Abstract
Objective:
This study aimed to evaluate the relation of Lactate dehydrogenase (LDH) levels with stage of the disease and it role in monitoring tumor response to therapy in lymphoma patients.
Methods.
LDH levels were evaluated on 65 diagnosed Algerian children and compared to healthy control.
Results:
Our results revealed that LDH levels were significantly higher in untreated children with both hodgkin’s and non hodgkin’s lymphomas compared to control. Moreover, it was observed that the higher is the stage of disease, the more serum LDH level will be. However, there was a significant fall in serum LDH activity by completion of the chemotherapeutic courses.
Conclusion:
LDH plays an important role in tumor initiation and maintenance. The elevated serum LDH may reflect, release of the enzyme from malignant cells and suggest that they may reflect tumor burden and therefore correlate with disease progression.
Author Contributions
Academic Editor: Fernando Luiz Affonso Fonseca, Head of Laboratório de Análises Clínicas da Faculdade de Medicina do ABC, Santo André, SP, Brazil.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2017 Galleze A, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Lymphoma is a group of malignancies characterized by malignant cell infiltrations of the lymphatic system. It represents the third most common cancer in children and has classically been divided into two distinct groups : Hodgkin’s lymphoma (HL) and non-Hodgkin’s lymphoma (NHL). HL is a common lymph node cancer of germinal center B-cell origin, which is characterized by malignant Hodgkin and Reed-Sternberg (HRS) cells mixed with a dominant background population of reactive lymphocytes and other inflammatory cells1. The most accepted histologic classification, the Rye classification system, defines four histologic subtypes of HL : lymphocytic predominace (LP), mixed cellularity (MC), lymphocytic depletion (LD), and nodular sclerosis (NS)2. NHL refers to a group of diverse tumors derived from cells of lymphocytic lineage. It occurs as clonal proliferations of single cells at any stage of lymphocyte development. In children, lymphoblastic lymphoma, Burkitt lymphoma and anaplastic large-cell lymphoma predominate3. There is male predominance in childhood NHL, except for primary mediastinal large B-cell lymphoma, which has nearly the same incidence in both sexe. In contrast to adult NHL, childhood NHL are typically high grade, always diffuse and often associated with extra-nodal diseases, but with intensive multiagent chemotherapy, the outcome is favorable4.
Lactate dehydrogenase (LDH), a cytoplasmic enzyme, reversibly catalyzes the conversion of pyruvate to lactate, which is the last step of glycolysis. Even under normal oxygen concentrations in malignancies, pyruvate transformation to lactate is upregulated5. The tumor microenvironment acidification can promote tumor progression and metastasis 6. There are many tissues in which LDH is widely expressed, such as heart,muscle, and various tumors, and it is detectable in serum. High serum lactate dehydrogenase (SLDH) levels have been reported as a poor prognostic indicator in non small cell lung cancer, malignant lymphoma, pancreatic carcinoma, and colorectal cancer7, 8, 9, 10. Furthermore, current European and American Joint Committee on Cancer (AJCC) recommend SLDH as a staging and progression marker in melanoma11. In addition, high LDH protein expression also correlates with poor outcome and metastasis in many solid tumors 12.
In this study, serum LDH level was measured in Algerian children with lymphoma and itsrelation with clinical presentation, stage of the disease and response to therapy was investigated in patients with Hodgkin's and non-Hodgkin's lymphoma.
