A, N P Friedman, M J Emerson, A H Witzki, Howerter A et al. (2000) The Unity and Diversity of Executive Functions and Their Contributions to Complex “Frontal Lobe” Tasks: A Latent Variable Analysis. , Cognitive 41(1), 49-100.
- 17.Karbach J, Kray J. (2009) How useful is executive control training? Age differences in near and far transfer of task-switching training. , Developmental Science.https://doi.org/10.1111/j.1467-7687.2009.00846.x.12 6, 978-990.
- 18.Dahlin E, A S Neely, Larsson A, Bäckman L, Nyberg L. (2008) . Transfer of Learning After Updating Training Mediated by the Striatum. Science.https://doi.org/10.1126/science.1155466 320(5882), 1510-1512.
- 19.Nouchi R, Taki Y, Takeuchi H, Hashizume H, Akitsuki Y et al. (2012) Brain Training Game Improves Executive Functions and Processing Speed in the Elderly: A Randomized Controlled Trial. , PLOS 7(1), 29676.
- 20.Klingberg T. (2010) Training and plasticity of working memory. Trends in Cognitive Sciences.https://doi.org/10.1016/j.tics.2010.05.002 14(7), 317-324.
- 21.A B Morrison, J M Chein. (2011) Does working memory training work? The promise and challenges of enhancing cognition by training working memory. , Psychonomic Bulletin & Review.https://doi.org/10.3758/s13423-010-0034-0.18(1): 46, 60.
- 22.P D Gajewski, Falkenstein M. (2018) ERP and Behavioral Effects of Physical and Cognitive Training on Working Memory in Aging: A Randomized Controlled Study [Research article].https://doi.org/10.1155/2018/3454835.
- 23.P D Gajewski, Falkenstein M. (2016) Physical activity and neurocognitive functioning in aging - a condensed updated review. European Review of Aging and Physical Activity13(1).https://doi.org/10.1186/s11556-016-0161-3.
- 24.E B Larson, Wang L, J D Bowen, W C McCormick, Teri L et al. (2006) Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. , Annals of Internal Medicine 144(2), 73-81.
- 25.S J Colcombe, K I Erickson, P E Scalf, J S Kim, Prakash R et al. (2006) Aerobic Exercise Training Increases Brain Volume in Aging Humans. The Journals of Gerontology: Series A.https://doi.org/10.1093/gerona/61.11.1166 61(11), 1166-1170.
- 26.K I Erickson, M W Voss, R S Prakash, Basak C, Szabo A et al. (2011) Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences.https://doi.org/10.1073/pnas.1015950108 108(7), 3017-3022.
- 27.Maass A, Düzel S, Goerke M, Becke A, Sobieray U et al. (2015) Vascular hippocampal plasticity after aerobic exercise in older adults. , Molecular 20(5), 585.
- 28.Kandola A, Hendrikse J, P J Lucassen, Yücel M. (2016) Aerobic Exercise as a Tool to Improve Hippocampal Plasticity and Function in Humans: Practical Implications for Mental Health Treatment. Frontiers in Human Neuroscience10.https://doi.org/10.3389/fnhum.2016.00373.
- 29.Boxtel M P van, F G Paas, P J Houx, J, J C Teeken et al. (1997) Aerobic capacity and cognitive performance in a cross-sectional aging study. Medicine and Science in Sports and Exercise 29(10), 1357-1365.
- 30.Liu-Ambrose T, M G Donaldson, Ahamed Y, Graf P, W L Cook et al. (2008) Otago Home-Based Strength and Balance Retraining Improves Executive Functioning in Older Fallers: A Randomized Controlled Trial. , Journal of the American Geriatrics Society.https://doi.org/10.1111/j.1532-5415.2008.01931.x 56(10), 1830.
- 31.K I Erickson, R S Prakash, M W Voss, Chaddock L, Hu L et al. (2009) Aerobic fitness is associated with hippocampal volume in elderly humans.Hippocampus.https://doi.org/10.1002/hipo.20547.19(10):. 1030-1039.
- 32.P D Gajewski, Falkenstein M. (2015) Lifelong physical activity and executive functions in older age assessed by memory based task switching. , Neuropsychologia, 73 (Supplement C):https://doi.org/10.1016/j.neuropsychologia.2015.04.031.195 207.
- 33.Thielen J-W, Kärgel C, B W Müller, Rasche I, Genius J et al. (2016) . Aerobic Activity in the Healthy Elderly Is Associated with Larger Plasticity in Memory Related Brain Structures and Lower Systemic Inflammation. Frontiers in Aging Neuroscience,8. https://doi.org/10.3389/fnagi.2016.00319 .
