Abstract
Objectives:
The aim of this study is to assess the correlation between the degree (percentage) of occlusion due to labial adhesions and vulvovaginitis.
Materials and Methods:
Prospective study of pre-pubertal females who attended our Division, during the past 7 years, due to labial fusion. Medical history, clinical examination and Q tip culture of vaginal fluid samples were performed, after separation of the labia. Treatment of isolated pathogens was administered, based on antibiogram results.
Results:
53 patients, with mean age 3.81 years (±0.88 years, SD:2.65, range:0.33-9.5 years) had a total of 89 vaginal specimens collected, as re-culture was performed in 17 patients due to labial fusion recurrence. In 32.08% no pathogen was isolated. Gardnerella vaginalis (24.51%) and Bacteroides spp (15.09%) were the commonest isolated pathogens of the rest 67.92%. Among labial fusion recurrences the commonest isolated pathogen was Gardnerella vaginalis (23.13%), while in 36.12% no specific pathogen was detected.
Conclusions:
The results suggest that non-specific vulvovaginitis is responsible for lower degree (<60% closure) of labial adhesions, as well as that recurrent infection plays a role in the formation of higher degree/percentage of labial closure, especially when there are one or more pathogens present. Furthermore, the presence of anaerobes at initial cultures, together with Gram negative bacteria at re-cultures, appear to further facilitate the creation of extensive adhesions.
Author Contributions
Academic Editor: Cornelia Amalinei, University of Medicine and Pharmacy Grigore T. Popa, Iasi, Romania
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2017 Efthimios Deligeoroglou, et al
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Labial adhesions are a common disorder in pre-pubertal females and especially in younger pre-pubertal girls (aged 3 months to 6 years), with a peak incidence at 13 to 23 months of age. Although adhesions occur less commonly after age 6, they may begin at any age and persist or recur until puberty1.Labial adhesions have been documented to occur in 0.6% to 5% of pre-pubertal girls, but some studies report that affect up to 38.9% of them to some degree2.
Labial fusion may be caused by inflammation or irritation of the vulva (eg, vulvovaginitis, recurrent diarrhea, and dermatologic disorders), tissue trauma, sexual abuse and low pre-pubertal estrogen levels3,while the protective effect of maternal estrogen makes them uncommon during the newborn period4. However, labial fusion has been noted in association with isolated premature thelarche, suggesting the existence of factors other than estrogen insufficiency5. Estradiol levels have been found to be slightly lower, but not statistically significant, in girls with labial fusion6.It has been suggested that the thin skin layer covering the labia minora could be denuded as a result of local irritation and scratching. The labia then adhere in the midline and as re-epithelialization occurs on both sides, the labia remain fused in the midline7.
The disorder is usually asymptomatic and is often first noticed by parents or pediatrician during a routine physical examination or may be detected while symptoms (including urinary retention, urinary tract infection, altered urinary stream, or pain with activity or rarely urinary outflow deflection or obstruction, leading to vaginal reflux of urine and subsequent vaginal leaking when the child stands after voiding) are being investigated8.On physical examination, positioning the child in a frog-leg position and using a pull-down procedure the labia majora are gently retracted caudally and laterally to better visualize the vagina. Labial adhesions are generally readily apparent as thin, pale, semi-translucent membranes covering the vaginal os between the labia minora. In severe cases, these adhesions almost entirely close the vaginal os. . Hymenal skin tags, imperforate hymen, introital cysts (paraurethral or Gartner duct cysts), ureterocele, urethral polyp, urethral prolapse, vaginal atresia and vaginal rhabdomyosarcoma should be included in the differential diagnosis. No specific imaging studies are required to evaluate labial adhesions.
Depending on the maturity of the child and the expectations of the parents, labial fusion can be managed either conservatively with the application of an estrogen containing cream or with surgical separation which can be performed in a physician's office using a topical anesthetic. Alternatively, labial adhesions can be treated in the operating room under general anesthesia. The procedure is short and usually just requires gentle traction by the surgeon once anesthesia has been instituted. If left untreated, labial adhesions usually spontaneously resolve at puberty, most likely as a result of increased estrogen levels.
