Abstract
Snake envenomations are responsible for a high percentage of deaths, as these toxic proteins induce severe local and systemic effects. In Brazil, the Bothrops genus is responsible for a satisfactory fraction of accidents, including Bothropsalternatus, recognized as urutu, whose venom is capable of inducing severe myotoxicity. In this work, the BaMtox toxin was purified through a combination of three chromatographic steps, ion exchange in DEAE-Sepharose, affinity in Benzamidine Sepharose 6B columns and reversed-phase HPLC chromatography on a C18 column. The BaMtox toxin has a molecular mass of approximately 14kDa and did not show phospholipase activity or hemorrhage. On the other hand, it induced edema and a significant increase in plasma levels of the creatine kinase enzyme. Thus, the protein called BaMtox is able to induce myotoxicity.
Author Contributions
Copyright © 2022 Lívia Maria Alves, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Animal venoms are toxic proteins produced by specialized glands and secreted at the time of the bit, sting or other sharp body features, it can cause intense physiological changes and pain or even death 1. Venomous animals include mammals (shrew and platypus), fishes (stingray and lionfish), amphibians (frog), invertebrates (spider, scorpion, snail, wasp, honey bee, ant, and jellyfish) and reptiles, as snakes and lizards 2.
Venomous snakes around the world include the families Viperidae, Elapidae and Colubridae 3. The Elapidae and Viperidae are by far the most important snake families out of the five ones capable of envenoming humans, being that in Brazil, envenoming snakes are represented by the genera Bothrops, Crotalus, Lachesis, and Micrurus4.
Within the Viperidae family, the genus Bothrops represents the most important group of Brazilian venomous snakes because its members are responsible for the majority of ophidian accidents in the Brazil 5. Among the bothropic snakes found in Brazil, Bothrops alternatus, also known as urutu, is a species involved in several snakebites 6.
The venom composition of Bothrops snakes is highly diverse, but proteomics-based approaches have allowed detailed identification of its major componentes, as SVMP (snake venom metalloprotease), PL (phospholipase, including PLA2 and PLB) SVSP (snake venom serine protease), CLEC (C-type lectin, including CLEC-like), LAAO (L-amino acid oxidase), Dis (disintegrin, including Dis/cysteine-rich fragment), VEGF (vascular endothelial growth factor), Pep (peptides, including vasoactive and natriuretic peptides, bradykinin-potentiating peptide, and inhibitory peptide) and other minor venom componentes 7.
The isolated or synergistic action of these enzymatic components of bothropic venoms are the main responsible for and local damage, as well as systemic changes. After the bite, several clinical manifestations appear at the site, such as edema e pain, common in inflammatory processes 8, as a direct or indirect consequence of the venom 9.
Initially, the inflammatory response is triggered by the activation of chemical sensors and mediators, which induce vasodilation and increase in vascular permeability, thereby increasing blood flow at the site of injury, generating hyperemia and consequent leakage of plasma components into the tissue. This disorder induces the progressive migration of defense cells (leukocytes) to the inflamed site to repair the injured tissue. The attraction of neutrophils, eosinophils and basophils (polymorphonuclear cells) coincides with the phagocytic action of the aggressive components inoculated at that location; they dominate early in the inflammatory process. Subsequently, the migration of macrophages and lymphocytes, mononuclear cells essential for the defense and repair of injured tissue, takes place; they appear after inflammation is established 7, drastically affecting tissue integrity, generating severe myonecrosis, coinciding with increased plasma levels of creatine kinase (CK), an indicator of muscle damage, which can lead to tissue loss or disability and even amputation of the affected limb 9. In the present study, we describe the isolation of a myotoxin isolated from the venom of the Bothrops alternatus snake capable of inducing myotoxicity.
Materials and Methods
Materials
Venom and Animals
Bioagents Serpentarium (Batatais, SP, Brazil) provided dehydrated Bothrops alternatus crude venom at room temperature, which was promptly stored at -20°C until use.
Experiments involving animals were carried out in accordance with recommendations of the National Council for the Control of Animal Experimentation (CONCEA) and were approved by the Ethics Committee on Animal Use from the Federal University of Uberlândia, Protocol Number 76/2011. Male isogenic mice (Balb/c, 20-25g, age 4 weeks) were kept under controlled conditions of temperature, humidity and light/dark cycles and they had free access to water and food until the end of the experiments.