Materials and Methods
This study was carried out with blood samples taken from 65 children, 32 NHL and 33 HD. The mean age was 11 years, while age range between 4 and 18 years. The mean age at disease onset was 7 ranging from 1 to 12 years. Patients came from the Department of Hematology, University Hospital Center of Beni Messous, Algeria. They had already a confirmed histologic diagnosis of lymphoma. The clinical data of age, gender, stage of disease and histologic subtype were recorded. Patients were treated for 14 weeks with LBM 2001 group B protocol (Prednisone, cyclophosphamide, vincristine, MTX) for non-Hodgkin's lymphoma and ABVD protocol (Adreamycien, bleomycin, vinblastine And dacarbazine) for hodgkin's disease. The patients received, according to their stage, two or three cures. The control group consisted of 75 unrelated healthy subjects, with no chronic or autoimmune disease and no personal or family history. The mean age was 13 years (Table 1). Serum LDH and other biochemical and hematological parameters were measured spectrophotometrically using biochemical analyzer. LDH level of > 470 U/L is considered high according to ILabTest TM LDH-P (Normal range is 235-470U/L). Statistical analysis was performed and results are expressed as mean±SD. Differences between lymphoma and control groups were assessed using the Student’s t test. Regression and correlation analyses between variables were performed by calculating Pearson’s correlation coefficients (r). P values of > 0.05 were considered not significant.
Table 1. Clinical characteristics of lymphoma patients| Characteristics | Patients (n=65) | |
| HL (n=33) | NHL (n=32) | |
| Age (years) | ||
| Mean | 11 | 11 |
| Range | 18-Apr | 18-Apr |
| Age at disease onset (years) | ||
| Mean | 7 | 7 |
| Range | 12-Jan | 13-Feb |
| Gender | 72.72 | 78.12 |
| Male (%) | 27.27 | 21.87 |
| Female (%) | ||
| Histological types | - | 37.5 |
| Thoracic (%) | - | 46.87 |
| Abdominal (%) | - | 15.62 |
| Maxillary (%) | 54.54 | - |
| Nodular sclerosis (%) | 18.18 | - |
| Lymphocyte-rich (%) | 27.27 | - |
| Mixed cellularity (%) | 0 | - |
| Lymphocyte-depleted (%) | ||
| Stage | 24.24 | 15.62 |
| I (%) | 51.51 | 37.5 |
| II (%) | 24.24 | 46.87 |
| III (%) | 0 | 0 |
| IV (%) | ||
Results:
Analysis of LDH concentration shows that the serum LDH level was significantly higher in untreated children with both hodgkin’s and non hodgkin’s lymphomas compared to control group (p<0.001). Furthermore, this level increased during disease growth time. When HL and NHL patients were separately studied against the control group the difference was significantly high, more remarkably in NHL patients. On the other hand, the mean serum LDH level in NHL patients was significantly higher than that of HL (646.72±51.02 vs. 557.85±44.16 , p=0.01) Figure 1.
Figure 1.Increased Serum LDH levels in patients compared to control. ***p < 0.001.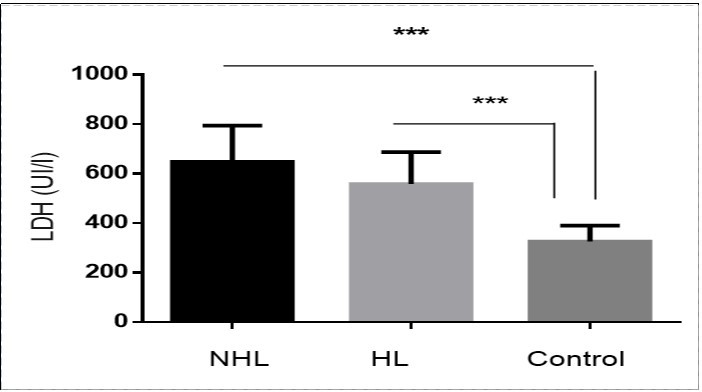
The effect of chemotherapeutic treatment on serum LDH level in HL and NHL patients is shown in Figure 2. As illustrated, there was a significant fall in serum LDH activity by completion of the chemotherapeutic courses in both HL and NHL groups of patients (360.85 ± 15.36 vs. 557.85 ± 44.16, p<0.001 ; 345.50± 21.49 vs. 646.72 ± 51.02 , p<0.001).
Figure 2.Deceased serum LDH levels in treated patients. ***P< 0.001.
