Abstract
Cassava is the fourth largest staple food after rice, wheat and maize. Cassava is produced in the tropical and sub-tropical countries. Currently, the global production of cassava is about 215,436,496 tons. Out of these, Nigeria accounts for 20.3%, being the largest producing nation. During processing of cassava flour (Gari), three main wastes are generated including cassava mill effluents (CME), solid and gaseous emission. This paper reviews the impacts of CME in Nigeria. The study found that CME’s physicochemical quality often exceeds the limit for effluents discharge onto land and surface water as recommended by Federal Environmental Protection Agency (FEPA), Nigeria. CME alters the quality of soil and water with regard to physicochemical, heavy metal and microbial characteristics. CME can induce toxicological effects on the environments and its biota including humans, fisheries, flora and fauna. The impacts are mostly associated with physicochemical (viz: odour, cyanide, acidic, dissolved oxygen, biological and chemical oxygen demand, conductivity) and heavy metals characteristics. Therefore, there is the need for treatment and sustainable management strategies of CME through biotechnological advancement.
Author Contributions
Academic Editor: Jasmin Mantilla Contreras, Department of Biology, University of Hildesheim, Germany
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2018 Sylvester Chibueze Izah,et.al
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Like rice and maize, cassava (Manihot esculenta Crantz) which belongs to the Euphorbiaceae family is a major staple food in Africa especially Nigeria. Cassava is a typical food security crop 1. According to Nwokoro et al. 2, Afuye and Mogaji 3, cassava is one of the most vital food crops consumed in developing countries especially in tropical areas. Its cultivation and processing into useful products such as gari and fufu4
Cassava is cultivated in over 80 countries of humid tropical region of the world 5. Cassava products are rich in carbohydrates 6, vitamins (mostly vitamins B and C), essential minerals and low in protein. The nutrient composition depends on the variety, age, and prevailing environmental condition including soil characteristics. Ukwuru and Egbonu 7 reported that cassava is a major source of energy for more than 2 billion people in the world especially in the tropical region. Cassava is consumed by more than 500 million people in developing nations 8 and about 300 million in the tropical countries 9.
Cassava is an annual crop that is propagated by stem and harvested between 7 – 13 months after planting depending on variety 10, 11. However, some farmers harvest cassava after 2 - 3 years of planting depending on their income 11. Cassava thrives well in warm, moist climate 12, but can tolerate harsh environmental conditions 13, 14, 15.
In Nigeria, cassava farming and processing into useful food items is a major source of livelihood to several families especially in rural areas 11, 16, 17, 18, 19, 20, 21, 22, 23. Moreover, smallholder processors have dominated the enterprises before the presidential cassava initiative of 2002-2003. According to Knipscheer et al. 24, smallholder cassava processors account over 80% of cassava production and processing into useful products for Nigeria. After petroleum, cassava is a major contributor of Nigeria’s gross domestic product (GDP) 25.
Basically, cassava tuber contains about 70% water 24, 26, 27. During cassava processing into gari, several by-products are derived including cassava peelings (21.8%), cassava mill effluents (CME) (16.2%), sieviates (7.5 %), air emission (19.8%), high quality cassava flour (25.0%) 28, 29. In Nigeria, these by-products (mainly solid and liquid wastes) are discharged into the ecosystem without treatment. Elijah et al. 30 opined that wastewater of cassava processing units could pose more intense problem in near future probably due to lack of effluent treatment facility, as effort of Nigerian Government is ongoing to boost cassava based products. These wastes stream could lead to environmental impacts especially on soil fertility, water and air quality. The solid wastes are consumed by domestic animals such as goat in some part of Nigeria. The liquid wastes are also consumed by domestic animals such as goat, but instances of toxicity leading to death of flora and fauna have been reported in literatures. Furthermore, CME contaminates agricultural farmland, surface water (creek, river, stream, pond etc) and percolates into sub-soil and groundwater resource 10. The discharge of effluents, sludge, and biosolid from food processing such as cassava on the land has been an age long practice 31. Sackey and Bani 32 have reported instances of CME flowing into vegetation, abandoned into living communities.
In Nigeria several varieties of cassava abound, but the two major cultivars cultivated are sweet and bitter variety. Bitter cassava is known to contain glucoside which forms hydrocacyanic acid during processing 9. Adeyemo 33, Abiona et al. 34, Kolawole 1, Eze and Onyilide 9, Arimoro et al. 35 reported that cassava contains cyanogenic glucoside viz: linamarin and lotaustralin which is stored in the vacuole of plant cell and are converted into hydrogen cyanide, and when it comes in contact with cell wall hydrolysis of linamarin and lotaustralin takes place. During processing (cooking, frying, boiling), the linamarin is reduced because it is hydrolyzed in the digestive system of humans and animals by indigenous microbial flora and in the process hydrogen cyanide (HCN) is released 8. Cyanide enters the human body through inhalation, ingestion and/ or skin contact and distribute round the body through the blood stream 36.
Typically, a life cycle assessment (LCA) framework of any processing outfit is used to evaluate the impacts associated with the life cycle of a product. Some of the major impacts that can be assessed include social, health, economic and environmental components. Among these impacts, environment components is frequently assessed and some of the notable area of evaluation include climate change, stratospheric ozone depletion, photochemical ozone creation, eutrophication, acidification, toxicological stress on human health, flora and fauna.
Nigeria being the largest cassava processing nation produces high amount of waste streams. The wastes need to be well utilized to avoid the attendant impacts associated with the various wastes stream. In view of the large quantity of wastes associated with cassava processing, this study assessed the impacts of cassava mill effluents in Nigeria.
The paper discusses issues like cassava processing value chain, uses and wastes streams, global cassava statistics, quality of CME (physicochemical, heavy metal and microbial), impact of CME on the environment i.e. soil, water and air, the toxicological impacts of CME including human, flora and fauna, fisheries, socioeconomics, etc.
Cassava Production Statistics
Cassava is a native of Central and South America 37, 38. Cassava took long time to spread to many tropical nations such as West India, South East Asia and West African countries including Sierra Leone, Liberia, Nigeria 9, Ghana, Democratic republic of Congo etc. According to Food and Agricultural Organization Statistics, global cassava production is 215,436,496 tons at as 2014 economic year. Of these, Nigeria account for 20.3% (54,831,600 tons). Various authors have reported that Nigeria is the largest cassava producing country 7, 10, 13, 16, 17, 18, 19, 20, 21, 22, 23. Indonesia and Thailand are the second and third largest cassava producing nations with domestic output of 30,022,052 tons and 23,436,384 tons respectively at 2014 economic year 48. Nigeria’s domestic production exceeds those of Indonesia and Thailand even after combining together. Angola is the 10th largest producer of cassava having global production of 7,638,880 tons 48 (Figure 1).