Methods
Purification of Toxin and Purity Analysis
The crude venom of Bothrops alternatus (200mg) was dissolved in 0.05M ammonium bicarbonate (AMBIC) pH 7.8, centrifuged at 4°C for 10 minutes at 5000xg, and the supernatant was purified in three steps. In the first stage, the supernatant was equilibrated with the s ame buffer and put to a 2.0x20.0cm ion exchange DEAE Sephadex A-25 column (Sigma-Aldrich). The AMBIC was eluted using a linear gradient from 0.05 to 1.0M at a flow rate of 20.0 mL/h, collecting 3mL each tube. In the second step, the fraction named D4 containing proteins with molecular weight of approximately 14kDa was lyophilized, and applied on a 1.8x4.5cm Benzamidine-Sepharose 6B affinity column (GE Healthcare Life Science), previously equilibrated with 0.05M Tris–HCl pH 7.6. The elution of proteins was performed using three steps; 0.05M Tris–HCl pH 7.6, 50mM Tris–HCl pH 7.6 plus 1M NaCl and 0.02M Glycine pH 3.2, at a flow of 3 mL/min. For purity analysis, the protein fraction D4B4 was lyophilized and diluted in milli-Q water before being submitted to a 0.46x15.0cm RP-HPLC system using a Shimadzu C18 column (GE Healthcare, Uppsala, Sweden), which was previously equilibrated with solvent A (trifluoroacetic acid 0,1%) and eluted with a concentration gradient of solvent B (70 percent acetonitrile) at 280m, the absorbance was measured. The Bradford 10 method was used to calculate the protein concentration.
Electrophoresis in Polyacrylamide Gel (SDS-PAGE)
The fractions produced in the first phase of purification and the toxin purified in the last step (D1 to D8 and BaMtox, 20µg) were separated on a 12 percent SDS-PAGE according to the Laemmli protocol 11. Bovine serum albumin (66kDa), ovalbumin (45kDa), glyceraldehyde-3-phosphate dehydrogenase (33kDa), trypsinogen (24kDa), trypsin inhibitor (20.1kDa), and alfa-lactalbumin (14.2kDa) were used as molecular markers, and the samples were heated at 100ºC for 5 minutes before being run under reducing Coomassie Brilliant Blue R.
Phospholipasic Activity
PLA2 activity was determined using the method, as previously described by De Hass & Postema 12, at the room temperature. The liberation of fatty acids was measured employing emulsion of egg-yolk, as substrate. Each assay was performed in triplicate, using 10mg of proteins (Bothropsalternatus snake venom and BaMtox purified). Results were expressed in mEqNaOH/mg/min.
Myotoxic Activity
Twelve Balb/c male mice (20-25g) were divided into three groups (n = 4) and implanted in the gastrocnemius muscle with 25µg of Bothrops alternatus snake venom or 25µg of BaMtox purified, both dissolved in 50µl sterile saline, or merely 50µl sterile saline. The animals were sedated ketamine (100mg/kg) and xylazine (10mg/kg), intraperitoneal route and their blood was obtained by heart puncture after three hours, after which they were euthanized by cervical dislocation. According to Mamede et al. 13 plasmatic levels of creatine kinase enzyme were measured using the Biotécnica Kit (Brazil) and expressed in units/L (U/L), with one unit equivalent to the synthesis of 1mol of NADH per minute at 30oC.
Figures and Statistical Analysis
The statistical analyses were examined using a t-test with a significance level of 5% for comparisons between groups. Data are expressed as arithmetic mean plus standard deviation. The graphs were made using the GraphPad Prism 5.0 software.
Results and Discussion
Three chromatographic stages were used to purify the BaMtox toxin (Figure 1). Initially, 200mg crude venom from Bothrops alternatus was subjected to ion exchange DEAE Sephadex A-25, yielding eight fractions, D1 to D8 (Figure 1A). The D4 fraction (Figure 1B) contains more low-molecular-mass proteins, which were concentrated and then put to a Benzamidine-Sepharose 6B plate. Five significant fractions called D4B1 to D4B5 (Figure 1C) were found during this chromatographic stage, with the fraction D4B4 showing the highest purity (Results not shown). Reverse-Phase HPLC chromatography was used to determine the purity of this fraction. The chromatographic profile indicated a single peak after the race (Figure 1D). In the presence of mercaptoethanol, 12% SDS-PAGE revealed that the toxin's molecular mass is estimated to be around 14kDa, and it was given the name BaMtox (Figure 1E).
