Abstract
Objectives
The present study addresses evaluation of acacia-guar gum combination as an enteric former for tablet coating aiming to add knowledge on how develop the ability of enteric forming ability of acacia-guar combination.
Methods
Five formulations of enteric coating solution incorporating guar gum as delayed release polymers along with film coating material acacia gum followed by CMC and glycerin as plasticizer and coloring agents were prepared to coat placebo tablet cores. Different enteric coating formulations organized in different acacia : guar gum ratios as 1:1, 1:2, 1:3, 1:4 and 1:6 were sprayed on placebo tablets surface resulted different delayed coated tablets (F1,F2,F3,F4 &F5) respectively. General appearance and physical parameters were evaluated of each. Enteric coated tablets that revealed promising properties were subjected to accelerated stability study for 3 months to explore the influences of physical aging on tablet coat properties.
Results
Physical parameters of enteric coated tablets post coating within the range of pharmacopeia specification. The disintegration test was carried out in pH 1.2 and pH 6.8 at 37ºC. F1, F2 and F3 enteric tablets disintegrated immediately with no acid resistance compared F4 and F5 enteric tablets showed good acid resistance coat with smooth tablet surfaces and no coat defects. F5 formula contain acacia: guar gum as 1:6 ratio showed delayed release for 30min in pH 1.2 and 15min in phosphate buffer. The study statistically analyzed and concluded that, an efficient and stable acacia-guar enteric coat is achievable with no effect on tablets physical parameters. Guar gum at 60% as a delayed tablet coating material capable of protecting the tablets core from being released in acidic media and be release in the alkaline buffer as well as stable coat under accelerated storage for three months.
Author Contributions
Academic Editor: Fatma Mohammed Mady, Department of Pharmaceutics, Minia University, Egypt.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2021 Suad Y. Alkarib, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
No author has any associations that may represent a potential conflict of interest.
Citation:
Introduction
Coating is one of the oldest pharmaceutical processes by which an essentially dry, outer layer of coating material is applied to the surface of a dosage form (sugar or polymeric coat). Coating can be applied to several kinds of solid dosage forms like tablets, pellets, pills, drug crystals, etc1. However a tablet is the most popular and preferred a pharmaceutical solid dosage form for oral administration all over the world 2 so that tablet coating can be described as a process of applying coating solution to a batch of tablets in a coating pan which are polymer and polysaccharide base with plasticizers and pigments 3. The coating problems tend to be visual or cosmetic defects that may not negatively impact product efficacy; their existence detracts from the awareness of overall product quality and may affect consumer/patient confidence 4. There are several techniques for tablet coating such as sugar coating, film coating and delayed or enteric coating. The advantages of tablet coating are taste masking, odor masking, physical and chemical protection, protects the drug in the stomach, and to control its release profile 1. A polymer used for film coating of tablets has great importance and one of the essential parts of the type of coating so that there are various polymers and which is responsible for effective, enteric or sustained release action 5. Delayed coatings are prepared from gastric resistant polymers which remain intact in acidic environment, but dissolve readily at the elevated pH of the small intestine. This property is related to the chemical structure of the applied polymer. The most effective enteric polymers contain many carboxylic acid groups with a pKa of 3-5 6.
In recent years, several tropical plants are sources of materials which possess properties that are of interest to the pharmaceutical, food and cosmetic scientists like the uses of natural plant-based ingredients. One group consists of polymeric plant metabolites, generally known as plant gums which can hydrate into gels or mucilages, hydrosoluble, swellable and are biopolymeric materials composed of complex hetero polysaccharides with proteinaceous material, in addition to some mineral element 7. They are biocompatible, non-toxic, readily accessible, economical and cost-effective, even for industrial scale production and safe for human consumption so preferred over the synthetic in various pharmaceutical applications 8 as suspending agents, emulsifiers, binders, disintegrants, bioadhesives, film formers or controlled release matrices , those include: tragacanth, acacia gum, karaya and guar gums.