Figure 1. First 10 largest cassava producing countries according to FAOSTAT 48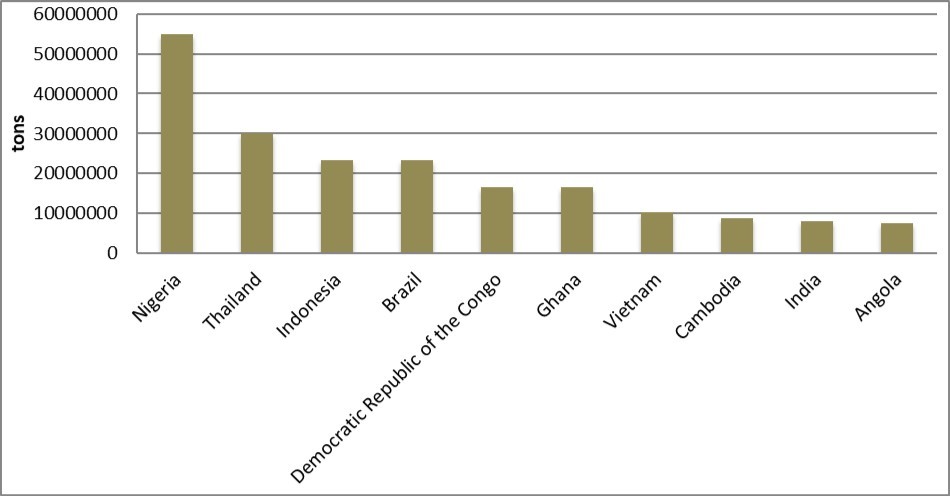
Nigeria being the largest cassava producing nation is motivated by several developmental programmes/policy interventions. A major boost in the Nigeria cassava production took place between the years 2002 – 2012. During this period several presidential interventions were taken for cassava production 49. Some of the intervention includes cassava bread policy 29, 49, 50, 51, 52, 53, biofuel policy 2007 54, 55, 56, replacement of paraffin cooking fuel with bioethanol 46, 57. These policies/interventions created the demand of several cassava based products. For instance, the policy of 10% cassava inclusion in bread production would have created a demand of 250,000 tons of high quality cassava flour which will need about 1 million tonnes of cassava tuber per annum 29, 49, 50, 51. Furthermore, the policy also increased its inclusion to 40%, which will require about 5.2 million tonnes of cassava tuber per annum 49. Based on the 10% bioethanol blend (E10), about 3-4 million tonnes of cassava tuber is required to produce about 32 million litres of bioethanol per annum 54. While the replacement of paraffin cooking fuel with bioethanol will require a demand of 3.75 billion litres of ethanol 46, 57, 58, which needs about 3.6 million tonnes of cassava tuber. Also, cassava action plan to diversify cassava subsector 59 also led to increase in Nigeria domestic cassava production 49.
Figure 2 and Figure 3 present production cassava output and hectares of land between 1961 to 2014 respectively. At as 2014, Nigeria cassava cultivated area was 7,102,300 hectares while the rest of the world production area is 40,632,527 hectares. As such Nigeria occupies 29.3% of global cassava plantation. Congo Democratic Republic, Brazil, Thailand and Indonesia occupy 2nd, 3rd, 4th and 5th position respectively of largest cassava area.
Figure 2. Cassava production statistics in Nigeria between 1961 – 2014 according to FAOSTAT 48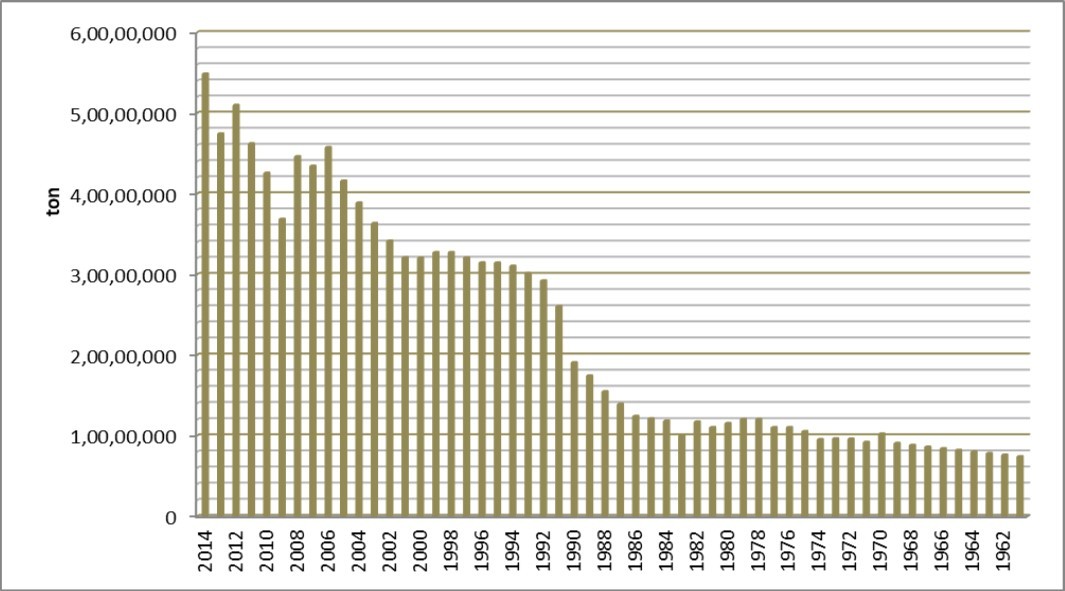
Figure 3. Cassava production area statistics in Nigeria in hectare between 1961 – 2014 according to FAOSTAT 60
Due to the different intervention programmes of the Federal government of Nigeria, private sector investment was included in the cassava sector 61 for the adoption of improved cassava yield variety 62. According to Aniedu and Omodamiro 63, about 6 new varieties of cassava processing pro-vitamin A (β Carotene) were released specifically for bread-making through research and development. Unfortunately, some of the intervention strategies have been abandoned probably due to change in regime. Ohimain 49 also reported that less emphasis on bioethanol led to decline in bioethanol projects.
Quality of Cassava Mill Effluents
Quality of CME is basically assessed in three broad groups. But, for convenience, they are classified into two groups viz: physicochemical and microbial quality. Physicochemical analysis examines the physical and chemical related parameters including appearance (turbidity), taste, colour, odour, pH, turbidity, total suspended solids, total hardness, total alkalinity, salinity, electrical conductivity, cyanide, nitrate, nitrite, sulphate, calcium, sodium, magnesium, potassium, carbonates, dissolved oxygen chemical and biological oxygen and heavy metals (iron, manganese, zinc, copper, cadmium, chromium, lead, silver, mercury etc). While the microbial quality assess the CME regarding population density of different microbial class (i.e. coliforms, total heterotrophic bacteria counts, total fungi, lactic acid bacteria counts, lipolytic, cellulolytic, phosphate solubilizing and nitrifying bacteria).
Physicochemical
CME is a colloidal suspension of fine particles of cassava i.e. starch in water. CME is highly acidic in nature containing high organic matter, suspended solid, sulphur dioxide and cyanide 32. The cyanide is reduced during cassava processing. Izah et al. 17 reported that heating, fermentation and addition of palm aid in the reductions of cassava cyanide content. Table 1 presents the characteristics of CME produced in cassava mills in Nigeria. Ehiagbonare et al. 64 reported that pH of CME discharged soil is 5.37 and pH of non-CME discharged soil is 6.04 with a value of cyanide content of 25.6ppm and 0.00 ppm for cyanide discharged and non-discharged soil. Based on the physicochemical quality (nutrient, heavy metals, oxygen-related parameters and general physicochemical parameters), instances of some parameters exceeding limits specified by Federal Environmental Protection Agency (FEPA) for all categories of effluents discharged into soil and surface water have been observed. Some of these parameters include biological Oxygen demand (BOD) 16, 65, 66, copper, lead and cadmium 21, 66, 67, 68, manganese 21, 66, 68, zinc 21, 67, 68, iron, chromium, silver and mercury 68. This suggests that contamination caused by CME in the environment is mostly from acidity, cyanide, heavy metals and odour.