Experimental Procedure
Prospective study of all pre-pubertal females, who visited (attended or referred by Pediatricians) our Department, due to the clinical finding of labial fusion. The work has been approved by the appropriate ethics committee of our Hospital and has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All parents or legal guardians of study participants have signed an informed consent form before entering the study. A thorough examination was performed and culture of vaginal fluid samples with a Q tip were taken, after separation of the labia with a cotton-tipped swab under topical anesthesia. The severity of adhesions ranged from 30% to 99% closure of the introitus and was assessed at the time of initial exam.
Materials and Methods
53 patients (all pre-pubertal) were included in the study. All girls were brought by parents or referred by other doctors immediately after recognition of labial adhesions. In all patients a full medical history was taken. Specific questions were made regarding hygiene habits, use of diapers, general activities of the child and presence of urinary symptoms. History of infectious diseases, especially gastroenteritis, respiratory tract infections and urinary tract infections of both the child and the family, as well as history of allergies, atopy, contact sensitivities and pharmacological treatment was also investigated. Previous treatments such as surgical lysis of adhesions or conjugated estrogen cream application were also taken under consideration. No labial adhesions family history or hormonal treatment during mothers’ pregnancy was reported by any parent of girls included in this study. The presence of anatomic anomalies such as fistulas, ectopic urethral opening and anomalies of the hymen were evaluated.
The patients were divided in two groups depending on the degree (%) of labial fusion, Group A: <60% and Group B: >60%. Treatment of isolated pathogens was applied, based on antibiogram results. Furthermore, pathogens were separated in four major categories: Gram (+), Gram (-), Anaerobes and Atypical. All statistical analysis was performed in SPSS 21 for Windows using chi-square test (χ2) for study data. All data are represented as mean values ± SD and the level of statistical significance was set at p<0.05.
Results
53 patients (all pre-pubertal), with mean age of 3.81 years (±0.88 years, SD:2.65, range:0.33-9.5 years) had a total number of 89 vaginal specimens collected, as in 17 (32.07% of study patients) culture repetition was indicated due to labial fusion recurrence (mean 2.11cultures). Mean total duration of labial adhesions in girls with re-cultures from time of first attendance until final treatment of adhesions was 1.27±0.35 years.
At initial cultures, in 32.08% was not isolated any pathogen, whilst from the rest 67.92% the commonest isolated pathogen was Gardnerella vaginalis (24.51%), Bacteroides spp (15.09%), Enterococcus faecalis (7.5%) and Escherichia coli (5.7%). In the rest 15.12% more than 20 different pathogens were isolated, including Staphylococcus aureus, Mycoplasma hominis, Streptococcus sanquis, Enterobacter cloacoe, Klebsiella pneumoniae spp, Clostridium perfingens, Bifidobacterium dysgalactiae spp equisimilis, Enterococcus casseliflarus/gallinarum, Prevotella melaninogenica, Prevotella oralis, Proteus mirabilis, Morganella morganii spp morganii, and Providencia rustigianni in order of decreasing frequency . (Table 1)
Among labial fusion recurrences the commonest isolated pathogen was Gardnerella vaginalis (23.13%), Bacteroides spp (13.64%) and Escherichia coli (9.11%) while in 36.12% no specific pathogen was detected. Other pathogens found were: Peptrostreptococcus spp, Pseudomonas aeruginosa, Staphylococcus epidermidis, Actinomyces meyeri, Gemella morbillorum and Proteus vulgaris in order of decreasing frequency. (Table 1)
