Abstract
Objective:
To utilize the effectiveness of MR-Enterography as a potential investigating marker for the diagnosis of Crohn’s disease.
Conclusion:
This article reviews the technique of performing MR enterography which aids in the diagnosis of Crohn’s disease, it also serves accurate information about the severity of the disease and complications of it that may guide for the surgical or medical treatment.
Author Contributions
Academic Editor: Shree Ram Singh, Mouse Cancer Genetics Program, National Cancer Institute, Frederick, MD, USA.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2014 Hasan Aydın, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Magnetic resonance enterography(MRE) is a new promising technique that may combine both the cross-sectional imaging, conventional enterography and enteroclysis1, 2, 3. MRE has become preferred approach for the diagnosis of inflammatory bowel diseases, tumors, malabsorbtion etc because of its high soft-tissue contrast resolution and multi-planar imaging capability1, 3, 4. Lack of radiation exposure and superposition of bowel loops, its use in pregnancy and in patients with iodine allergy are the other advantages of MRE2, 5. The other MRI based techniques are the routine dynamic abdominal MRI and MR Enteroclysis which present the disadvantages of high costs, motion artefacts and long scanning time, motion artefacts can be eliminated by some fast imaging sequences2, 4, 5, 6 MR Enteroclysis has the disadvantage of nasojejunal intubation which is performed under flouroscopy and exposure to ionizing radiation, MRE eliminates this disadvantage with the administration of oral contrast agent3, 5, 6, 7.A few limitations for MRE are reported up to now, those are: Patients intolerance for drinking the Oral contrast agents(OCA), nausea and vomit of the patients, patients dyscomfort in the magnet and claustrophobia, general contraindications against MR imaging and magnetic field due to inappropriate instruments of the patients against the magnet like metalic prosthesis and implants, cardiac pacemakers, metalic sutures,etc.
Adequate small bowel distention is vital for an optimal enterographic examination as collapsed or contracted small bowel loops may mimic some pathologies, mask some intraluminal lesions or may cause overdiagnosis with exaggerating of wall thickness3,6, 7, 8. OCA has been administered to ensure the optimal bowel distention, they are classified into three groups: Negative-Positive- Biphasic intestinal contrast agents4, 5. Negative OCA’s are hypointense in both T1 and T2 weighted(W) images, positive OCA’s are hyperintense in T1 and T2W images, biphasic OCA’s are hypointense on T1W and hyperintense on T2W images2, 4, 5. Negative ones are, CO2-02-Iron oxide particules-oral superparamagnetic agents and perfloroctylbromide, positive agents are; Gadolinium chelates , ferric/manganese ions, milk, blackberry juice, gren tea and ice cream, biphasic ones are: Water, mannitol, sorbitol, lactulose, polyethylene glycole, low dose barium, manganese compounds and etc5. All those agents may prevent the rapid absorbtion of water, increase bowel distention due to their osmotic characteristics, nausea-vomiting-diarrhea and abdominal dyscomfort are the side effects of OCA’s4, 5.
Capsule endoscopy, balloon-assissted endoscopy, CT enterography and enteroclysis, conventional endoscopy with biopsy,conventional small intestine graphy are the other examination techniques for the diagnosis of Crohn’s disease 3, 9.These methods require high technical expertise, time consuming CT-based techniques cause radiation exposure, endoscopic approaches and conventional scopic bowel graphy with oral Barium contrast can be associated with false positive findings , incomplete bowel wall evaluation and risk of bowel injury3, 5, 9. MRE has precise superiority over all those diagnostic methods with higher sensitivity and specificity without any ionizing radiation exposure.
In this review; the utility of MRE, its routine applications and performance in the daily practice will be emphasized for the diagnosis of Crohn’s disease and patient’s follow-up.
MR-Enterography Technique and Protocol
In our protocol; We need at least 6 hours of fasting from every patient, 5 gr.of methylcellulose is mixed with 500 ml water, then 667 mg/ml Osmolac, Biofarma, Turkey which constitutes about 150-200 mg Lactulose and 250 mg/ml E.Z-CAT, E-Z-EM, Quebec,Canada which involves 250 ml Barium with sorbitol, are added to the first mixture in which water and methycellulose exist. Afterwards, cold water is added to the mixture to make it about 1.5 lt. We request from each patient to drink this mixture with a rate of 300 ml/10 min. so in about 50 min, all the enterographic OCA will be finished by the patient. This mixture is a biphasic OCA, well tolerated by the patients with less side effects including nausea-vomiting and abdominal ache. It provides an adequate bowel wall distention which aids in the visualization of intestinal folds and loops4, 5, 10.