- 34.M W Voss, Heo S, R S Prakash, K I Erickson, Alves H et al. (2013) The influence of aerobic fitness on cerebral white matter integrity and cognitive function in older adults: Results of a one-year exercise intervention. Human brain mapping.https://doi.org/10.1002/hbm.22119. 34(11):. 2972-2985.
- 35.M W Voss, R S Prakash, K I Erickson, Basak C, Chaddock L et al. (2010) Plasticity of Brain Networks in a Randomized Intervention Trial of Exercise Training in Older Adults. Frontiers in Aging Neuroscience,2.https://doi.org/10.3389/fnagi.2010.00032.
- 36.C W, N C Berchtold, Christie L-A. (2007) Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends in Neurosciences.https://doi.org/10.1016/j.tins.2007.06.011.30(9):. 464-472.
- 37.Bherer L, K I Erickson, Liu-Ambrose T. (2013) A Review of the Effects. of Physical Activity and Exercise on Cognitive and Brain Functions in Older Adults [Research article]. https://doi.org/10.1155/2013/657508 .
- 38.P M Greenwood, Parasuraman R. (2010) Neuronal and Cognitive Plasticity: A Neurocognitive Framework for Ameliorating Cognitive Aging. Frontiers in Aging Neuroscience,2. https://doi.org/10.3389/fnagi.2010.00150
- 39.Rahe J, Petrelli A, Kaesberg S, G R Fink, Kessler J et al. (2015) Effects of cognitive training with additional physical activity compared to pure cognitive training in healthy older adults. Clinical Interventions in Aging.https://doi.org/10.2147/CIA.S74071 10, 297.
- 40.Shah T, Verdile G, Sohrabi H, Campbell A, Putland E et al. (2014) A combination of physical activity and computerized brain training improves verbal memory and increases cerebral glucose metabolism in the elderly. , Translational 4(12), 487.
- 41.Linde K, Alfermann D. (2014) Single Versus Combined Cognitive and Physical Activity Effects on Fluid Cognitive Abilities of Healthy Older Adults: A 4-Month Randomized Controlled Trial With Follow-Up. , Journal of Aging and Physical Activity.https://doi.org/10.1123/JAPA2012-0149 22(3), 302-313.
- 42.Theill N, Schumacher V, Adelsberger R, Martin M, Jäncke L. (2013) Effects of simultaneously performed cognitive and physical training in older adults. , BMC Neuroscience.https://doi.org/10.1186/1471-2202-14-103.14 103.
- 43.Shatil E. (2013) Does combined cognitive training and physical activity training enhance cognitive abilities more than either alone? A four-condition randomized controlled trial among healthy older adults. Frontiers in Aging Neuroscience5. https://doi.org/10.3389/fnagi.2013.00008
- 44.C A Frantzidis, A-K I Ladas, A B Vivas, Tsolaki M, P D Bamidis. (2014) Cognitive and physical training for the elderly: Evaluating outcome efficacy by means of neurophysiological synchronization. , International Journal of 93(1), 1-11.
- 45.Z S Nasreddine, N P Chertkow, H. (2017) Normative data for the Montreal Cognitive Assessment (MoCA) in a population-based sample. Neurology. Consulté à l’adressehttp://n.neurology.org/content/normative-data-montreal-cognitive-assessment-moca-population-based-sample-0.
- 46.Mitrushina M, Satz P, Chervinsky A, D’Elia L. (1991) Performance of four age groups of normal elderly on the rey Auditory-Verbal learning test. , Journal of Clinical Psychology.https://doi.org/10.1002/1097-4679(199105)47: 47(3), 351-357.
- 47.R M. (1958) Validity of the Trail Making Test as an Indicator of Organic Brain Damage. Perceptual and Motor Skills. 8(3), 271-276.
- 48.Cardebat D, Doyon B, Puel M, Goulet P, Joanette Y. (1990) [Formal and semantic lexical evocation in normal subjects. Performance and dynamics of production as a function of sex, age and educational level]. , Acta neurologica Belgica 90(4), 207-217.
- 49.L A Erdodi, C A Abeare, J D Lichtenstein, B T Tyson, Kucharski B et al. (2017) Wechsler Adult Intelligence Scale-Fourth Edition (WAIS-IV) processing speed scores as measures of noncredible responding: The third generation of embedded performance validity indicators. Psychological Assessment.https://doi.org/10.1037/pas0000319.29(2):. 148-157.