Table 1. The commonest isolated pathogens at initial cultures and re-cultures categorized as Gram (+), Gram (-), Anaerobes and Atypical. No pathogens were found in 32.08% and 36.12% of initial cultures and re-cultures respectively. In parentheses are included the number of girls affected by each pathogen. 13 girls at initial cultures and 14 at re-cultures have been affected by >1 pathogen.| Commonest isolated pathogens at initial cultures | ||||
| Gram (+) -0.127 | Gram (-) -0.1042 | Anaerobes (18.79%) | Atypical (26.01%) | No Pathogen (32.08%) |
| Enterococcus faecalis (3), | Escherichia coli (3) | Bacteroides spp (8) | Gardnerella vaginalis (13) | |
| Staphylococcus aureus (1) | Enterobacter cloacae (1) | Clostridium perfingens (1) | Mycoplasma hominis (1) | |
| Streptococcus sanguis (1) | ||||
| Other (Bifidobacterium dysgalactiae ssp equisimilis, Enterococcus casseliflavus/gallinarum) (1) | Other (Proteus Mirabilis, Providencia rustigianni, Morganella morganii spp morganii, Klebsiella pneumonia) (2) | Other (Prevotella melanonogenica, Prevotella oralis) (1) | ||
| Commonest isolated pathogens at re-cultures | ||||
| Gram (+) -0.1 | Gram (-) -0.1311 | Anaerobes (17.64%) | Atypical -0.2313 | No Pathogen (36.12%) |
| Enterococcus faecalis (1) | Escherichia coli (3) | Bacteroides spp(4) | Gardnerella vaginalis -8 | |
| Staphylococcus epidermidis (1) | Pseudomonas aeruginosa (1) | Clostridium perfingens (1) | ||
| Peptrostreptococcus spp (1) | ||||
| Other (Gemella morbillorum) (1) | Other (Proteus vulgaris) (1) | Other (Actinomyces meyeri) (1) | ||
At initial cultures, in Group A included 31 patients (58.49% of all patients). Among them, in 15 patients (28.31% of all and 48.38% of Group A) was not isolated any pathogen, in 11 patients (20.75% of all patients and 35.48% of Group A patients) was found 1 pathogen in each culture, while only in 5 patients (9.43% of all patients and 16.14% of Group A patients) were isolated more than one pathogens. In Group B were included 22 patients (41.51% of all patients) and among them in 2 patients (3.77% of all patients and 9.09% of Group B patients) was not isolated any pathogen, in 12 patients (22.65% of all patients and 54.54% of Group B patients) was isolated one (1) pathogen, while in 8 patients (15.09% of all patients and 36.37% of Group B patients) were isolated more than one pathogens.
In 17 patients culture repetition was indicated due to labial fusion recurrence and a total number of 36 vaginal specimens were collected. In Group A were included 21 patients (58.33% of all re-cultures). Among them, in 12 patients (33.34% of all and 57.15% of Group A) was not isolated any pathogen, in 7 patients (19.44% of all patients and 33.33% of Group A patients) was shown 1 pathogen in each culture, while only in 2 patients (5.55% of all patients and 9.52% of Group A patients) were isolated more than one pathogens. In Group B were included 15 patients (41.67% of total re-cultures) and among them in 1 patient (2.78% of all patients and 6.66% of Group B patients) was not isolated any pathogen, in 2 patients (5.56% of all patients and 13.34% of Group B patients) was isolated one (1) pathogen, while in 12 patients (33.33% of all patients and 80% of Group B patients) were isolated more than one pathogens. The above results are summarized in Table 2.
A high statistically significant difference (p<0.001) was found between Group A and Group B patients when no pathogen was present at initial cultures, while there was no difference (p>0.05) between the two groups when 1 or more pathogens were found. This result suggests that non-specific vulvovaginitis is responsible for lower degree (<60% closure) of labial adhesions. At initial cultures, a high statistically significant difference (p<0.001) was also found in Group B patients between no pathogen presence and one or more isolated pathogens, while no statistically significant difference found (p>0.05) in Group A patients between no pathogen and 1 or more isolated pathogens. The above results indicate that 1 or more pathogens are correlated with higher degree (>60%) of labial closure (Table 2).