20 mg intravenous(IV) Buscopan, Hyoscine-N-butyl bromide can be administered to prevent intestinal spasm and abdominal dyscomfort of the patients, 1 mg Glucagon subcutaneously or 0.3 mg IV Scopolamine can also be used as spasmolytic agents4, 5.Current MRE protocol is: Fat-saturated(FS) T2 weighted(W) sequences in axial and coronal planes, T2W gradient echo breath-hold sequence in axial plane, precontrast T1W-WATS and fat-saturated 3D-T1W gradient-echo sequence in coronal planes, post-contrast dynamic T1W-gradient echo sequences in axial and coronal planes2, 5, 7, 10, 11, 12, 13.Post-contrast dynamic images are often obtained at arterial-portal and late venous phases. The all MRE acquisitions are about 20-25 min of duration per patient.All MR-Enterography procedure was performed at 1.5 T Magnet, Achievva HB, Philips,Netherland..
Diffusion-weighted imaging(DWI) and magnetization transfer imaging(MTI) are the new imaging sequences and techniques that can be performed for the accurate diagnosis of Crohn’s disease2, 9, 10. In case of active inflammation, restricted diffusion on high-b-value is conducted at DWI in case of Crohn’s disease, whereas infrequent for ulcerative colitis. To my experience, DWI may play a collaborating role in the imaging of patients who can’t tolerate OCA’s or in patients in whom IV contrast agent use is contraindicated5, 9.MTI may reflect the enteric fibrosis and stricture development in Crohn’s disease via transfer of energy from the free water protons inside the lumen, to the macromolecules especially for the collagen fibers at the bowel wall9, 10, 11, 12. Motility of the small intestine can be visualized by cine MRI via fast T2W images or true fast steady-state precession imaging, abnormal bowel motility and inflammatory activity in Crohn’s disease can be shown, based on wall thickness, ulceration and T2 signal intensity 9, 11, 12, 13 (Figure 1a-b).
Assessment of Crohn’s Disease, its Clinical and Imaging Findings.
Crohn’s disease is a chronic inflammatory bowel disease with transmural invasion, causes damage to bowel wall with progression from inflammation to fibrosis over time6, 9,12, 13, 14, 15, 16, 17.It is mostly seen in young adults between 15-30 years of age and slightly predominant in females, can be seen in any part of bowel, but predominant in terminal ileum with invasion to ileoceccal valve, forms enteric abcesses and via fistulization, invades adjacent bowel loops and wall2, 6, 7, 10, 13, 14, 18. It has three main subgroups: Ulcerative-fistulizing and stenosing types, ulcerations of hypertrofied ileum and ileoceccal valve are predominant in ulcerative form, entero-enteric and entero-colic fistulas are the characteristic findings of fistulising form and stenotic segments with skip lesions and dilated pre-obstructed segments are the pathognomonic presentations of stenosing form6, 11, 14,16, 17, 18, 19, 20. Surgical approaches are most evident at stenosing form whereas medical conservative therapy aids more in ulcerative form. In fistulising form, both medical and surgical therapies can be applied6, 7, 8, 9, 10, 11.