Figure 1.Purification steps of BaMtox from Bothrops alternatus snake venom. (A)Chromatographic profile of crude venom by ion exchange on a DEAE Sephadex A-25 column. The column was equilibrated 0.05M AMBIC buffer. The elution occurred with the same buffer and linear gradient of 0.05 to 1.0M. (B) Electrophoresis in polyacrylamide gel 12% SDS-PAGE. Lines: (1) Molecular marker. (2-9): D1 to D8 fractions, respectively. (C) Rechromatographic profile of D4 fraction on a Benzamidine Sepharose 6B column. The column was equilibrated with 0.05M Tris–HCl pH 7.6, and the elution was 0.05M Tris–HCl pH 7.6, 0.05M Tris–HCl pH 7.6 plus 1M NaCl and 0.02M Glycine pH 3.2. (D) Rechromatografic profile on a RP-HPLC system using a Shimadzu C18 column of fraction D4B4. The column was previously equilibrated with solvent A (trifluoroacetic acid 0,1%), and eluted with a concentration gradient of solvent B (70% acetonitrile, 0.1% trifluoroacetic acid) of 0-100%. (E) Electrophoresis in polyacrylamide gel 12% SDS-PAGE. Lines: (1) Molecular marker. (2) BaMtox (20mg), respectively.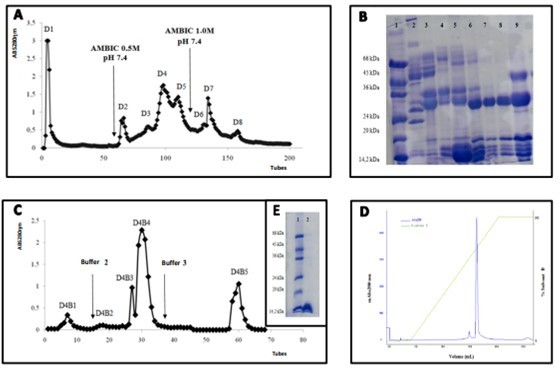
Recent research has shown that snake venoms often contain toxins of low molecular mass, of approximately 14kDa, that cause change in the sting site, which play a major role to the complexity of ophidian envenomation 14, 15, 16. Furthermore, many of these toxins have acidic characteristics 16 or basic 15, 16, 17 with or without catalytic activity.
The myotoxic effect of the BaMtox toxin was evaluated after the administration of 25µg in the gastrocnemius muscle (route i.m.) of Balb/c mice, and after 3 hours, it was possible to observe a significant increase in the plasma levels of the enzyme creatine kinase, when compared to the negative control, who were injected only saline 0.9% (Figure 2), suggesting myonecrosis. In these animals, intense edema was also observed (data not shown).
Figure 2.Myotoxic activity induced by Bothrops altenatus snake venom and BaMtox. Balb/c male were injected intramuscular route with (25μg/50μL) venom or (50μg/50μL) BaMtox. The negative control animals were injected with saline (50μL) by the same route. After 3h, the level of plasmatic creatine kinase was measured. Results are reported as mean ± SD (n = 4). (a) Absence of statistical difference compared to negative control. (b) Statistically significant increase in relation to negative and positive controls, p < 0.05; t-test.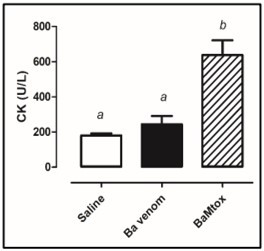
As for the effect of the crude venom, under the same conditions, there was no significant increase in the serum concentration of the creatine kinase enzyme, when compared to the negative control (Figure 2), indicating low myotoxicity.
Garcia-Denegrini et al. 18, researching the myotoxic effect of a Bothropsalternatus snake from Argentina, observed, under the same experimental conditions, low myotoxicity of this venom, but with a significant increase in the activity of the plasma creatine kinase enzyme, compared to the control group, injected only with saline.
These variations in enzymatic activity within the same species may be linked to the different origins of snakes. In other studies, the results were similar to those obtained in this work that several snake venoms induce destabilization of the muscle fibers with consequent leakage of creatine kinase to plasma 14, 19, 20, 21, 22. These same authors attribute this degeneration to the presence of low molecular weight proteins, as PLA2s, in the venom of the snakes.
These molecules are referred to as myotoxins and represent a group of venom proteins with skeletal muscle specificity, affecting muscle fibers, without causing significant damage to other tissues such as connective tissue, nerves and blood vessels 23.
According to these authors, there are three patterns of development of myotoxicity caused by snake venoms. The first type has the ability to depolarize skeletal muscle cells and induce an influx of Na+, increasing the concentration in the intracellular medium, generating a destabilization of sodium channels, but its degenerative mechanism, myonecrotic, is not fully understood 24. The second type are non-enzymatic proteins, found in many elapid snake venoms 25. They display general membrane-permeabilizing action, causing damage to the skeletal muscle 26. Finally, the third type is widely distributed in snake venoms and is represented by the phospholipases A2 (PLA2s), acting directly on the phospholipids present in the membrane of the muscular cells, destabilizing the lipid bilayer, causing tissue degeneration.