Acacia gum (also known as gum Arabic) is gummy exudates from the stem and branches of Acacia Senegal, (Fam. Leguminoseae). The tree is indigenous to West and East Africa. Various quality grades of acacia gum are commercially available, but only the best qualities are suitable for pharmaceutical uses should preferably be colorless, normally solid, brittle and inodorous. The gum is soluble in 2 parts of water, forming a mucilage, but insoluble in alcohol 9 which is a branched-chain, complex polysaccharide, either neutral or slightly acidic 10. It has an approximate molecular weight of 250,000. The most fundamental property of gum Arabic its water solubility and high viscosity but does not thicken the product. It has approved by FDA as pharmaceutical products that use in a wide variety of applications mainly as tablet binder, film forming agent, emulsifier, and suspending agent 11. Gum Arabic is a multifunctional hydrocolloid with a highly branched arabinogalactan protein complex; it is often described as globular in shape. This structure makes it a good film-former, this encouraged the choice of gum Arabic to apply as a coating solution at 10% to 15% 12 and from its film forming property and leaves a shiny film coat when coated on tablets surface 13.
Guar gum is an extractive polysaccharide gum derived from the endosperm of the leguminous plant, Cyamopsis tetragonolobus (Leguminoseae). Their chemistry is not complex, the major compound being galactomannan 9 which hydrates rapidly in cold or hot water and produces a uniform and high viscous gel with relatively low concentrations that aids in thickening a wide variety of food categories 14. It has better bio-degradability and bio-compatibility so guar gum finds application in various industries but it should be chemically modified to overcome uncontrollable rate of viscosity, uncontrollable rate of hydration, and instability of its solution for a long time and susceptibility to microbial contamination 15. Since the guar is neutral, which give solutions that are sensitive to pH are usually characterized by the presence of carboxyl or sulphate groups. Guar solutions have an almost constant viscosity over pH range of about 1.0-10.5. However the maximum hydration takes place at pH 8.0-9.0. Slowest hydration is at pH, above 10.0 and below 4.0 16. Guar gum and their derivatives were evaluated as coating material by coating dummy tablets but produces films that lack in clarity and have poor tensile strength hence was modified chemically for improving its coat forming properties. To overcome their limitations, a combined coating formulation was developed 17 as well as improving the mechanical properties of the compressed coated tablets 18.
The aim of the present study was to evaluate the acacia and guar gum combination as delayed release coat on tablets with no effect on tablets physical parameters and no change with time and storage conditions.
Materials and Methods
The uncoated placebo tablets were obtained from Azal pharmaceuticals company Ltd. -Khartoum, Sudan. Natural polymers acacia (Alnasr gum Arabic dry powder) and guar gum were obtained from Sudan central of gum Arabic – Khartoum.
Other chemicals and materials for coating formulations that obtained from the Amipharma laboratories Ltd.- Sudan and Karary University.
Preparation of Different Enteric Coating Solutions
Placebo tablets were coated by traditional coating method based on previous conducted studies 12, five different formulations of coating solutions were prepared contain different percentages of guar gum (Table 1) with acacia which is film coat former at 10% and improve adhesion properties of guar gum on tablet surface. Each components of enteric formulation, was accurate weighted and transferred into a beaker. Distilled water was added up to 100% of coating solution and solution mixture were homogenized for 45 minutes.
Table 1. General enteric coating solution formula| No. | Coating materials | Coating materials (%) | Function | ||||
| F1 | F2 | F3 | F4 | F5 | |||
| 1 | Acacia gum | 10 | 10 | 10 | 10 | 10 | Film former |
| 2 | Guar gum | 10 | 20 | 30 | 40 | 60 | Delayed release polymer |
| 3 | Maize Starch | 2 | 2 | 2 | 2 | 2 | Opcaifer |
| 4 | Carboxy methyl cellulose CMC | 3 | 3 | 3 | 3 | 3 | Plasticizer |
| 5 | Glycerin B.P | 3 | 1 | 1 | 1 | 1 | Plasticizer |
| 6 | Titanium dioxide | 1 | 1 | 1 | 1 | 1 | Opcaifer |
| Water up to | 100 | 100 | 100 | 100 | 100 | ||
Enteric Coating Process of Placebo Tablets
In pan coating equipment, a number of placebo tablets (200g) were positioned on a cleaned and dedusted pan coater. Enteric coating solution was filled into spray gun of coating pan (Sams, Bombay, India) and sprayed at rate of 1ml/min every 3 minutes on tablet surface and the pan rolled at 5 rounds/ min. A tube of preheated air at 50º C was inserted within the pan to allow for the solvent evaporation. The process continued until the coating solution used up and elegant coated tablet were prepared 19. Coating process was repeated for each enteric coating solution formulations (F2), (F3), (F4) and (F5). Five delayed placebo coated tablets were produced.