Figure 1a.Diffuse mucosal involvement and increased wall thickness with loss of valvula conniventes in the ileal segments on T2W coronal images after OCA administration, seen on 30 years old female with moderate Crohn’s disease.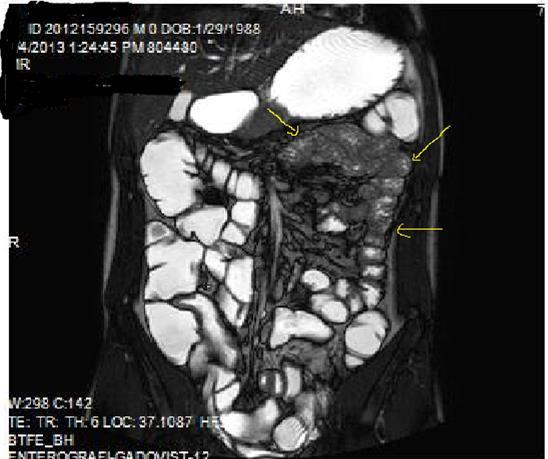
Figure 1b.Diffuse bowel wall enhancement in the small intestine due to Crohn’s disease on the Post-contrast T1W coronal sequence.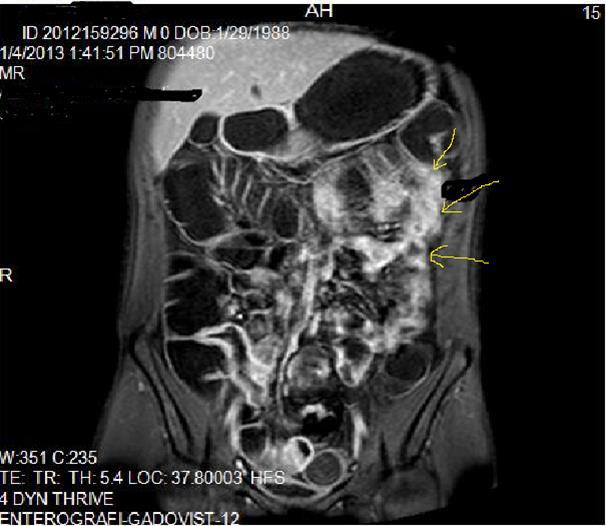
Pathognomonic signs of Crohn’s disease are; Bowel wall thickness must be higher than 2 mm in small intestine, 3 mm in colon, valvula conniventes has to be less than 3 mm, and small intestine diameter will be less than 3 cm, long segment intestinal infiltration, creeping fat sign, loss of haustration in colonic segments, skip lesions between normal and diseased segments and transmural invasion of bowel wall2, 5, 12, 14,18, 19, 20. In endoscopy; Aftos ulcerations and erosions are found out initially in the normal bowel mucosa than at later stages, mural stratification with longitudinal and transverse ulcerations are observed7, 14, 20, 21. On MRI; High bowel wall enhancement is particular at active inflammation, increased signal at bowel wall and adjacent mesentery on T2W images, strictures-fistulization and abcesses are the main complications and hyperintense on T2W images, asymmetrically thickened mucosal folds and lymphadenopathy, mesenteric stranding of the effected bowel wall and prominent draining vessels of the inflamed intestinal loops(comb sign), presence of intramural fat at chronic phase and target sign due to wall thickening at acute phase, surrounding peri-intestinal edema in acute phase are the main findings of Crohn’s disease7, 12, 14,18, 19, 20, 21 (Figure 2a-b)
Mucosal irregularities and ulcerations, strictures and fistulas at chronic stage can also be visualized by enteroclysis3, 4, 14, 15, 22 (Figure 3a-b-c).Clinical signs of Crohn’s disease are; Abdominal ache, diarrhea, fever, loss of weight, malnutrition, hemorrhage and fistulas, its extra-intestinal manifestations are: Arthritis, iridocyclitis, erthytema nodosum, aftoz stomatitis, cholelithiasis, primary sclerosing cholangitis and ankylosing spondylitis8, 9, 10, 11, 12, 13, 14, 15, 16,20, 21, 22.In the differential diagnosis; Ulcerative colitis(UC), ischemic colitis(IC), infectious-fungal and tuberculous(Tbc) enteritis, radiation enteritis, Lymphoma, Behcet’s disease have to be thought5, 6, 7,18, 19, 20, 21 . UC involves the entire colon, small intestine involvement is extremely rare, it tends to involve only the mucosal part of bowel with continuous pattern and skip lesions-transmural pattern of Crohn’s disease involvement can never be seen in UC, fistulization and abscess formations are not evident, terminal ileum involvement of UC is named as backwash ileitis, UC is a pre-malignant inflammatory disease which often causes rectal adenocarcinoma, Malignant transformation of Crohn’s disease is extremely rare 6, 8, 10, 14, 18, 19
Figure 2a.Concentric luminal narrowing, mural and transmural involvement of terminal ileum due to Crohn’s disease in T2W coronal sequence after OCA application.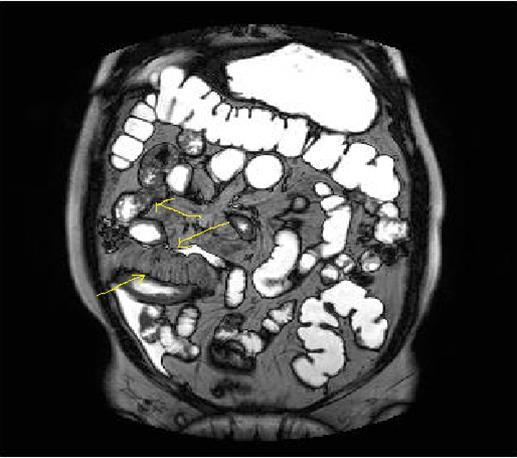
Figure 2b.Curvi-lineer, diffuse peripheral enhancing bowel wall in the terminal ileum on axial T1W post-contrast image.