Table 2. Statistically significant differences between Group A and Group B and numbers of isolated pathogens at initial cultures and re-cultures. The only statistically significant difference that was not found (p>0.05) was in Group A patients between no pathogen and 1 or more isolated pathogens both in initial cultures and re-cultures. p values are based on chi-square test.| Different categories of isolated pathogens at initial cultures | |||||
| Groups | Patients | No pathogen | ≥1 pathogen(s) isolated | ||
| No Pathogen | 1 pathogen | >1 pathogens | p (ϰ2) | ||
| A | 31 (58.49%) | 15 (28.31% of all and 48.38% of Group A) | 11 (20.75% of all and 35.48% of Group A) | 5 (9.43% of all and 16.14% of Group A) | >0.05 |
| B | 22 (41.51%) | 2 (3.77% of all and 9.09% of Group B) | 12 (22.65% of all and 54.54% of Group B) | 8 (15.09% of all and 36.37% of Group B) | <0.001 |
| Total | 53 | 17 (32.08%) | 23 (43.4%) | 13 (24.52%) | |
| p (ϰ2) | <0.001 | >0.05 | >0.05 | ||
| Different categories of isolated pathogens at re-cultures | |||||
| Groups | Patients | No Pathogen | ≥1 pathogen(s) isolated | ||
| No Pathogen | 1 pathogen | >1 pathogens | p (ϰ2) | ||
| A | 21 (58.33%) | 12 (33.34% of all and 57.15% of Group A) | 7 (19.44%of all and 33.33% of Group A) | 2 (5.55% of all and 9.52% of Group A) | >0.05 |
| B | 15 (41.67%) | 1 (2.78% of all and 6.66% of Group B) | 2 (5.56% of all and 13.34% of Group B) | 12 (33.33% of all and 80% of Group B) | <0.001 |
| Total | 36 | 13 (36.12%) | 9 (25%) | 14 (38.88%) | |
| p (ϰ2) | <0.001 | 0.022 | <0.001 | ||
Analyzing re-cultures a high statistically significant difference (p<0.001) between Group A and Group B patients was found again when no pathogen was present, confirming the previous finding. Further analysis of the re-cultures shows that there is statistically significant difference (p=0.022) between Group A and Group B patients when one pathogen was found and a high statistically significant difference (p<0.001) when more than one pathogens was present. These last results suggest the importance of recurrent infection, especially with more than one pathogens, in the formation of higher degree/percentage of labial closure. In re-cultures, a high statistically significant difference (p<0.001) in Group B patients between no pathogen presence and one or more isolated pathogens was observed, while no statistically significant difference was found (p>0.05) in Group A patients between no pathogen and 1 or more isolated pathogens and this was in agreement with the above mentioned result of the initial cultures (Table 2).
Furthermore, pathogens were separated in four major categories: Gram (+), Gram (-), Anaerobes and Atypical (Table 3). Comparing the initial cultures of groups A and B, only the presence of anaerobes in the culture appears to be statistically significant in the formation of extensive adhesions (p=0.0053). At re-cultures the afore mentioned finding is still statistically
significant (p=0.016), but Gram negative bacteria appear also to have statistically significant contribution (p=0.022) in the formation of adhesions that exceed 60%. (Table 3, Figure 1 & Figure 2)
Figure 1.Groups of labial closure percentage and major categories of pathogens at initial cultures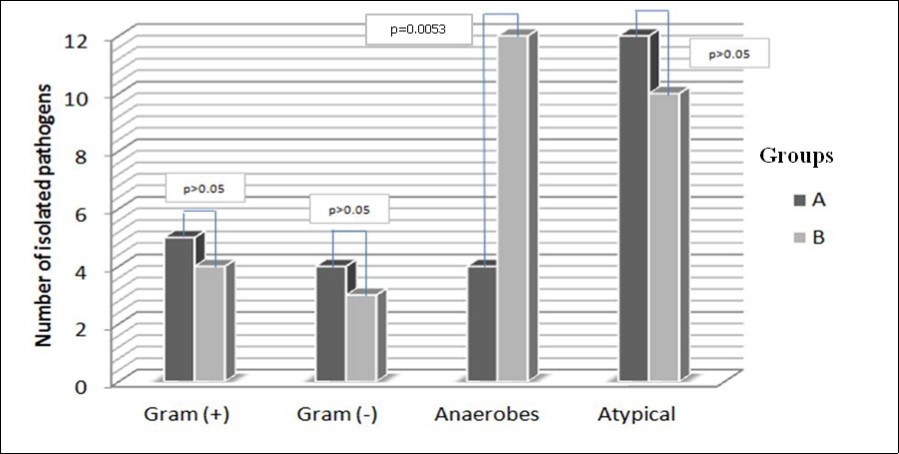
Figure 2.Groups of labial closure percentage and major categories of pathogens at re-cultures
| Groups of labial closure percentage and major categories of pathogens at initial cultures | ||||
| Groups | Cultures with Gram (+) | Cultures with Gram (-) | Cultures with Anaerobes | Cultures with Atypical |
| A | 5 | 4 | 4 | 12 |
| B | 4 | 3 | 12 | 10 |
| p (ϰ2) | >0.05 | >0.05 | 0.0053 | >0.05 |