The relation of extent of the disease represented by stage of lymphoma to the mean serum LDH level is shown in Figure 3. It was observed that the higher is the stage of disease, the more serum LDH level will be. This difference was notably high among NHL groups. In fact, The serum LDH level was significantly higher in patients in stage III + IV than in patients in stage I + II in HL (735.38 ± 73.40 vs. 501.04 ± 28.26, p<0.0001) and NHL patients (771.07 ± 49.49 vs. 537.00 ± 37.88, p<0.0001). The survival rate for five years after the treatment was 93.75%. The analysis of LDH levels of diagnosed patients against their survival rates, showed that those with higher LDH levels were exposed to early relapse of their disease. However, there was no significant correlation between LDH level before the treatment with survival rate.
Figure 3.Increased serum LDH level in patients in stage III + IV, ***p < 0.001.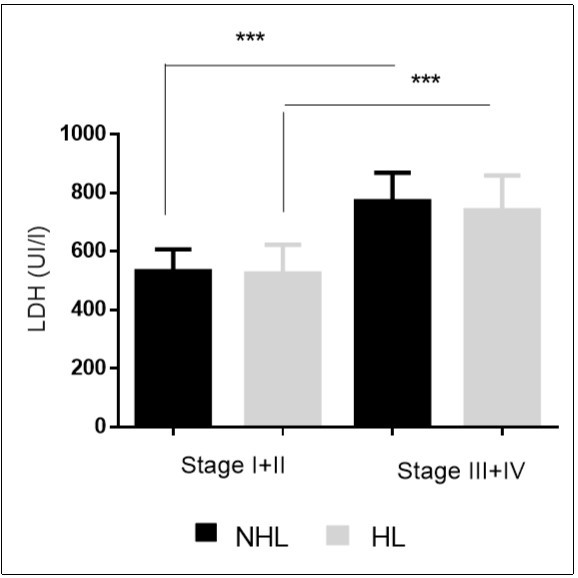
Liver and kidney function tests and blood cell count in total HD and NHL patients were compared to control. The levels of albumin and hemoglobin were significantly lower in lymphoma patients compared to control (p<0.001)( Figure 4). However, the total WBC count and Erythrocyte sedimentation rate were significantly higher in lymphoma patients compared to control (p<0.001)( Figure 5).
Pearson correlation analysis of serum LDH among HL and NHL patient groups showed no correlation between serum LDH levels and biochemical or hematological parameters (Table 2).
Figure 4.Decreased levels of albumin and hemoglobin in patients.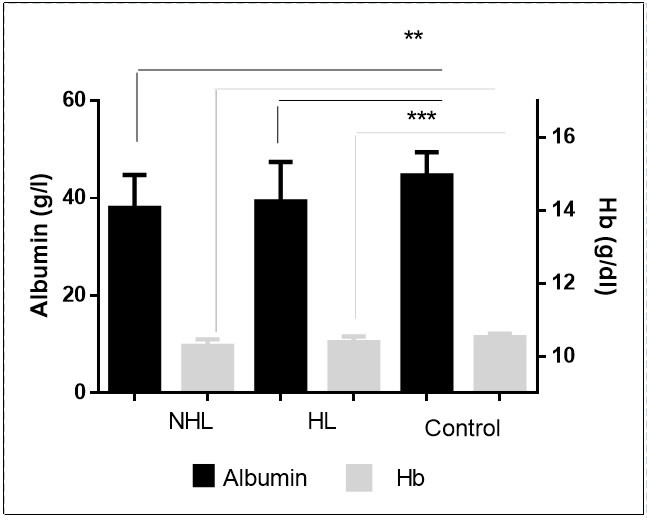
Figure 5.Increased Total WBC count and ESR in patients.
| NHL | HL | |
| PAL | 0.03 | -0.12 |
| ALT | 0.09 | 0.32 |
| AST | -0.04 | 0.03 |
| Alb | -0.03 | 0.08 |
| UA | 0.22 | 0.26 |
| Urea | -0.04 | 0.22 |
| Creat | -0.18 | -0.08 |
| WBC | -0.08 | 0.1 |
| RBC | -0.03 | -0.14 |
| Hb | -0.12 | -0.03 |
| Platelets | 0.35 | 0.29 |
| ESR | -0.18 | -0.19 |
Discussion
Among biochemical parameters, lactate dehydrogenase (LDH) represents a very valuable enzyme in patients with malignant lymphomas. The serum level has been considered as very important in the evaluation of disease extension in non- Hodgkin's lymphomas (NHL) and Hodgkin's disease (HD)13.