Figure 3a-3b-3c.Diffuse mucosal involvement and extreme luminal narrowing in the terminal ileum due to Crohn’s disease on T2W coronal sequence after OCA administration, stricture and string sign near ileo-ceccal valve in Figure 3b.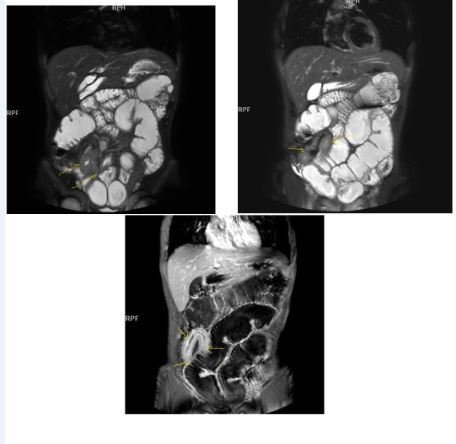
IC is particularly seen in elder patients with predominant vascular changes, especially thrombosis of mesenteric arteries, resulting the reduced blood flow to small intestine, presents low perfusion and loss of contrast enhancement on the bowel wall with submucosal edema or hemorrhage6, 7, 8. Infectious involvement of bowel loops may also mimic Crohn’s disease, Tbc enteritis mainly involve ceccum, then comes the terminal ileum, transverse and star shaped ulcerations on the intestinal mucosa with more evident bowel wall thickening, low segment concentric stenosis, are the major pathognomonic findings of it, lung involvement-calcified mesenteric lymphadenopathy and peritonitis are the common manifestations of Tbc24, 25, 26. In the infectious enteritis, short clinical history and increased peristaltism of involved segments are the major clues in the differential diagnosis, radiation enteritis mostly causes luminal narrowing, mural thickening, adhesions and obstruction, abnormal contrast enhancement in the thickened intestinal wall, especially at terminal ileum and distal colon can frequently be observed14,23, 24, 25, 26, 27. Lymphomas are also most commonly seen in ileum, luminal dilatation with mucosal necrosis and asymmetric bowel wall thickening are the major characteristics of lymphoma’s, moderate contrast enhancement of intestinal wall, preserved mesenteric tissues with lymphadenopathies , long segment involvement, increased wall signal on T2W images are the other common findings, obstruction of intestinal lumen due to lymphoma is quite rare14, 22, 23, 26. Behcet’s disease often presents aftos lineer ulcerations in the terminal ileum and ileoceccal valve, also regard genital-oral and orbital ulcerations but histopathological differentiation from Crohn’s disease, can be necessary in most of the cases due to their similar involvement patterns 14, 26, 28.
Potentials of MR-Enterography in the Diagnosis of Crohn’s disease
Standard MR imaging protocole generally include T2W-enhanced and unenhanced T1W sequences, their importance is mainly based on presenting mural and mucosal characteristics, including ulceration-wall thickness and mural involvement, seen with T2 signal intensity and contrast enhancement, histologically active Crohn’s disease has to be an inflammatory transmural process2, 3, 4, 5, 6, 7,14, 15, 16, 17, 18, 19. Active disease activity is also consistently associated with extramural inflammation such as comb sign, lymph node involvement, fat wrapping and edema of the bowel wall11, 14, 15, 20, 29. Due to the lack of OCA application, standard MR imaging has a limited role in the diagnosis, management and follow-up of Crohn’s disease2, 3, 6. At this point of view, MRE may be currently used for accurate diagnosis and treatment of inflammatory bowel diseases(1, 2, 3, 4,7, 8, 9. Most standard MRE protocols achieve adequate intestinal distention via OCA, mural involvement and enhancement can be depicted by suboptimal bowel distention but, however mucosal ulcerations, skip lesions or strictures may be compromised3, 6, 7, 8, 9, 10.