According to CAMEY et al. 27Bothropsalternatuscrude venom presents low phospholipasic activity when compared to the venom of snakes of the same genus. The BaMtox expressed no phospholipase activity (data not shown) under the conditions and concentrations tested (10 to 25μg). Several authors isolated toxins with similar characteristics to those presented in this research 28, 15, 29, 30, 17. Although the Bothropsalternatus has a low content of PLA2 (5.2%) 31. Echeverría et al. 32showed that this venom was able to promote proinflammatory effects in vivo and in vitro assays provoking permeability of the cell, cellular extravasation, increase of proinflammatory cytokines liberation as IL-1, IL-12 and TNF-alfa and also activates lipid metabolism in macrophages stimulated with B. alternatusvenom, induction of the expression of enzymes related to lipid signaling (such as cPLA2 and COX-2) and promotes a release of arachidonic acid and others fatty acids.
Lomonte & Rangel 23 attribute this condition to molecules recognized as PLA2-homologues. They have functional molecular architecture of PLA2. However, in its catalytic site there are exclusive amino acids, causing them to be catalytically inactive, but without loss of the capacity to generate severe damages to the musculature.
Conclusion
The BaMtox was purified in three chromate graphic steps and demonstrated an absence of enzymatic specific activity under the conditions assayed. In other hand, it induced the release of the creatine kinase enzyme of muscle to the plasma, indicating muscle fiber injury. Despite of myotoxic effect induced by this toxin, future studies to elucidate its physicochemical and functional characteristics would be useful for a better understanding of the dynamics of envenomation caused by the Bothropsalternatussnake venom.
Financial Support
The authors are grateful to the: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for financially supporting this study, Programa Institucional de Bolsas de Iniciação Científica (PIBIC), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) and Universidade Federal de Uberlândia.
References
- 1.Kenneth V K, Scott A W, Tamara L S. (2009) Reptile venom glands. In: Mackessy SP (ed) Handbook of venoms and toxins of reptiles. , Raton, CRC, Boca 65-66.
- 2.Chan Y S, RCF Cheung, Xia L, Wong J H, Ng T B et al. (2016) Snake venom toxins: toxicity and medicinal applications. , Appl Microbiol Biotechnol 100, 6165-6181.
- 3.McLane M A, Joerger T, Mahmoud A. (2008) Disintegrins in health and disease. Front Biosci. 13, 6617-6637.
- 4.Frare B T, YKS Resende, Dornelas B C, Jorge M T, VAS Ricarte et al. (2019) . , Clinical, Laboratory, and Therapeutic Aspects of (South American Rattlesnake) Victims: A Literature Review. Biomed Research International 1-7.
- 5.Brasil 2019b.SINANWEB - Acidente por Animais Peçonhentos [WWW Document]. Ministério da Saúde. URL.http://portalsinan.saude.gov.br/acidente-por-animais-peconhentos. accessed 03.03.22.
- 6. (2017) World Health Organization. Guidelines for the production, control and regulation of antivenom Immunoglobulins. Technical Report Series
- 7.CCN Mamede, BBS Simamoto, DFC Pereira, Costa J O, MSM Ribeiro et al. (2020) hyperalgesia and myonecrosis induced by Brazilian bothropic venoms: overview of the last decade. Toxicon. 187, 10-18.
- 8.Burin S M, Menaldo D L, Sampaio S V, Frantz F G, Castro F A. (2018) An overview of the immune modulating effects of enzymatic toxins from snake venoms. , Int J Biol Macromol 109, 664-671.
- 9.Bustillo S, Fernández J, Chaves-Araya S, Ângulo Y, Leiva L C et al. (2019) Isolation of two basic phospholipases A2 fromBothropsdiporussnake venom: Comparative characterization and synergism between. Asp49 and Lys49 variants. Toxicon 68, 113-121.
- 10.Bradford M M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of proteindye binding. Anal Biochem. 72, 248-254.
- 11.Laemmli U K.Cleavage of structural proteins during the assembly of the head of bacteriophage T4. , Nature1970; 227, 680-685.
- 12.De Haas GH, Postema N M, Nieuwenhuizen W. (1968) Purification and properties of phospholipase A from porcine pancreas. , Biochim Biophys Acta 24, 103-117.
- 13.CCN Mamede, MRD Queiroz, Fonseca K C, NCGD Morais, SAG Filho et al. (2012) Histological and ultrastructural analyses of muscle damage induced by a myotoxin isolated fromBothrops alternatussnake venom. Protein Pept Lett. 20, 192-199.