Table 1. Physiochemical properties of cassava mill effluents| Parameters | 69 | *** 65 | 66 | 67 | 68 | 70 | Effluent limitation in Nigeria for all categories of industries 71 | ||
| Limits for discharge into surface water | Limits for discharge into surface water | ||||||||
| - | - | - | - | - | CME discharged point | Control | - | - | |
| Total dissolved solid, mg/l | 799 | - | - | - | - | - | - | NS | NS |
| Total suspended solid, mg/l | 789 | - | - | - | - | - | - | NS | NS |
| Nitrogen, mg/l | 0.19 | - | - | - | - | - | - | NS | NS |
| Phosphorus, mg/l | 0.18 | - | - | - | - | - | - | NS | NS |
| Total solid, mg/l | - | 14.30 | - | 5600 | NS | NS | |||
| Total hardness, mg/l | - | - | - | 75.00 | 280 | 196 | NS | NS | |
| Appearance/colour, TCU | - | - | - | Turbid | - | - | 7 (lovibonds) | NS | |
| Odour | - | - | - | Unpleasant | - | - | NS | NS | |
| Conductivity, µS/cm | - | - | 1550 | - | - | NS | NS | ||
| pH | 5.07 | 2.50 – 4.20 | 4.6 | 4.1 | 3.96 | - | - | 6-9 | 6-9 |
| Cyanide, mg/l | - | 0.65* | 54.1 | 685.0 | 6.3 | 6.5 | 0.1 | NS | |
| BOD, mg/l | - | 13.0-73.0 | 70.0** | - | - | 1.2 | 0.0 | 30 | 50 |
| COD, mg/l | - | 320-365 | - | - | - | - | - | NS | NS |
| Dissolved oxygen, mg/l | - | 1.10-2.60 | - | - | - | - | - | NS | NS |
| Redox potential, mV | - | 61-97 | - | - | - | - | - | NS | NS |
| Hydrocyanic acid | - | 54.10-63.20 | - | - | - | - | - | NS | NS |
| Temperature, ºC | - | - | - | - | - | 24.5 | 24.5 | <40 | <40 |
| Turbidity, NTU | - | - | - | - | - | 24.0 | 5.5 | - | - |
| Chloride, mg/l | - | - | - | - | 516.30 | 24 | 5.5 | 600 | 600 |
| Calcium, mg/l | 1.48 | - | 62.25 | 94.30 | - | - | NS | NS | |
| Potassium, mg/l | 0.58 | - | 50.9 | - | - | - | - | - | NS |
| Aluminum, mg/l | - | - | - | - | 71.50 | - | - | NS | NS |
| Magnesium, mg/l | 0.82 | - | 25.25 | - | 110.90 | - | - | 200 | NS |
| Sodium, mg/l | 1.20 | - | 120.4 | 146.2 | - | - | - | NS | NS |
| Cadmium, mg/l | - | - | 0.19 | 1.98 | 0.11 | - | - | 0.05 | NS |
| Copper, mg/l | 1.83 | - | 1.91 | 2.5 | 2.60 | 0.00 | 0.00 | <1 | NS |
| Manganese, mg/l | - | - | 0.71 | - | 7.10 | 0.00 | 0.00 | 5 | NS |
| Lead, mg/l | - | - | 9.45 | 8.31 | 1.82 | 0.25 | 0.15 | <1 | NS |
| Zinc, mg/l | 1.07 | - | 0.00 | 4.1 | 5.90 | 0.00 | 0.00 | <1 | NS |
| Iron, mg/l | 2.00 | - | 2.35 | - | 30.9 | 2.3 | 1.5 | 20 | NS |
| Mercury, mg/l | - | - | - | - | 1.05 | - | - | 0.05 | NS |
| Chromium, mg/l | - | - | - | - | 1.14 | - | - | <1 (as trivalent and hexavalent) | NS |
| Silver, mg/l | - | - | - | - | 8.20 | - | - | 0.1 | NS |
Microbiology of CME
Microbes are often described as ubiquitous organisms due to the ability to thrive in nearly all environments under different conditions. Some of the environments could be stressful to the microbes. For instance, CME is acidic in nature and contain high cyanide content. This makes it toxic to some certain group of life. However, some still survive under this environment. These microbes are also transferred to the environment (soil or surface water) receiving the CME via discharge. The microbial diversity associated with CME in its environment and populations from cassava mills are presented in Table 2 and Table 3 respectively. The microbial diversity comprises of several microbial genera including Neisseria, Streptococcus, Staphylococcus, Bacillus, Entrobacter, Proteus, Lactobacillus, Pseudomonas, Micrococcus, Saccharomyces, Penicillium, Aspergillus and Mucor. The occurrence of some of the microbes is associated to processing environment and equipment used in processing including water used in washing, knife/cutlass used peeling, bag used in storing prior to pressing and hygienic status of the processors.
Table 2. Diversity of Microbes associated with cassava mill effluents| Point of discharge of CME into surface water | cassava mill effluents | Soil receiving CME and CME its-self | Soil receiving cassava mill effluents | |||||
| 72 | 73 | 64 | 65 | 70 | 74 | 10 | 9 | 75 |
| - | Marexalla | - | - | - | - | - | - | - |
| - | Acineto bacter | - | Neisseria | - | - | Chromobacterium sp | - | Streptococcus sp |
| E. coli | E. coli | - | Streptococcus | - | E. coli | E. coli | E. coli | - |
| Shigella sp | Carynebacterium | - | - | - | - | - | Proteus sp | Proteus mirabilis, P. vulgaris |
| - | Klebsiella | - | - | - | Klebsiella oxytoca | Klebsiella sp | Klebsiella sp | Klebsiella sp |
| Staphylococcus | Staphylococcus | Staphylococcus aureus; S. epidermidis | - | - | - | - | Staphylococcus aureus | Staphylococcus sp |
| Bacillus | Bacillus | Bacillus | Bacillus | - | Bacillus subtilis, B. macerans | Bacillus sp | Bacillus sp | Bacillus sp |
| Proteus sp | Entrobacter | Entrobacter aerogens | - | Proteus mirabilis | - | - | Entrobacter sp | - |
| Lactobacillus | Lactobacillus | - | - | - | - | - | ||
| Flavobacterium | Alcaligenes | Flavobacterium | - | Flavobacterium aquatile | - | - | - | - |
| Pseudomonas | Pseudomonas | Pseudomonas | Pseudomonas aeruginoa , P. putida, P. Cepacia , P.luorescens | Pseudomonas aeruginosa | Pseudomonas sp | Pseudomonas aeruginosa | - | |
| Micrococcus | Micrococcus | Micrococcus | Micrococcus | - | - | Micrococcus sp | - | - |
| Candida | Saccharomyces | Saccharomyces cerevisae | - | - | - | Yeast | - | - |
| Penicillium | - | Penicillium oxialicum ; P. notatum | - | - | Penicillium | Penicillium sp | Penicillium sp | Penicillium sp |
| Aspergillus | - | Aspergillus | - | - | Aspergillus | Aspergillus sp | Aspergillus sp | Aspergillus sp |
| Geotricum | - | Mucor | - | - | - | Mucor sp | - | Mucor sp |
| - | - | - | - | - | Rhizopus sp | Rhizopus sp | Rhizopus sp | Rhizopus sp |
Microorganisms present in soil could be affected by the toxicity of the CME effluents. As such, the density of the microbes typically reduces as compared to non-effluent soil and surface water. The reduction in population of microbial parameters including total heterotrophic bacteria, total coliform, E.coli counts, Staphylococci counts, fecal coliform counts, lipolytic bacteria, cellulolytic bacteria, phosphate solubilizing bacteria, nitrifying bacteria and total fungi (Table 3). The decline in microbial population due to the effect of CME could also affect the environment receiving the effluents (soil and water).