Evaluation of placebo Tablets Physical Tests Pre and Post Enteric Coating
Tablet Weight Variation
The weight variation test was performed as per procedure of IP. 20 tablets of placebo and of enteric coated tablets were accurate weighted. The mean weight (mg) and standard deviation (± SD) were determined 20.
Tablet Diameter, Thickness and Hardness
10 tablets of placebo tablets and of enteric coated tablets were evaluated for diametrical crushing strength test using hardness tester (TDTF YD-35, China). Device measures tablet diameter (mm), thickness (mm) and the force required to break the tablet in Newton (N) were determined for each 21.
Tablet Friability
10 uncoated placebo tablets and 10 tablets of enteric coated tablets were subjected to the official friability testing where tablets accurate weighed and placed in the drum of tablet friability tester (TDTF FT 2000SE, China ) that operates at 25 round/min for 4 minutes 21.
After de-dusting, tablets were weighted and difference in weight expressed as a percentage of the initial weight. The United State Pharmacopoeia USP states that the friability value of tablets should be less than 1% .
Tablet Disintegration Test
The disintegration test was performed to check the intactness of enteric coat in acidic medium which preventing the release completely and gets dissolved in the phosphate buffer 22. Disintegration test was performed according to the general monograph of tablets that described in appendix XIIA of Ph. Eur 21. Six of uncoated placebo tablets and of delayed coated tablets placed into each of the six tubes of the disintegration test apparatus (TDTF ZBS- 6E, China) and perforated auxiliary discs were placed on the top, then motor rotation speed to the minimal value up and up down. Firstly the basket rack was positioned in a beaker of 0.1(N) HCl at 37 ± 0.5 °C. The time for enteric coated tablet to withstand in acid media was determined (min). Secondly another six tablets of each was placed in each of the six tubes of cylindrical glass and was inserted into the beaker of 0.02 M phosphate buffer at 37 ± 0.5 °C. The time for a tablet to completely disintegrate was determined (min).
Short Term Stability Studies 23
Short term stability study as per ICH guidelines24 was subjected at a temperature 40º ºC and 75% RH of humidity for three months (90 days). Sufficient numbers of coated tablets (10) were individually wrapped using aluminum foil and packed in amber color screw cap bottle and kept in stability chamber 25. General appearance and/or pharmaceutical tablets tests were evaluated every month.
Data Analysis
Different parameters between different formulations were carried out using statistical package for social science SPSS version 20 at 95% confidence intervals. P-value < 0.05 was considered statistically significant.The physical tests results of enteric coated tablets products pre and post enteric coating process were analyzed by one way variance of analysis (ANOVA), in addition to data of acacia-guar enteric coated tablets F4 and F5 before and after the three month of stability study were analyzed (paired sample t test).
Results and Discussion
Evaluation of placebo Tablets pre and post Enteric coating
Quality can be defined as the measurement of excellence or state of being free from defects, deficiencies, and significant variations. In a pharmaceutical sense, quality means checking and directing the degree of superiority of processes and products. It is the purpose of the pharmaceutical operations to produce medication of superior efficacy, safety and elegance and to provide assurance to physician, pharmacist and the consumer 26.