MRE examines; the number, distribution, shape and thickness of the plicae of intestine, diameter of the intestinal lumen and presence of intra or extra-luminal mass, thickness and contour of colonic wall, presence of abnormal bowel wall enhancement, mural or transmural inflammation, edema or hyperemia of bowel wall, irregularity and ulceration of mucosa, halo or target sign, stenosis or perforation of intestinal segments, mesenteric lympadenopathies, fistula formation, vascular engorgement and presence of other extra-intestinal pathologies 7, 12, 14,16, 17, 18, 19, 20. Summary of MRE findings in Crohn’s disease are as follows:
Possible Findings on T1W Images at Coronal and Axial Plains:
Bowel wall thickening with increased enhancement in the delayed images, precise stranding which extends into the mesenteric border fat, increased size and number of vessels, accordion-like compression and symmetric thickening of folds involving the mesenteric side of the small bowel having a tethered appearance, reactively enlarged adjacent mesenteric nodes2, 7, 12, 14, 18.
Possible Findings on T2W Images at Coronal and Axial Plains:
Bowel wall thickening with increased luminal and extra-luminal signal changes on FS images, showing active inflammation, edema in the adjacent intraperitoneal and mesenteric spaces, active inflammation; bowel wall thickening and enhancement on post-contrast T1W images plus high signal intensity on T2W- FS images7, 9, 12, 14, 17, 18, 19, 20, 21 (Figure 4a-b).
Chronic disease without active inflammation; Bowel wall thickening and enhancement on postgadolinium T1W images plus low signal intensity on T2W-FS images with possible stenosis and obstruction due to fibrosis, chronic disease with active inflammatoryexacerbations;these conditions can overlap with active inflammation, require longitudinal repeated scanning7, 12, 14, 20,26, 27, 28, 29, 30 (Figure 5a-b, Figure 6a-b)
Figure 4a.Mucosal involvement and lack of opacified ileal segments with loss of intestinal folds and increased bowel wall thickness due to Crohn’s disease on T2W coronal sequence after OCA, presented at 37 years old male with moderate Crohn’s disease.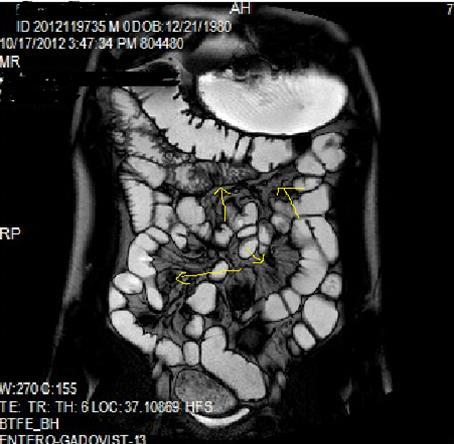
Figure 4b.Mucosal, luminal and bowel wall involvement in Crohn’s disease with skipped lesions, seen at T2W axial image after OCA, presented at 45 years old male with severe inflammatory disease.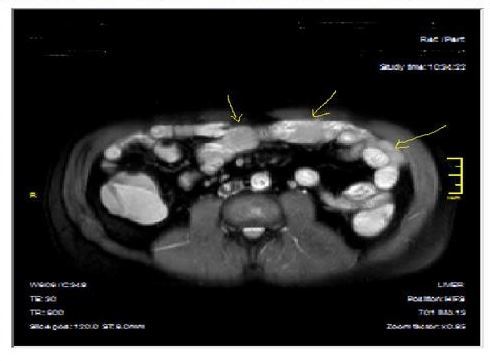
Figure 5a.Enhancing bowel segments due to Crohn’s disease, on T1W post-contrast axial image, luminal enhancement is predominant, shown at 62 years old female with mild disease status.
Figure 5b.İrregular bowel wall and luminal enhancement of ileum, with loss of valvula conniventes on T1W coronal post-contrast sequence, seen on 26 years old male with moderate inflammatory bowel disease.