Table 3. Density of microorganisms associated with cassava mill effluents| Samples source | Total bacterial load | Coliform counts | E.coli counts | Staphylococci count | Fecal coliform counts | Total fungi | Lipolytic bacteria | Cellulolytic bacteria | Phosphate solubilizing bacteria | Nitrifying bacteria | References |
| CME; expressed as cfu/ml | |||||||||||
| CME | 1.0 x103 | - | - | - | - | - | - | - | - | - | 70 |
| CME | 106-1015 | - | - | - | - | - | - | - | - | - | 65 |
| Surface water receiving CME; expressed as cfu/ml | |||||||||||
| 10m upstream discharge point | 3.2 x106 | 2.0 x104 | - | - | 1.1 x104 | - | - | - | - | - | 72 |
| Point of discharge | 2.1 x107 | 5.5 x105 | - | - | 1.4 x106 | - | - | - | - | - | |
| Point of discharge | 4.8 x105 | 2.3 x103 | - | - | - | - | - | - | - | - | 73 |
| 120 meter away from CME discharge point | 4.7 x104 | 2.2 x102 | - | - | - | - | - | - | - | - | |
| Soil receiving cassava mill effluents expressed as cfu/g | |||||||||||
| Edge of CME discharge pit | 3.7 x104 | - | - | - | - | - | 0.9 x101 | - | 2.2 x102 | 0.4 x101 | 76 |
| Control | 6.7 x106 | - | - | - | - | - | 2.2 x103 | - | 2.3 x104 | 2.9 x103 | |
| Discharge point | 19 x106 | 18 x106 | 13 x106 | 0 | - | - | - | - | - | 64 | |
| Control | 4 x106 | 1 x106 | 0 | 21 x106 | - | - | - | - | - | ||
| Point of discharge | 9.5 x104 | - | - | - | - | - | - | - | - | - | 70 |
| 6feet away from the point of discharge | 7.0 x104 | - | - | - | - | - | - | - | - | - | |
| Impacted soil at 0-20cm | 4.2 x106 | - | - | - | - | 4.2 x106 | - | - | - | - | 75 |
| Impacted soil at 20-40cm | 3.9 x106 | - | - | - | - | 2.1 x106 | - | - | - | - | |
| Impacted soil at 40-60cm | 2.3 x106 | - | - | - | - | 0.2 x106 | - | - | - | - | |
| CME contaminated soil for 1 – 6 weeks | 5.60-5.76 Log | 4.56-4.71 Log | 2.39-2.56 Log | - | 3.47-3.65 Log | - | - | - | - | - | 9 |
| Control | 5.84 Log | 4.28 Log | 2.14 Log | - | 3.19 Log | - | - | - | - | - | |
| Discharge point | 1.3 – 3.61 x108 | - | - | - | - | 1.84 x104 – 2.2 x108 | - | - | - | - | 74 |
| Control | 1.0-3.3 x108 | - | - | - | - | 2.0x x102 – 2.0 x106 | - | - | - | - | |
| Impacted soil (dry season) | 3.7 x104 | - | - | - | - | - | 0.9 x101 | 2.4 x103 | 2.4 x102 | 0.6 x101 | 77 |
| Control | 6.7 x106 | - | - | - | - | - | 2.2 x104 | .3 x104 | 2.9 x104 | 2.4 x104 | |
| Impacted soil (wet season) | 4.3 x104 | - | - | - | - | - | 1.1 x101 | 2.8 x103 | 2.5 x102 | 1.1 x101 | |
| Control | 2.1 x107 | - | - | - | - | - | 2.4 x104 | 2.9 x104 | 3.3 x104 | 3.2 x104 | |
| Point of discharge | 1.2 – 1.6 x106 | - | - | - | - | 2.3 – 2.9 x106 | - | - | - | - | 10 |
| 5meters away from dumpsite | 2.1-2.7 x106 | - | - | - | - | 3.5 – 3.9 x106 | - | - | - | - | |
| 50 m away from dumpsite | 3.0 - 3.4 x106 | - | - | - | - | 4.2 – 4.6 x106 | - | - | - | - | |
Impacts of Cassava Mill Effluents
Generally, cassava processing units generate large volumes of effluent 1, 16, 17, 21, 28, 70, which contain highly lethal substances, mobile in soil, affect biodiversity including marine lives, benthic macro-invertebrates, fisheries, microbes, plants 70, human, domestic animals (goat and sheep), fauna and flora, and affect water and soil physicochemical parameters 64. The current trend is direct discharge of CME onto soils and nearby surface water including rivers and streams 78. Probably due to high cyanogenic contents, chemical oxygen demand (COD), BOD, total suspended solid, total dissolved solid, colour, the receiving water bodies could get polluted and its suitability is hindered for downstream utilization such as drinking and washing. CME can also cause alteration in aquatic ecology, plant and animal composition and distribution, and human health.
Air
CME is typically known to cause bad odour. Ehiagbonare et al. 64 reported that foul odour of CME can be perceived as far as 90.3-102.3m of its source. In a developing country like Nigeria, air quality studies is still at infant stage and government agencies have not seen food processing sectors like cassava processing as a major area for which limits need to be established. During cassava processing, odour emanates from the decomposition of nutrients and this could be highly offensive 79.
Soil
The soil plays several functions to human and their abiotic components including social, ecological and economical 18. The soil is a platform through which the several economic activities take place including construction works 18. The soil harbors several economic microbes (including aerobic and anaerobic) and they play essential roles in transformation, biodegradation and mineralization. The soil also contains several minerals and organic matter needed by plant growth. There are different strata found in the soil. Every strata supports plant growth in addition to several other functions. Soil is the top layer of the lithosphere formed during weathering 77, 80 and combination of weathering, geologic materials and microbial interactions 18. The soil is a major recipient of agricultural and industrial wastes 81. These wastes stream often alter the physicochemical and microbial community of soil 9.
CME is a major waste from cassava processing, an agro business in Nigeria 16, 17, 18, 19, 20, 21, 22, 23, Cassava processing alters soil microbial characteristics (Table 2 and Table 3), physicochemical properties (Table 4), Heavy metals concentration (Table 5), cation and anions exchange (Table 6), soil enzymatic activity (Table 7), soil particle size, bulk density and porosity (Table 8) 10, 64, 67, 69, 74, 75, 77, 78, 81, 82, 83, 84, 85. The influence of CME on the soil parameters could be due to the fact that it contains cyanide which is highly lethal, fairly mobile in soil and damages microorganisms 75, 86. Also, due to the acidic condition of CME, it causes acidification 87. As such, toxicity of CME is associated with its cyanide content and acidity 87. Alteration in soil physical, chemical and microbial parameters have both adverse and beneficial effects. The increase in soil nitrate due to CME could enhance the aeration processes 82, 83, 84, 85, 86, 87, 88.