- 14.Terra ALC, Moreira-Dill L S, Simões-Silva R, JRN Monteiro, WLG Cavalcante et al. (2015) Biological characterization of the Amazon coralMicrurusspixiisnake venom: Isolation of a new neurotoxic phospholipase A2. , Toxicon 103, 1-11.
- 15.Almeida J R, Lancellotti M, Soares A M, Calderon L A, Ramirez D et al. (2016) CoaTx-II, a new dimeric Lys49 phospholipase A2fromCrotalusoreganusabyssussnake venom with bactericidal potential: Insights into its structure and biological roles. , Toxicon 120, 147-158.
- 16.Rey-Suaréz P, Núñes V, Saldarriaga-Córdoba M, Lomonte B. (2017) Primary structures and partial toxicological characterization of two phospholipases A2from Micrurus mipartitus and Micrurus dumerilii coral snake venoms. , Biochimie 137, 88-98.
- 17.Grabner A N, Alfonso J, Kayano A M, Moreira-Dill L S, APA Santos et al. (2017) BmajPLA2-II, a basic Lys49-phospholipase A2homologue fromBothropsmarajoensissnake venom with parasiticidal potential. , Int J Biol Macromol 102, 571-581.
- 18.Garcia-Denegri M E, Acosta O C, Huancahuire-Vega S, Souza D M, Marangoni S et al. (2010) Isolation and functional characterization of a new acidic PLA(2) Ba SpII RP4 of theBothropsalternatussnake venom from Argentina. Toxicon.56: 64-74.
- 19.Jiménez-Charris E, Montealegre-Sanchéz L, Solano-Redondo L, Castro-Herrera F, Fierro-Pérez L et al. (2016) Divergent functional profiles of acidic and basic phospholipases A2in the venom of the snakePorthidiumlansbergiilansbergii. , Toxicon 119, 289-298.
- 20.NDR Prado, Pereira S S, Silva M P, MSS Morais, Kayano A M et al. (2016) Inhibition of the Myotoxicity Induced byBothropsjararacussuVenom and Isolated Phospholipases A2by Specific Camelid Single-Domain Antibody Fragments. Plos One. 11, 1-22.
- 21.AYL Lim, Singhi P N, Isbister G K. (2016) Severe rhabdomyolysis from red-bellied black snake (Pseudechisporphyriacus) envenoming despite antivenom. , Toxicon 117, 46-48.
- 22.Oliveira J S, Sant’anna L B, MCO Junior, PRM Souza, ASA Souza et al. (2017) Local and hematological alterations induced by Philodryas olfersii snake venom in mice. , Toxicon 132, 9-17.
- 23.Lomonte B, Rangel J. (2012) Snake venom Lys49 myotoxins: From phospholipases A2to non-enzymatic membrane of disruptors. Toxicon. 60, 520-530.
- 24.Rizzi C T, JLC Souza, Schiavon E, Cassola A C, Wanke E. (2007) Troncone LRP. Crotamine inhibits preferentially fast-twitching muscles but is inactive on sodium channels. Toxicon. 50, 553-562.
- 25.Kini R M, Doley R Structure. (2010) function and evolution of three-finger toxins: Mini proteins with multiple targets. Toxicon. 56, 855-867.
- 26.Li Q, Colberg T R, Ownby C J. (1993) A simple and rapid method for isolating small myotoxins from rattlesnake venoms. Toxicon. 31, 1197-1201.
- 27.Cambey K U, Velarde D T, Sanchez E F. (2002) Pharmacological characterization and neutralization of the venoms used in the production of Bothropic antivenom in Brazil. Toxicon. 40, 501-509.
- 28.Furtado J L, Oliveira G A, Pontes A S, Setúbal S S, Xavier C V et al. (2014) Activation of J77A.1 macrophages by three phospholipases A2isolated fromBothropsatroxsnake venom. , Biomed Research International 1-13.
- 29.Corrêa E A, Kayano A M, Diniz-Souza R, Setúbal S S, Zanchi F B et al. (2016) Isolation, structural and functional characterization of a new Lys49 phospholipase A2homologue fromBothropsneuwiediurutuwith bactericidal potential. , Toxicon 115, 13-21.
- 30.Carregari V C, Dai J, Verano-Braga T, Rocha T, Ponce-Soto L A et al. (2016) Revealing the functional structure of a new PLA2K49fromBothriopsistaeniatasnake venom employing automatic "de novo" sequencing using CID/HCD/ETD MS/MS analyses. , Journal of Proteomics 131, 131-139.