- 50.A K Troyer, Leach L, Strauss E. (2006) Aging and Response Inhibition: Normative Data for the Victoria Stroop Test. , Aging, Neuropsychology, and Cognition.https://doi.org/10.1080/13825589096818713(1): 20, 35.
- 51.M P Lawton, E M Brody. (1970) Assessment of older peaople : Self-maintaining activities of daily living. , Nursing Research 19(3), 278.
- 52.D M McNair, R J Kahn. (1984) Self-assessment of cognitive deficits. In. , New Canaan, CT: Mark Powley.https://doi.org/10.2466/pms.1958.8.3.271.137–143
- 53.J A Yesavage, T L Brink, T L Rose, Lum O, Huang V et al. (1982) Development and validation of a geriatric depression screening scale: A preliminary report. , Journal of Psychiatric Research.https://doi.org/10.1016/0022-3956(82)90033-4 17(1), 37-49.
- 54.D J Buysse, C F Reynolds, T H Monk, S R Berman, D J Kupfer. (1989) The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Research.https://doi.org/10.1016/0165-1781(89)90047-4.28(2):. 193-213.
- 55.J E Ware, Kosinski M, S D Keller. (1996) . A 12-Item Short-Form Health Survey: Construction of Scales and Preliminary Tests of Reliability and Validity. Medical Care 34(3), 220.
- 56.Ranchet M, Paire-Ficout L, Marin-Lamellet C, Laurent B, Broussolle E. (2010) Impaired updating ability in drivers with Parkinson’s disease. , Journal of Neurology, Neurosurgery & Psychiatry 2009-10.
- 57.B A Eriksen, C W Eriksen. (1974) Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception & Psychophysics.https://doi.org/10.3758/BF03203267.16(1):. 143-149.
- 58.Bunting M, Cowan N, J S Saults. (2006) How Does Running Memory Span Work? Quarterly journal of experimental psychology (2006).https://doi.org/10.1080/17470210600848402. 59(10):. 1691-1700.
- 59.Unsworth N, G J Spillers. (2010) Working memory capacity: Attention control, secondary memory, or both? A direct test of the dual-component model. , Journal of Memory and Language.https://doi.org/10.1016/j.jml.2010.02.001 62(4), 392-406.
- 60.G E Smith, Housen P, Yaffe K, Ruff R, R F Kennison et al. (2009) A Cognitive Training Program Based on Principles of Brain Plasticity: Results from the Improvement in Memory with Plasticity-based Adaptive Cognitive Training (IMPACT). , Study. Journal of the American Geriatrics Society.https://doi.org/10.1111/j.1532-5415.2008.02167.x 57(4), 594-603.
- 61.G W Rebok, Ball K, L T Guey, R N Jones, Kim H-Y et al. (2014) for the ACTIVE Study Group. , Journal of the American Geriatrics Society.https://doi.org/10.1111/jgs.12607.62(1): 16, 24.
- 62.P L Ackerman, Kanfer R, Calderwood C. (2010) Use it or Lose it? Wii Brain Exercise Practice and Reading for Domain Knowledge. Psychology and aging.https://doi.org/10.1037/a0019277. 25(4):. 753-766.
- 63.J E Karr, C N Areshenkoff, Rast P, M A Garcia-Barrera. (2014) An empirical comparison of the therapeutic benefits of physical exercise and cognitive training on the executive functions of older adults: a meta-analysis of controlled trials. , Neuropsychology.https://doi.org/10.1037/neu0000101 28(6), 829-845.
Cited by (3)
- 1.Mundada Purva H, Dadgal Ragini M, 2022, Comparison of Dual Task Training Versus Aerobics Training in Improving Cognition in Healthy Elderly Population, Cureus, (), 10.7759/cureus.29027
- 2.Rieker Jennifer A., Reales José M., Muiños Mónica, Ballesteros Soledad, 2022, The Effects of Combined Cognitive-Physical Interventions on Cognitive Functioning in Healthy Older Adults: A Systematic Review and Multilevel Meta-Analysis, Frontiers in Human Neuroscience, 16(), 10.3389/fnhum.2022.838968
- 3.Dimachki Samar, Tarpin-Bernard Franck, Croisile Bernard, Chainay Hanna, 2022, Study design and protocol of a low to high intensity computer-based cognitive training at home in supplement to standard care in patients with AD, BMJ Open, 12(6), e050993, 10.1136/bmjopen-2021-050993