In this study, we have analazed the relation between serum LDH level, stage and response to treatment on 33 patients with HL and 32 patients with NHL. The LDH level was significantly elevated in lymphoma patients compared to control. This finding agrees with that, reported by other investigators14, 15.This intense LDH level observed in patients during cancerous conditions may be the result of high glycolytic rate16. In fact, the high glycolytic rate is important for rapidly proliferating cancers
Protein kinases, various hormones and growth factors have been shown to regulate LDH gene expression20, 21. In most cases, these factors induce a shift towards A-subunit containing isoenzymes which can derive more energy under anaerobic conditions. Furthermore, it has been shown that TNFα is capable of inducing LDH-A expression in cultured Sertoli cells22. Human lymphoma cells obtained from fresh tumor samples have been shown to produce TNF23. It is therefore possible that the serum LDH alterations observed in lymphoma patients may be due to the production of inflammatory cytokines. The importante of serum LDH as a direct indicator of tumor burden has already been pointed out in other clinical studies24, 25. Indeed, mechanisms for energy production involved in cell duplication require a high LDH cell content and renewal of NAD resynthesis, in support of a continuing glycolysis. For a given tumor bulk LDH production is conceived as being proportional to its metabolic and proliferative activity. Accordingly, high LDH production suggests either large tumor bulk or a fast proliferation in a smaller tumor, and this could explain an aggressive course and a poorer response to therapy26, 27.
In addition, the tumor cells may produce LDH enzyme to respond to the increase of lactic acid, resulting in oxidtive reductive reaction to become pyruvic acid. High LDH level describes high aggressiveness and proliferation of the tumor cells. Therefore, LDH level in NHL is a marker of cell turnover and correlated with tumor burden. Several studies showed correlation between LDH level and the result of therapy. High LDH level often provides less response to the result of therapy, often relapses, and has a potential of metastasis28.
Lactate efflux provokes a local inflammatory response that attracts immune cells such as macrophages, which secrete cytokines and growth factors that drive tumor cell growth and metastasis30, 31. Indeed, the inflammatory response is often necessary for tumor progression, and elevated numbers of inflammatory cells, such as tumor-associated macrophages, connote poor prognosis31. Furthermore, lactate in the tumor cell milieu impairs the adaptive immune response, disabling immune surveillance 32, 33, 34. Thus, lactate also appears to promote tumorigenesis via non–tumor cell mediated effects on the inflammatory and immune responses.
Conclusion
Lactate dehydrogenase (LDH) plays an important role in tumor initiation and maintenance since its ability to function in anaerobic metabolism helps cancer cells grow even after their rapid proliferation leads to low-oxygen conditions in the tumor microenvironment. The elevated serum LDH could be used to select patients who will benefit from more intensified treatment.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 2.Franziska C Eberle, Mani Haresh, Elaine S Jaffe. (2009) Histopathology of Hodgkin’s Lymphoma. , Cancer J 15, 129-137.
- 3.Truong T H, Weitzman S, Arceci R J. (2013) . Non-Hodgkin Lymphoma of Childhood. Neoplastic Diseases of the Blood 1049-1072.
- 4.Truong T H, Weitzman S, Arceci R J. (2013) . Non-Hodgkin Lymphoma of Childhood. Neoplastic Diseases of the Blood 1049-1072.
- 5.W H Koppenol, P L Bounds, C V Dang. (2011) Otto Warburg’s contributions to current concepts of cancer metabolism. , Nature Reviews Cancer 11(5), 325-337.
- 6.D A Tennant, R V Dur´an, Gottlieb E. (2010) Targeting metabolic transformation for cancer therapy.Nature Reviews Cancer. 10(4), 267-277.