Table 4. Physicochemical parameters of soil receiving cassava mill effluents| Sample source | Depth, cm | Temp. ºC | pH | Conductivity, µS/cm | CN-, mg/kg | % organic carbon | % Total Nitrogen | % Organic matter | Exch acidity, Cmol/kg | Available phosphorus, ppm | % Moisture | Ammonium, ppm | Oil content, mg/kg | Reference | ||||
| Edge of discharge point | - | - | 10.3 | - | 5.2 | 41.3 | 2.1 | - | - | 1.78 | - | 5.2 | - | 76 | ||||
| Control | - | - | 7.3 | - | 0.72 | 24.2 | 3.07 | - | - | 5.61 | - | 0.72 | - | |||||
| Impacted soil | 0-20 | - | 4.9 | - | 9.06 | 1.99 | 0.98 | 5 | - | 180 | - | - | - | 75 | ||||
| Impacted soil | 20-40 | - | 5.2 | - | 12.7 | 1.5 | 0.32 | 1.02 | - | 15 | - | - | - | |||||
| Impacted soil | 40-60 | - | 5.16 | - | 18 | 1.15 | 0.12 | 1.2 | - | 15.11 | - | - | - | |||||
| Impacted soil | - | - | 6.3 | 33.4 | 3 | - | - | - | - | - | - | - | 1 | 9 | ||||
| Control | - | - | 7.1 | 16.6 | <0.01 | - | - | - | - | - | - | - | 1 | |||||
| Contaminated soil | - | - | 3.3-3.6 | - | - | - | 0.097-0.116 | - | - | 0.061-0.088 | 13.40-13.90 | - | 92 | |||||
| Control | - | - | 4.5-5.6 | - | - | - | 0.171-0.196 | - | - | 0.033-0.051 | 10.58-10.92 | - | ||||||
| Contaminated soil | - | 20.65-26.52 | 4.00-4.78 | 160.0-192.0 | - | 1.21-2.36 | 0.31-0.44 | - | - | - | - | - | - | 74 | ||||
| Control | - | 25.00-27.52 | 5.58-6.60 | 150-187 | - | 0.21-1.36 | 0.11-0.44 | - | - | - | - | - | - | |||||
| Point of CME discharge | 0-15 | - | 3.89-4.00 | 136.2-958.0 | - | 0.02-1.17 | 0.08-0.12 | - | - | **46.75-90.75 | - | - | - | 83 | ||||
| 15-30 | - | 4.02-4.92 | 172.8-517.0 | - | 0.12-1.09 | 0.09-0.12 | - | - | **43.82-86.60 | - | - | - | ||||||
| 30-45 | - | 4.83-5.41 | 151.8-427.0 | - | 0.70-1.56 | 0.08-0.11 | - | - | **45.72-87.50 | - | - | - | ||||||
| Control | 0-15 | - | 6.96 | 44.2 | - | 0.7 | 0.11 | - | - | **68.30 | - | - | - | |||||
| 15-30 | - | 7.4 | 75.7 | - | 0.47 | 0.11 | - | - | **70.20 | - | - | - | ||||||
| 30-45 | - | 7.81 | 37.03 | - | 0.78 | 0.1 | - | - | **69.20 | - | - | - | ||||||
| Discharge point | Top | - | 6.62 | 60.5 | 221.5 | 0.24 | 0.02 | 0.41 | 1.77 | 27.44 | - | - | - | 87 | ||||
| Bottom | - | 6.02 | 41.7 | 220.54 | 0.07 | 0.02 | 0.11 | 1.78 | 30.94 | - | - | - | ||||||
| Control | Top | - | 6.15 | 33 | 143.52 | 0.25 | 0.02 | 0.42 | 1.96 | 34.38 | - | - | - | |||||
| Bottom | - | 6.1 | 51.5 | 157.88 | 0.71 | 0.02 | 1.15 | 1.76 | 22.46 | - | - | - | ||||||
| Discharge point | - | 25.8 | 8.9 | - | ***0.19 | *47.43 | 22.47 | - | ^1.03 | - | 19.71 | 7.92 | - | 82 | ||||
| 5 meter away from discharge point | - | 29.4 | 8.2 | - | ND | *43.10 | 19.59 | - | ^1.52 | - | 17.92 | 7.01 | - | |||||
| Control | - | 28.3 | 6 | - | - | *21.91 | 7.23 | - | ^1.15 | - | 16.41 | 22.15 | - | |||||
| Distance from discharge point in meters | 0 | - | 5.22 | - | - | - | 0.17 | 1.3 | 2.68 | 32.05 | - | - | - | 81 | ||||
| 100 | - | 5.2 | - | - | - | 0.2 | 1.25 | 2.63 | 32 | - | - | - | ||||||
| 200 | - | 5.29 | - | - | - | 0.16 | 1.52 | 2.69 | 34.01 | - | - | - | ||||||
| 300 | - | 5.32 | - | - | - | 0.15 | 1.38 | 2.6 | 33.05 | - | - | - | ||||||
| 400 | - | 5.31 | - | - | - | 0.09 | 1.58 | 2.52 | 36.41 | - | - | - | ||||||
| Depth | Fe, mg/kg | Cd, mg/kg | Cu, mg/kg | Mn, mg/kg | Cr, mg/kg | Zn, mg/kg | Pb, mg/kg | References | |
| Edge of discharge point | - | - | 0.012 | 0.112 | - | 0.03 | 1.41 | 0.108 | 76 |
| Control | - | - | 0.012 | 0.12 | - | 0.01 | 1.37 | 0.097 | |
| Contaminated soil | - | 0.118-0.147 | - | 0.149-0.158 | - | - | 25.40-32.24 | 0.106-0.636 | 92 |
| Control | - | 0.034-0.064 | - | 0.01-0.052 | - | - | 14.67-17.84 | 0.009-0.042 | |
| Discharge point | (average of 0 – 45cm) | 84.88-139.28 | 0.006-0.04 | 0.146-0.643 | 1.729-3.420 | 0.002-0.022 | 0.618-1.684 | - | 83 |
| Control | (average of 0 – 45cm) | 84.88 | 0.002 | 0.235 | 0.314 | 0.016 | 0.506 | - | |
| Contaminated soil | - | 4.58-12.0 | - | 1.69-2.9 | 0.05-0.69 | - | 1.43-1.89 | - | 74 |
| Control | - | 4.33-12.6 | - | 1.09-2.12 | 0.01-0.61 | - | 1.33-1.78 | - |
| Sample source | Depth | Na+ Cmol/kg | K+ Cmol/kg | Ca2+ Cmol/kg | Mg2+ Cmol/kg | CEC Cmol/kg | Nitrate, mg/kg | Phosphate ion, mg/kg | Sulphate, mg/kg | Chloride, mg/kg | Aluminum ion, mg/kg | Reference |
| Edge of discharge point | - | 0.11 | 0.23 | 2.1 | - | - | - | - | - | - | - | 76 |
| Control | - | 0.09 | 0.42 | 1.47 | - | - | - | - | - | - | - | |
| Impacted soil | 0-20cm | 0.92 | 0.92 | 5.3 | 3.0 | 15.0 | - | - | - | - | - | 75 |
| Impacted soil | 20-40cm | 0.80 | 0.87 | 2.5 | 1.5 | 14.6 | - | - | - | - | - | |
| Impacted soil | 40-60cm | 0.56 | 0.76 | 2.0 | 1.8 | 8.84 | - | - | - | - | - | |
| Contaminate soil | - | 92.0 | 4.0 | 167.0 | 85.0 | - | 0.35 | 0.52 | 13.0 | - | - | 9 |
| Control | - | 106.0 | 5.0 | 203.0 | 89.0 | - | 0.32 | 0.36 | 11.0 | - | - | |
| Contaminated point | - | 42.9-56.1 | 2.2-3.5 | 31.04-36.14 | 1.23-1.64 | - | - | - | 0.05-0.081 | - | 2.46-4.40 | 92 |
| Control | - | 11.9-16.8 | 0.40-0.78 | 10.63-12.19 | 0.71-0.88 | - | - | - | 0.184-0.195 | - | ND | |
| Contaminated point | - | - | - | - | - | - | 4.00-7.00 | 5.24-7.22 | 4.00-7.9 | 47.17-59.33 | - | 74 |
| Control | - | - | - | - | - | - | 0.67-8.00 | 5.22-6.88 | 3.0-7.4 | 40.67-49.00 | - | |
| Discharge point | Top | 0.21 | 0.10 | 4.77 | 1.48 | 8.43 | - | - | - | - | - | 87 |