Immediate release placebo tablets characterized by smooth surface with uniform white color (Figure 2 ) and release in acid and alkaline buffer (Table 2). General appearance and physical tests results pre and post tablet coating were listed in Table 2. Uncoated and delayed coated tablets complies the IP standard. Tablet thickness (3-4 mm) , hardness slightly increased attributed to nature of polymers. The average percent deviation was less than ±10% indicated weight uniformity. Friability less than 1% of all tablets, ensuring the tablets were mechanically stable. Acacia- guar enteric coat no effect on tablet physical characteristics.
Table 2. General appearance and physical tests parameters of uncoated and coated placebo tablets within different formulations| Tablets' physical parameter | UncoatedPlacebo tablets(Mean ± SD) | Enteric coated placebo tablets | ||||
| F1 | F2 | F3 | F4 | F5 | ||
| Weight (g) | 130.4±8.14 | 140.5±6.42 | 147.2±5.87 | 138.2±7.01 | 149.5±7 | 149.1±7.01 |
| Diameter (mm) | 7.38± 0.06 | 7.43±0.08 | 7.37±0.41 | 7.39±0.05 | 7.42±0.02 | 7.73±1.43 |
| Thickness (mm) | 3.64±0.12 | 3.23±0.15 | 3.22±0.31 | 3.81±1.76 | 3.46± 0.04 | 3.71±0.38 |
| Hardness (N) | 82.6±46.6 | 94.5±56.6 | 88.8±53.3 | 80.1±45.2 | 109.8±15.6 | 100.3±41.4 |
| Friability (%) | 0.15± 0.01 | 0.31±0.01 | 0.15±0.01 | 0.38±0.03 | 0.05±0.01 | 0.11±0.02 |
| Acid resistance pH 1.2 (min.) | 0:37 | Failed | Failed | Failed | 13:41±0.44 | 34:27±2.08 |
| Disintegration in pH 6.8 (min.) | 0:30 | 0: 32± 0.2 | 0:41±0.01 | 0: 51±1.8 | 7 : 25±0.06 | 16:34 ±8.2 |
| Surface | Smooth | Rough | Uneven | Uneven | Smooth | Smooth |
| Shape | Convex | Convex | Convex | Convex | Convex | Convex |
| Colour | Uniform | Irregular | Uniform | Irregular | Uniform | Uniform |
| Defects | None | Cracked coat | Brittle coat | Easily broken coat | None | None |
Disintegration Test
In vitro disintegration study of delayed coated tablets was performed in 0.1 M HCl followed in 0.02 M phosphate buffer resulted in Table 2. Polymer ratios, delayed polymer (%) and disintegration medium affect disintegration time 25. F1, F2 and F3 coated tablets no acid resistance was showed whereas F4 and F5 showed delayed release in pH1.2 for 15- 30 min and 10- 20 min in pH 6.8 (Figure 1). Acacia –guar gum coat can protect tablets cores from acid media and mask their odor and taste. Figure 2.
Figure 1. Disintegration test within different acacia- guar delayed coated formulations in acidic pH (1.2) and in alkaline pH 6.8
Figure 2. uncoated placebo tablets
General appearance of placebo tablets pre and post coating listed in Table 2. F1 showed dry, weak or cracked surface and easily detached surface (Figure 3). F2 and F3 no smooth surface was showed attributed to cracks on tablets surface (Figure 4 and Figure 5). Smooth tablet surface with no defects and uniform color was resulted in F4 and F5 enteric tablets (Figure 6 and Figure 7). F4 and F5 enteric formulations contain acacia :guar ratio as 1:4 and 1:6 respectively were delayed release coat forming with good appearance and no effect on tablets properties. F5 formula with further development and optimization to be used for preventing the drug release in upper part of GIT and be released in the small intestine.
Figure 3. Delayed coated tablets (F1)
Figure 4. Delayed coated tablets (F2)
Figure 5. Delayed coated tablets (F3)
Figure 6. Delayed coated tablets (F4)
Short Term Stability Studies
General appearance and physical tests parameter changes of F4 and F5 coated tablets after three months storage resulted in (Table 3). The inconsequential increase or decrease at third month storage by no more than 5 % of parameters initial values. Acacia-guar gum delayed coat no effect on tablets pharmaceutical parameters, not affected by storage conditions and be stable (Figure 8) attributed to a little impact on different parameters of delayed coated tablets, at least under this study conditions.