- 7.Danner B C, Didilis V N, Wiemeyer S, Stojanovic T, Kitz J et al. (2010) Long-term survival is linked to serum LDH and partly to tumour LDH- 5 in NSCLC. , Anticancer Research 30(4), 1347-1351.
- 8.Yi J H, Kim J H, Baek K K, Lim T, Lee D J et al. (2011) ElevatedLDHandparanasal sinus involvement are risk factors for central nervous system involvement in patients with peripheral T-cell lymphoma. Annals of Oncology. 22(7), 1636-1643.
- 9.Tas F, Aykan F, Alici S, Kaytan E, Aydiner A et al. (2001) Prognostic factors in pancreatic carcinoma: serum LDH levels predict survival in metastatic disease. , American Journal of Clinical Oncology 547-550.
- 10.Fahmueller Y N, Nagel D, Hoffmann R T, Tatsch K, Jakobs T et al. (2012) Predictive and prognostic value of circulating nucleosomes and serum biomarkers in patients with metastasized colorectal cancer undergoing Selective Internal Radiation Therapy. , BMC Cancer 12(5), 1471-2407.
- 11.Garbe C, Peris K, Hauschild A, Saiag P, Middleton M et al. (2012) Diagnosis and treatment of melanoma. European consensus-based interdisciplinary guideline-update. , European Journal of Cancer 48(15), 2375-2390.
- 12.Kolev Y, Uetake H, Takagi Y, Sugihara K. (2008) Lactate dehydrogenase-5 (LDH-5) expression in human gastric cancer: Association with hypoxia-inducible factor (HIF-1𝛼) pathway, angiogenic factors production and poor prognosis. Annals of Surgical Oncology. 15(8), 2336-2344.
- 13.Tahannejad Z, Dayer D, Samie M. (2012) . The levels of Serum Alkaline Phosphatase and Lactate Dehydrogenase in Hodgkin Lymphoma. IJBC 3, 125-128.
- 14.Akinlolu A A, Salau B, Akingbola T. (2011) Evaluation of Lactate Dehydrogenase Activities in Adult Nigerians Affected with Hematological Malignancies. The FASEB Journal. 25, 686-8.
- 15.Lu R,Jiang M,Chen Z,Xu X,Hu H,Zhao X,Gao X,Guo L. Lactate dehydrogenase 5 expression in Non-Hodgkin lymphoma is associated with the induced hypoxia regulated protein and poor. prognosis.PLoSOne.2013 ; 8(9):e74853. doi: 10. 1371 / journal . pone 0074853-23.
- 16.Surya Surendren P, Jayanthi G, K R Smitha. (2012) . In Vitro Evaluation of the Anticancer Effect of Methanolic Extract of Alstonia scholaris Leaves on Mammary Carcinoma. Journal of Applied Pharmaceutical Science 02(05), 142-149.
- 17.Seyfried T N, Flores R E, Poff A M, D’Agostino D P. (2014) Cancer as a metabolic disease: implications for novel therapeutics. , Carcinogenesis 35(3), 515-27.
- 18.Lei Y, Xiaohong X, Chunlei P, Jinzhi W, Zhuchen S et al.Prognostic values of serum LDH and β2-MG in patients with non-Hodgkin’s lymphoma. , Chinese-German Journal of Clinical Oncology.2009 ; 8(6), 353-355.
- 19.Suzuki K,Terui Y,Nishimura N,Mishima Y,SakajiriS,Yokoyama M,Takahashi S,TsuyamaN,Takeuchi K,HatakeK. Prognostic value of C-reactive protein, lactase dehydrogenase and anemia in recurrent or refractory aggressive lymphoma.JpnJ. Clin Oncol.2013 ; 43(1), 37-44.
- 20.Li X,Qin C,Burghardt R,Safe S. Hormonal regulation of lactate dehydrogenase-A through activation of protein kinase C pathways. in MCF-7 breast cancer cells.BiochemBiophysResCommun.2004 ; 320(3), 625-34.