| Bottom | 0.25 | 0.08 | 2.66 | 2.15 | 6.76 | - | - | - | - | - | ||
| Control | Top | 0.25 | 0.09 | 2.75 | 2.20 | 7.25 | - | - | - | - | - | |
| Bottom | 0.21 | 0.09 | 5.55 | 2.40 | 10.02 | - | - | - | - | - | ||
| Discharge point | - | 30.64 | 13.92 | 12.27 | 9.63 | ^17.13 | 10.33 | 10.97 | 107.01 | 0.39 | 82 | |
| 5 meter away from discharge point | - | 30.31 | 12.99 | 13.01 | 8.97 | ^19.14 | 11.27 | 10.98 | 103/81 | - | 0.20 | |
| Control | - | 30.11 | 6.51 | 4.10 | 7.16 | ^19.13 | 8.30 | 3.92 | 106.14 | - | 0.68 | |
| Point of discharge | 0-15 | - | - | - | - | 0.49-1.63 | - | - | - | - | - | 83 |
| 15-30 | - | - | - | - | 0.80-1.00 | - | - | - | - | - | ||
| 30-45 | - | - | - | - | 0.72-0.98 | - | - | - | - | - | ||
| Control | 0-15 | - | - | - | - | 0.84 | - | - | - | - | - | |
| 15-30 | - | - | - | - | 0.85 | - | - | - | - | - | ||
| 30-45 | - | - | - | - | 0.83 | - | - | - | - | - |
| Sample source | Depth | Acid phosphatase, µmol-pNP/g | Alkaline phosphatase, µmol-pNP/g | Dehydrogenase, mg/g/6h | Lipase, µMFFA/g | Urease, mgNH4+-N kg/2h | Cellulase, mg/g/6h | Reference |
| Edge of discharge point | - | 0.41 | 6.7 | 16.10 | - | 5.1 | - | 76 |
| Control | - | 3.6 | 3.4 | 37.50 | - | 2.2 | - | |
| Discharge point | - | 13.21 | 8.92 | 7.89 | 0.63 | 29.14 | - | 82 |
| 5 meter away from discharge point | - | 13.11 | 8.72 | 8.88 | 0.93 | 27.77 | - | |
| Control | - | 18.33 | 6.71 | 28.43 | *1.83 | 22.15 | - | |
| Contaminated soil | Dry season | 2.4 | 2.8 | 16.0 | *1.1 | 5.8 | 2.1 | 77 |
| Control | 3.4 | 3.1 | 33.50 | *2.4 | 2.7 | 3.2 | ||
| Contaminated soil | Wet season | 2.6 | 2.9 | 20.17 | *1.4 | 5.2 | 2.9 | |
| Control | 3.6 | 3.7 | 34.32 | 2.5 | 2.8 | 3.2 |
| Sample source | Depth, cm | % sand | %silt | % clay | References |
| Impacted soil | 0-20 | 90 | 15.4 | 11.0 | 75 |
| Impacted soil | 20-40 | 90 | 8.6 | 7.03 | |
| Impacted soil | 40-60 | 70.4 | 12.0 | 17.6 | |
| Contamination point | - | 86-89 | 4 | 7-8 | 74 |
| Control | - | 76-87 | 3-4 | 5-6 | |
| Point of CME discharge | 0-15 | 72-92 | 0-2 | 7-26 | 83 |
| 15-30 | 69-81 | 0-2 | 18-23 | ||
| 30-45 | 71-81 | 1-10 | 15-28 | ||
| Control | 0-15 | 78 | 2 | 20 | |
| 15-30 | 65 | 4 | 31 | ||
| 30-45 | 76 | 2 | 22 |
Typically soil contamination affects the alteration of soil’s fauna and flora, thereby leading to low productivity due to reduced fertility. Instances of CME leading to reduction in height and leaves of Telfairiaoccidentalis Hook F 69 and root of Allium cepa L 68 have been reported. Cyanide is a metabolic poison and has the tendency to reduce the biomass of microorganisms within the impacted soil. Due to acidic and high cyanide content (i.e. cynogenic glucoside such as linmarin) of CME, i could reduce the activities of most microbes involve in biogeochemical nutrient cycling 77. For instance, chemolithotrophic, acetogenic and methanogenic microbes that play essential role in carbon cycle, non-symbiotic microbes includingAzotobacter, Beijerinckia, Cyanobacteria, Clostridium, denitrifying bacteria includingPesudomonas, Bacillus licheniformis, Paracoccusdenitrificiens, symbiotic bacteria such as Rhizobium, Bradyrhizobium; nitrifying bacteria such as Nitrobacter, Nitrospira that play essential role in nitrogen cycle, Beggioatoa, Thiobacillus, purple and green phototrophic bacteria that play essential role in sulphur cycle could be impacted upon. The groups of microbes that can be affected by cynogenic glucoside found in CME include nitrifying, lipolytic, cellulolytic, phosphate solubilizing and total heterotrophic bacteria 76, 77.
CME typically reduces the concentration of acid and alkaline phosphate, dehydrogenase, lipase, cellulose and lipase, and elevates urease concentration 77, 82. The alteration could also affect soil geotechnics.
Instance of heavy metal contamination of soil due to CME has been documented by Aiyegoro et al. 89, Izah et al. 18. High concentration of heavy metal especially non-essential metals has the tendency to affect soil native micro-biota 89. Recently, the role of essential heavy metals has been comprehensively documented by Izah et al. 90. Some of these heavy metals have the tendency to persist in the environment for a long time 91 as they have low degradation potentials.
Water Quality
Like food, water is also a prime resources required by living things for the sustenance of life 93. Water resources include surface (creek, creeklets, rivers, ocean, stream, pond, rivulets), ground and rain water 94. Water can also be classified according to salt concentration viz: marine, brackish or estuarine and freshwater 95.
Water resources are majorly contaminated by anthropogenic activities 96, 97, 98. CME could lead to reduction of oxygen demand in water 28. CME has the tendency to turn water brownish/milkish thereby impacting on the colour and turbidity level. Omotioma et al. 72 reported that colour of water sample at the source of CME has 9 Hazen unit being higher than the control samples (4 – 6 Hazen unit) respectively. Acidification have widely been reported in CME due to low pH (<5) 16, 27, 99, 100, 101. As the water becomes acidic due to the presence of CME, the turbidity also elevated. Table 9 present variations associated in the physicochemical characteristics of surface water receiving CME. Microbial quality of the water receiving the effluent are also altered (Table 2 and Table 3).