| Groups of labial closure percentage and major categories of pathogens at re-cultures | ||||
| Groups | Cultures with Gram (+) | Cultures with Gram (-) | Cultures with Anaerobes | Cultures with Atypical |
| A | 4 | 2 | 3 | 9 |
| B | 3 | 7 | 9 | 7 |
| p (ϰ2) | >0.05 | 0.022 | 0.016 | >0.05 |
Discussion
The correlation between labial fusion and vulvovaginitis has been reported in several studies1, 2, 9, 10, 11. All our study patients were pre-pubertal, hence hypoestrogenism was a predisposing factor for labial adhesion formation through the mechanism of facilitated inflammation due to the decreased estrogen bioavailability.
Even though, vulvovaginitis has been reported as a causative factor of labial adhesions in several studies1, 3, 9, using pubmed as our primary database, we did not find any study correlating labial fusion with specific pathogens causing vulvovaginitis.. In our study the results indicate that the percentage of labial closure is correlated with the severity of co-existing vulvovaginitis and the number of pathogens that were isolated, as well as with the ability of specific pathogens to lead or not to vaginal inflammation. An important aspect that was observed in our study is that recurrent inflammation, especially with one or more specific pathogens, is associated with higher degree of labial closure, while non-specific vulvovaginitis is responsible for lower degree (<60% closure) labial adhesions. The presence of anaerobes at initial cultures appears to be statistically significant in the formation of extensive adhesions while at re-cultures both the anaerobes and Gram negative bacteria appear to have statistically significant contribution to this end.
Recurrence of labial adhesions is common, as was also seen in our study. It has been reported in as many as 11.6 - 14% of cases12, 13; however, the true recurrence rate may be higher with longer follow-up14. This percentage was 32.07% in our study. Nevertheless, the correlation between labial fusion recurrences and isolated pathogens is not yet known.
Labial adhesions can be managed either surgically or conservatively. Surgical separation of labial fusion is commonly performed in a physician's office using a topical anesthetic and this is based on the following considerations: the density of the adhesions, the patient’s level of maturity and the patient’s ability to tolerate an in-office procedure15. Furthermore, the severity of labial fusion is correlated with recurrent vaginal infections and urinary difficulties15 and that was the reason why we proceeded to surgical separation of labial adhesions, in order to avoid possible urinary difficulties correlated with the percentage of labial closure, as well as to investigate the possible pathogens involved in the formation of labial fusion.
On the other hand, labial adhesions can often be managed either with periodic observation, in which case spontaneous resolution has been reported in as many as 80% within 1 year1, or with estrogen cream. This cream is directly applied to the labia minora and can be used twice daily for 2-4 weeks. A literature review of 2007 by Tebruegge et al.12 reported that the success rate of topical estrogen intervention in girls with labial adhesions is typically about 90%, with published success in case series reports ranging from 46.7-100%12. Adverse systemic effects of estrogen cream are rare and reversible once medication is discontinued, while estrogen cream application often causes temporary hyperpigmentation of the skin in the area of application. It is important to reassure parents that hyperpigmentation normally fades after therapy ends. The use of steroid betamethasone 0.05% cream has also been described4.