- 21.FinotF,Masson R,DesmotsF,Ribault C,Bichet N,VericatJA,LafougeP,Guguen-GuillouzoC,Loyer P. Combined Stimulation with the Tumor Necrosis Factor α and the. Epidermal Growth Factor Promotes the Proliferation of Hepatocytes in Rat Liver Cultured Slices. 2012.Int JHepatol.2012 ; 785-786.
- 22.Nehar D, Mauduit C, Boussouar F, Benahmed M. (1998) Tumor necrosis factor alpha-stimulated lactate production is linked to lactate dehydrogenase A expression and activity increase in porcine culture Sertoli cells. , Endocrinology 138, 1964-1971.
- 23.Voorzanger N, Touitou R, Garcia E, Delecluse H J, Rousset F et al.Interleukin (IL)-10 and IL-6 are producedin vivoby non-Hodgkin’s lymphoma cells and act as cooperative growth factors.CancerRes.1996;. 56, 5499-5505.
- 24.Shanique R Palmer.Lori A Erickson, Ilia Ichetovkin, Daniel. Svetomir N Markovic. (2011)CirculatingSerologicandMolecularBiomarkersinMalignantMelanoma.Mayo Clin Proc 86(10), 981-990.
- 25.GobbiPG BergonziM, Bassi E, MerliF CorianiC, StelitanoC IannittoE, Federico M.Tumor burden in Hodgkin’s lymphoma can be reliably estimated from a few staging parameters.OncolRep.2012. 28(3), 815-20.
- 26.Relander T, Johnson N A, Farinha P, Connors J M, Sehn L H et al. (2010) Prognostic factors in follicular lymphoma.J. , ClinOncol 28(17), 2902-13.
- 27.Federico M, Molica S, Bellei M, Luminari S. (2009) . Prognostic factors in low-grade non-Hodgkin lymphomas.CurrHematolMaligRep 4(4), 202-10.
- 28.Lee H, Yuh Y, Kim S. (2009) lactate dehydrogenase (LDH) level as a prognostic factor for the patients with advanced gastric cancer. , JCO 28(17), 2902-2913.
- 29.Yabu M, Shime H, Hara H, Saito T, Matsumoto M et al. (2011) IL-23-dependent and –independent enhancement pathways of IL-17A production by lactic acid.IntImmunol. 23(1), 29-41.
- 30.Shime H, Yabu M, Akazawa T, Kodama K, Matsumoto M et al. (2008) Tumor-secreted lactic acid promotes IL-23/IL-17 proinflammatory pathway.JImmunol. 180(11), 7175-7183.
- 31.Dietl K, Renner K, Dettmer K, Timischl B, Eberhart K et al. (2010) Lactic acid and acidification inhibit TNF secretion and glycolysis of human monocytes.JImmunol. 184(3), 1200-1209.
- 32.Fischer K, Hoffmann P, Voelkl S, Meidenbauer N, Ammer J et al. (2007) Inhibitory effect of tumor cell-derived lactic acid on human T cells.Blood. 109(9), 3812-3819.
Cited by (3)
- 1.Khalil Marwa Mohammed Ibrahim Mohammed, Sohaib Ahmed, Mansour Manal Monir, El Sayed Ramadan Genena Shaimaa, 2021, The role of ZNF384, DNAH17, and NDST2 gene expression in non-Hodgkin's lymphoma patients, Gene Reports, 25(), 101354, 10.1016/j.genrep.2021.101354
- 2.Ahmadi Salman, Yousef Mardoukhi Mohammad Saba, Salehi Mahmoud, Sajjadi Sharareh, Keihan Amir Homayoun, 2019, Molecular dynamics simulation of lactate dehydrogenase adsorption onto pristine and carboxylic-functionalized graphene, Molecular Simulation, 45(16), 1305, 10.1080/08927022.2019.1632447
- 3.Sharma Akshay, Sharma Anshu, 2021, ROLE OF SERUM LDH LEVELS IN PROGNOSTICATION OF PATIENTS DIAGNOSED OF HODGKIN'S LYMPHOMA: A PROSPECTIVE COHORT STUDY, GLOBAL JOURNAL FOR RESEARCH ANALYSIS, (), 13, 10.36106/gjra/1809573