Table 9. Physicochemical characteristics of surface water receiving cassava mill effluents| Parameters | 102 | 73 | 72 | 3 78 | 103 | ||
| Discharge point | Discharge point | 120meter away from discharge point | Discharge point | 10meter away from discharge point | Nigerian drinking water limit | ||
| Water type | Pond | Surface water | Surface water | Surface water and close to well water | |||
| Ph | 4.2 | 8.0 | 6.7 | 5.3 | 6.3 | 7.50-8.00 | 6.5-8.5 |
| Total cyanide, mg/l | 39.0 | - | - | - | - | - | 1.0 |
| Temperature, ºC | - | 29.1 | 28.4 | 27.5 | 24.5 | - | Ambient |
| TDS, mg/l | - | 1240 | 256 | 930 | 515 | 269-735 | 500 |
| TSS, mg/l | 4030 | 73 | 51 | 3890 | 2238 | 40-181 | NS |
| Total solid, mg/l | - | - | - | - | - | 309-845 | NS |
| DO, mg/l | 4.05 | 4.3 | 6.8 | 4.4 | 12.3 | - | NS |
| BOD, mg/l | 430.7 | 8.3 | 0.08 | 532.3 | 36.3 | 90-290 | NS |
| COD, mg/l | 1236 | - | - | 1019.7 | 65.3 | NS | |
| Total hardness, mg/l | 91.2 | - | - | 81.5 | 61.8 | 166-365 | 150 |
| Conductivity, µS/cm | 1822 | - | - | 1749 | 686 | 400-1170µmhos/cm | 1000 |
| Turbidity, mg/L SiO2 | 15.5 | - | - | 13.8 | 3.5 | - | 5NTU |
| Colour, Hazen units | - | - | - | 9 | 4 | - | 15TCU |
| Phosphate, mg/l | - | 0.10 | 0.08 | 15.2 | 3.5 | - | NS |
| Total Phosphorus, mg/l | - | - | - | - | - | 1-4 | NS |
| Total Nitrogen, mg/l | - | - | - | - | - | - 1.1 | NS |
| Nitrate-N, mg/l | - | - | - | - | - | 3.3- 83.7 | 0.2 |
| Alkalinity as CaCO3, mg/l | - | - | - | - | - | 195-376 | NS |
| Chloride, mg/l | - | - | - | - | - | 31.4- 162.0 | 250 |
| Nitrate, mg/l | - | 1.14 | 1.04 | 12.0 | 1.9 | - | 50 |
| Sulphate, mg/l | - | 26.8 | 26.1 | - | - | - | 100 |
| Sodium, mg/l | - | 0.85 | 0.84 | - | - | 21 – 91 | 200 |
| Potassium, mg/l | - | 1.45 | 1.42 | - | - | 15 – 59 | NS |
| Magnesium, mg/l | - | 1.05 | 1.04 | - | - | 8.6-21.4 | 0.20 |
| Calcium, mg/l | - | 25.0 | 22.1 | - | - | 13.6 – 52.0 | NS |
| Lead, mg/l | 0.05 | - | - | 0.02 | 0.01 | 0.00 | 0.01 |
| Cadmium, mg/l | - | - | - | 0.00 | 0.00 | - | 0.003 |
| Mercury, mg/l | 0.03 | - | - | 0.00 | 0.00 | - | 0.001 |
| Iron, mg/l | 0.04 | - | - | - | - | 0.27-1.92 | 0.3 |
| Boron, mg/l | - | - | - | - | - | 0.02 – 0.19 | NS |
High concentration of total suspended solid, BOD, COD and complex polymers and minerals in CME suggests that it could contaminate water sources causing eutrophication in the process without treatment. Also, runoff associated with CME discharge may result to percolation into the ground water thereby causing contamination.
Toxicological Impacts Associated with Cassava Mill Effluents
CME elicit several toxicological impacts which are caused by the presence of cyanogenic glucoside; lionamann (synthesized from valine, amino acid) and lotaustralin (synthesized from isoleucine, also an amino acid) 9, 79. This section of the paper is organized into various impacts associated with CME toxicity.
Food Resources (Flora and Fauna)
Due to acidic nature of CME, it is toxic to household animals, fisheries and other organisms 1. Most of the human food resources are found in the environment including water and land. Acidification of water and soil leads to loss of viable food resources. It could lead to decline in abundance and composition of fisheries over a long period of time which could have adverse impact on human who depend on these fishes as source of protein. However, most communities aligning water bodies depend on fisheries from the wild as source of livelihood. For instance, in Bayelsa state, Niger Delta region of Nigeria, fishing is a major source of livelihood of the indigenous people inhabiting of the area.
Soil containing decomposed CME could reduce crop yield and death of live-stocks as well. Otunne and Kinako 104 reported that CME without palm oil led to death of some plant species such as Sidaacuta, Mimosa pudica, Euphorbia hirata, Tridaxprocumbens at exposure and 20% of Chromelaenaodorata survived at 75% CME exposure. In addition, authors have also reported that CME without palm oil kills domestic animals such as sheep and goats and do not affect cat, fowl and pig 64, 79. CME has the tendency to inhibit growth and germination of seedlings 92. Nwakaudu et al. 92 reported that CME could affect height, leaf colour of maize. CME containing palm oil does not induce mortality in domestic animals such as cat, goat, sheep, fowl and pig 104. This could be due to antioxidant potentials of palm oil 105 – 109.
Furthermore, cyanide toxicity also occurs in insects. Notable symptoms associated with cyanide toxicity in Malacosoma americanum, an eastern tent caterpillar include darkening of muscle tissue, congestion or hemorrhage of the lungs, patechination of the tracheal mucosa and a frothy bloody damage (i.e. mouth and nostrils) 110, 111.
Fisheries
Due to the tendency of CME to cause acidification in the aquatic ecosystem, it could have both short and long term impacts on the aquatic biodiversity including fisheries (shelled and fin), plankton (zooplankton and phytoplankton), benthic organisms and macrophytes. On fisheries, it could lead to disease condition, egg damage, high mortality rate of oyster and species of crustaceans that are acid tolerant on short term basis. Furthermore, it could lead to loss of habitat and growth abnormalities in fisheries including bivalve, oysters, fin fish, decline in spawning potential probably due to stress, destruction of fish eggs leading to reduction in composition and abundance on long term impacts. Furthermore, acidification could lead to acid tolerance plankton communities.
Increased light penetration potential due to acidification of the water could ultimately enhance water temperature which could lead to variation in spawning period, species distribution and abundance and migration of aquatic organisms over a long period of time. In addition, the presence of toxic substances resulting from acidification could have long term impacts on biodiversity especially aquatic flora.
In toxicological studies, fisheries have been widely used for assessing pollution in water bodies. Notable fish indices used in assessing pollution in aquatic ecosystem include enzymes, biochemical, metabolites, electrolytes, haematological, histopathological and behavioral response, organosomatic and mortality rate 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124. Several fish species have been studied depending on its availability and region. Among the family that had been widely studied is Clariidae. Of all the species, Clariasgariepinus and Heterobranchusbidosalisare commonly used for toxicology study. This could be due to the ability to withstand stress and unfavorable conditions. However, Clariasgariepinus is a common fish found in most surface water in Nigeria. This fish species have been widely described as a common Niger Delta fish 125, 126.
Studies on effect of CME on some indices (mortality, behavioral, enzyme, histopathology and haematology) (Table 10) causes toxicological abnormality in fish 33, 127, 128, 129. The toxicity in fisheries exposed to CME is most likely to be caused by cyanide content and acidity. According to Adeyemo 33 cyanide is a potent respiratory poison and could kill life in aquatic ecosystem especially fisheries. Small concentration of cyanide could elicit physiological and pathological effects in fisheries 33. However, alteration in the various indices could lead to stress, disease and even death. This is because the toxic component could impede the metabolic and physiological responses. For instance, CME could decrease mean cell volume in the blood and haemoglobin content which is an indication of shrinkage of red blood cell caused by microcytic or hypoxia 33.