Moreover, the doctor should encourage parents to use the pull-down maneuver, in order to facilitate gentle takedown of adhesions. Additionally, avoiding exposure to possible irritants (e.g., strong detergents, bubble baths and harsh soaps) may be beneficial and parents should understand that they must continue to apply an emollient, even after the labia have separated, to prevent recurrence of labial adhesions. A limitation of this study is the small sample size (53 pre-pubertal girls), therefore larger multicenter studies should be set, based on specific pathogens and the severity of labial adhesions.
Conclusion
In conclusion, labial adhesions are a common disorder in pre-pubertal females and especially frequent in girls aged between 3 months and 6 years old, which may persist or recur until puberty. Labial fusion may be caused by inflammation or irritation of the vulva eg. vulvovaginitis (which has also been reported in several studies) and low pre-pubertal estrogen levels. Recurrence of labial adhesions is common, as was also seen in this study. According to the results of this study, non-specific vulvovaginitis is responsible for lower degree (<60% closure) of labial adhesions. The results also suggest the importance of recurrent infection in the formation of higher degree/percentage of labial closure, especially when there are one or more pathogens present. Furthermore, the presence of anaerobes at initial cultures together with Gram negative bacteria at re-cultures, appear to further facilitate the creation of extensive adhesions. Finally, labial fusion may be the cause, but also the result of vulvovaginitis and it is highly recommended that cultures should always be taken each time labial adhesions are separated.
Acknowledgments:
The author(s) declare(s) that there are no acknowledgments regarding the publication of this paper.
Funding:
The author(s) declare(s) that there is no funding regarding the publication of this paper.
References
- 1.J L Bacon. (2002) Prepubertal labial adhesions: evaluation of a referral population. , Am J Obstet Gynecol 187, 327-31.
- 2.J B Myers, C M Sorensen, B P Wisner, Furness P D 3rd, Passamaneck M. (2006) Betamethasone cream for the treatment of pre-pubertal labial adhesions. , J Pediatr Adolesc Gynecol 19, 407-11.
- 3.J S Sanfilippo.Vulvovaginitis. In:Nelson WE,Behrman RE,Kliegman RM,(eds).Nelson. Textbook of Pediatrics 15th Ed. Saunders WB, Philadelphia,1996;1555 .
- 4.A K Leung, W L Robson, Tay-Uyboco J. (1993) The incidence of labial fusion in children. , J Paediatr Child Health 29, 235-6.
- 5.Papagianni M, Stanhope R. (2003) Labial adhesions in a girl with isolated premature thelarche: the importance of estrogenization. , J Pediatr Adolesc Gynecol 16, 31.
- 6.M K Caglar. (2007) Serum estradiol levels in infants with and without labial adhesions: the role of estrogen in the etiology and treatment. , Pediatr Dermatol 24, 373.
- 7.S J Emans, M R Laufer, D P Goldstein. (1998) Pediatric and Adolescent Gynecology. , Lippencott- Raven, Philadelphia
- 8.D A Gaudens, Moh-Ello N, Fiogbe M, Bandre E, B M Ossoh. (2008) Labial fusion in the paediatric surgery department of Yopougon University hospital (Cote d'Ivoire): 108 cases. , Sante 18, 35-8.
- 9.K, Sonika A, Charu C, Sunesh K, Neena M. (2006) Labial adhesions in pubertal girls. , Arch Gynecol Obstet 273, 243-5.
- 10.J Nurzia M, M Eickhorst K, K Ankem M, G Barone J. (2003) The surgical treatment of labial adhesions in pre-pubertal girls. , J Pediatr Adolesc Gynecol 16, 21-3.
- 11.Mayoglou L, Dulabon L, Martin-Alguacil N, Pfaff D, Schober J. (2009) Success of treatment modalities for labial fusion: a retrospective evaluation of topical and surgical treatments. , J Pediatr Adolesc Gynecol 22, 247-50.
- 12.Tebruegge M, Misra I, Nerminathan V. (2007) Is the topical application of oestrogen cream an effective intervention in girls suffering from labial adhesions?. , Arch Dis Child 92, 268-71.
- 13.Soyer T. (2007) Topical estrogen therapy in labial adhesions in children: therapeutic or prophylactic?. , J Pediatr Adolesc Gynecol 20, 241-4.