Table 3. general appearance and physical tests changing profile of F4 and F5 coated tablets investigated at accelerated stability study for 3 months.| Tablet physical test & observed change | Enteric coated group (F4) | Enteric coated group (F5) | ||
| Zero months | Third month | Zero months | Third month | |
| Weight variation (mg) | 149.5±7 | 145.9±9.2 | 149.1 ±7.01 | 149.4±5.1 |
| Diameter (mm) | 7.42±0.02 | 7.23± 0.06 | 7.73±1.43 | 7.64±1.85 |
| Thickness (mm) | 3.46± 0.04 | 3.53±0.02 | 3.71±0.38 | 3.85±54 |
| Hardness (N) | 109.8± 15.6 | 125.5±8,2 | 100.3±41.4 | 127.2±36.7 |
| Friability (%) | 0.05±0.01 | 0.03±0.1 | 0.11±0.02 | 0.02±0.1 |
| Acid resistance pH 1.2 (min.) | 13: 41± 0.44 | 15:12 ±0.02 | 34:27 ±2.08 | 31:22 ±4.1 |
| Disintegration in pH 6.8 (min.) | 7: 25±0.06 | 10:06±3.1 | 16:34 ±8.2 | 15:58±0.7 |
| Appearance | Smooth surface | Smooth surface | Smooth surface | Smooth surface |
| Colour | Uniform | Uniform | Uniform | Uniform |
| Defects | None | None | None | None |
Figure 8. Comparison of physical tests parameters of enteric coated tablets within F4 and F5 pre and post 3 months of at accelerated stability study.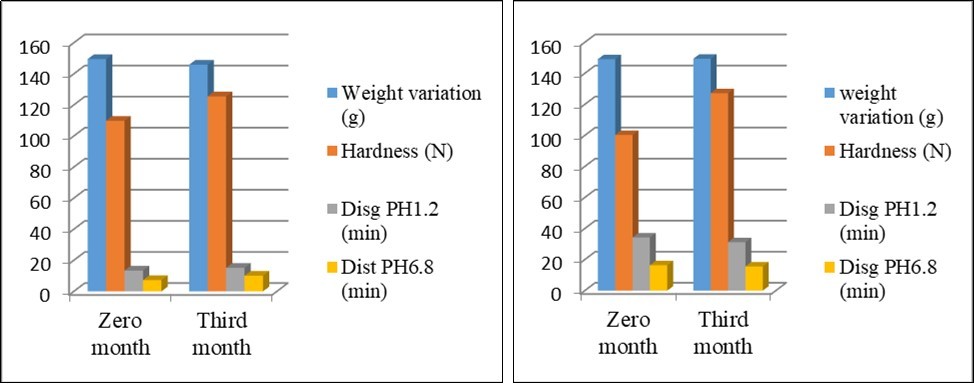
Data Analysis
There is no significant difference (0.999 > 0.05) in physical tests parameters of placebo tablets before and after tablet enteric coating between different enteric coated tablets. Acacia-guar gum enteric coat no effect on tablet physical characteristics.
There is no significant difference of F4 (0.228 > 0.05) and F5 (0.408 > 0.05) in pharmaceutical evaluation results pre and post three months stability study. That concluded the temperature and humidity have no effect on acacia-guar delayed coat and a long shelf life under this test conditions.
Conclusion
In conclusion, the benefits of enteric tablet coatings based on combined approach of using acacia with guar gum as natural polymer suggests the formulator toward using safest natural materials to coat active ingredient in the tablet cores, which has bitter taste or affected by stomach acid media, leads to non-toxicity, cost effectiveness and non hazardous to humans and the environment.
Acknowledgments
This work was supported by Azal Pharma Ltd.- (Khartoum, Sudan) and the laboratory staff of College of Pharmacy at karary University- Sudan for providing laboratory facilities to carry out the experiments. Finally much thankful to Wafra Pharma Ltd.- (Khartoum, Sudan) for area of work of coating process.