Table 10. Toxicological response of fisheries (Clarias gariepinus) to cassava mill effluents| Parameters/ response | Exposure period/ and bioassy | Findings | Implication | Reference |
| Mortality | 72 hours in a non-renewable bioassay system | LC50 value of 96.937mg/ml at 24hr and 9.795 mg/ml at 48 hour | CME lead to death over a prolong period of time | 127 |
| 2, 5, 10, 15mls of CME was separately injected to the fish and exposed for 96 hours | Mortality was 0% at 2ml, 20% at 5ml, 50% at 10ml and 100% at 15ml | CME could cause death in fisheries | 33 | |
| Varying concentration of the effluent in static bioassay for 96 hours | LC50 value of 4.28ml/L at 96 hour | CME could cause death in fisheries | 128 | |
| Behavioural | 72 hours in a non-renewable bioassay system | Stressful behavioural changes characterized by erratic swimming, vertical swimming, gasping, and body discolouration. | Alteration in behavioral changes | 127 |
| 2-15ml of CME was injected to the fishes and exposed for 96 hours | Reduced swimming activity and body discolouration | Induces behavioral response | 33 | |
| Varying concentration of the effluent in static bioassay for 96 hours | sudden change in response (viz: erratic swimming, occasional gasping for breath and frequent surfacing) to the environment | Induces behavioral change | 128 | |
| Enzyme | 72 hours in a non-renewable bio assay system | Significant elevation of serum aspartate amino transferase and alanine transferase concentrations; and apparent increase alkaline phosphatase | Alteration in enzyme activity | 127 |
| Histopathological | 2-15ml of CME was injected to the fishes and exposed for 96 hours | Severe necrosis, hypertrophy and vacuolation of hepatocytes, haemorrahagic patches on the ventral surface of the fishes and anoxia | Causes histopathological effects | 33 |
| The fish was exposed to varying concentration (0.020ml/L, 0.016ml/L, 0.012ml/L, 0.008ml/L, and 0.004ml/L) of CME for 14 days. | Degenerative changes were congestion, vacuolization of hepatocyte, cellular infiltration and necrosis. Furthermore, the liver revealed slight vacuolated cells which is an indication of fatty degeneration of hepatocytes | Histological degradation | 129 | |
| The fish was exposed to varying concentration of the effluents (4.0, 4.5, 5.0, 5.5 ml/L) for 96 hours | Degeneration of filament,fragmentation of the lamella, vacuolation of the filaments, erosion of the gills and they showed the sign of necrosis and slight congestion of the gills. Furthermore, it also showed hydropic and cellular arrangement degeneration of the liver at high concentration | Alter gills and liver functions | 128 | |
| Haematological | 2-15ml of CME was injected to the fishes and exposed for 96 hours | Causes significant decline of pack cell volume, haemoglobin, mean cell volume, red blood cells (at 5-10ml injection), mean cell haemoglobin concentration (at 10ml injection), and significant elevation in white blood cell, apparent decline in mean cell haemoglobin and neutrophil, and elevation in lymphocyte and eosinophil (at 5-10ml injection) and unaffected monocyte | Adversely affects most haematological indices in fisheries | 33 |
| The fish was exposed to varying concentration of the effluents (4.0, 4.5, 5.0, 5.5 ml/L) for 96 hours | Causes reduction in packed cell volume, Red blood cells andHaemoglobin concentration | Induces some blood parameters | 128 |
Furthermore, due to eutrophication caused by CME, oxygen content could be reduced. This could eventually affect rate of respiration and photosynthesis, thereby inducing behavioural responses leading to mortality 127. Typically, eutrophication could increase growth of aquatic plants and marsh transformation 79. The nutrient level in CME could also intensify the rate of eutrophication. Typically various nitrogenous compounds are found in CME including nitrate, nitrite etc. and these compound could be toxic to fisheries especially at high concentration over a prolonged period of time.
Microorganisms
Acidification in environment (soil and water) water could impact microscopic organisms including bacteria and fungi. In acidic environment, non-acid tolerant microbes do not thrive well. Presence of cyanide resulting from the discharge of untreated CME into soil could prevent oxidation/reduction process in non-resistant microbes, thereby leading to decline in productivity probably due to the effect on soil microorganisms 82. Similarly, Ezeigbo et al. 10 have reported that high cyanogenic glycoside limit the growth of microbes. This is a typical observation in CME contaminated soil and non-polluted soil (Table 3).
Due to availability of microbial isolates in CME, it’s a medium through which pathogenic (bacteria and fungi) diseases can be transmitted 9. This typically occurs when microbial contaminated CME come in contact with human skin for some microbes or incidentally ingested. Some of the microbes found in CME could induce health impacts when unintentionally ingested especially in immune-compromised patients.
The presence of coliforms in CME suggests fecal contamination and is mostly of the genus Enterobacter and Escherichia. The contamination could also be from the environment i.e. soil or water used for washing prior to processing.
Impact on Human
Like fisheries, cyanide content could affect human. But humans are not sensitive to cyanide as compared to fisheries 33. Odour pollution may trigger unpleasant sensation which could have adverse physiological reactions and olfactory functions 79. Some of the adverse response associated with odour pollution include breathing and sleeping difficulty, coughing, stomach and loss of appetite, eye, nose and throat irritation, disturbance from external environment, annoyance etc 79, 130. According to Ero and Okponmwense 79, hydrogen cyanide, a type of cyanide found in CME contains toxic materials that could cause partial blindness in human exposed to environment containing decomposing CME.
In addition, Uhegbu et al. 111 reported CME dumpsite could significantly influence cyanide concentration in some common root crops of Nigeria including Dioscoreadumetorum (domestic yam), Dioscoreadumetorum(wild yeam), Dioscorearotundata (white yam), Dioscoreaalata(water yam), Xanthosomasagittifolium(red cocoyam), Colocasia esculenta (white cocoyam), Ipomeabatatas (red sweet potato), Ipomeabatatas (white sweet potato). Consumption of food material containing high concentration of cyanide has some health implications. When food containing high cyanide ion concentration is ingested, they are absorbed by the gastro intestinal tracts and could lead to nutritional neuropathies such as tropical ataxic neuropathy and epidemic spastic paraparesis 111, 131. This disease affects the spinal cord. Oluwole et al. 132 reported that ataxic polyneuropathy occurrence is endemic area in south west Nigeria, which is associated to cyanide exposure from cassava foods. Famuyiwa et al. 131 also reported that tropical ataxic neuropathy is associated to chronic cyanide intoxication.
Over time, oral ingestion of cyanide can lead to neurological health issues. In man, cyanide toxicity is characterized by hyperventilation, headache, collapse and coma, nausea and vomiting, generalized weakness, perhaps with convulsion and then respiratory depression 111. When cyanide combines with noxious gases such as carbon monoxide, hydrogen sulphide and azide they inhibit cytochrome oxidase activities, thereby hindering the mitochondrial oxidation and phosphorylation which could eventually prevent ATP formation (Uhegbu et al., 2012).
Socioeconomic Impacts
Odour from cassava processing mill has socioeconomic influence in the society. Ero and Okponmwense 79 opined that odour from CME could worsen or down grade community pride especially in communities with high rate of cassava processing, interfere with human relation leading to unhealthy annoyance, discourage capital investment leading to slow growth in such community outside cassava processing. Sackey and Bani 32 also reported that sanitation and environmental challenges associated with cassava wastes and CME could have adversely affected processors and the larger community of the processing mills.
Conclusion and Future Direction
Nigeria is the largest cassava producing nation. Cassava cultivation and processing is a major source of livelihood to several families especially in rural area. This study reviews the impacts of CME in Nigeria and found that it causes air, soil and water pollution, and toxicological responses in human, fisheries, flora and fauna. Despite these impacts, management and treatment strategies are poor in developing countries like Nigeria. Therefore, there is the need for treatment of CME prior to discharge and/ or utilization through biotechnological advancement.
Acknowledgements
This paper is part of PhD project work of S.C. Izah supervised by Dr S.E. Bassey and Prof. E.I. Ohimain at the Niger Delta University, Wilberforce Island, Nigeria. The author wish to thank Dr. Arun Lal Srivastav Environmental Sciences & Disaster Management Unit at Chitkara University, Solan for proofreading this manuscript.