Figure 6a.Diffuse homogeneous contrast enhancement on the bowel wall and mucosa due to Crohn’s disease, seen at T1W post-contrast axial image, indicated at 23 years old male with mild disease status.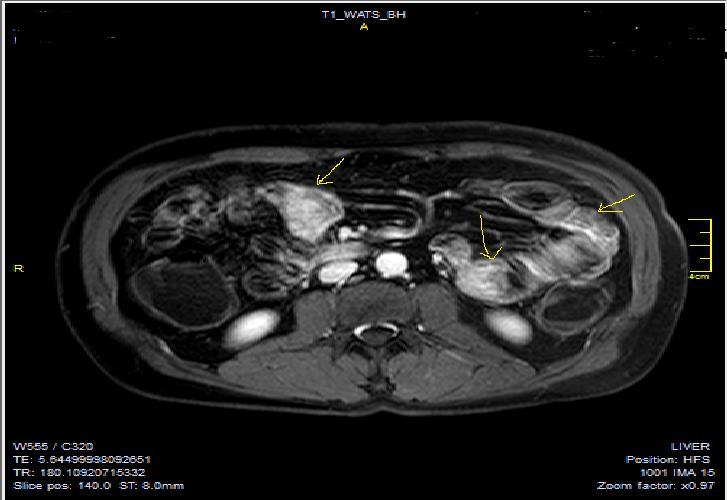
Figure 6b.Heterogeneous bowel wall and mucosa involvement with collaborating skip lesions, specific for Crohn’s disease, predominant in ileum and terminal ileum which are shown at T2W coronal sequence after OCA, regarded at 39 years old female with severe disease.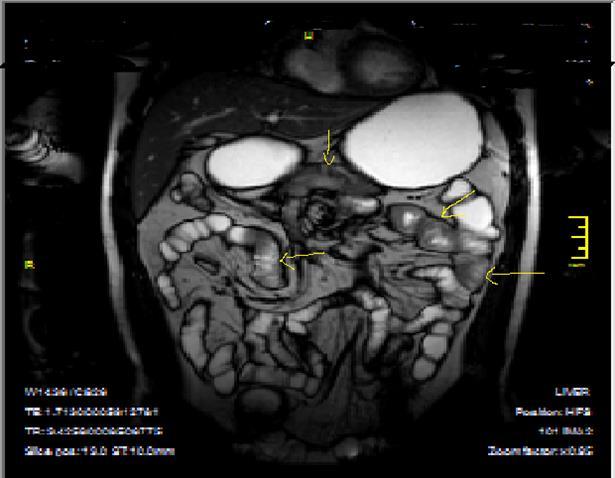
Complications of Crohn’s Disease:
Tethering and strictures, bowel obstruction, extra-enteric collections and abscesses, peri-anal fistulae, visualized by pelvic MRI, extra-enteric complications of Crohn’s disease or other causes of abdominal pain visualized on
MRI, including liver or gallbladder disease (sclerosing cholangitis), mesenteric vascular thrombi, abdominal masses, tumors,and pancreatic abnormalities7, 14, 16, 17, 18, 19, 20, 21.
MRE may also be used for the following clinical applications in Crohn’s disease: Evaluation of the extent of small bowel disease at diagnosis, evaluation of disease burden in symptomatic patients to direct therapeutic management, evaluation of fibrostenotic disease,which may respond better to surgery than to the conservative medical therapy, confirmation of clinical remission and consideration for escalation of medical therapy if there is persistent
submucosal disease despite clinical remissions, evaluation of intra-abdominal complications, including fistulae, tethering, stenosis, and abscesses, evaluation of perianal disease7, 9, 11,16, 17, 18,20, 31.
A number of imaging features may lead to incorrect diagnoses when interpreting MR enterographic images. Although submucosal edema is often present in acute inflamed bowel segments, it is not unusual to misdiagnose extensive submucosal edema as a high-grade obstruction. In such cases, acute inflammation may be present to some extent. The transition between acutely inflamed small bowel and noninflamed but obstructed edematous small bowel can be difficult to delineate3, 7, 14, 20, 29, 30. To my experience, noninflamed obstructed bowel typically has a conspicuous submucosal layer with very low signal intensity on T1W images and very high, bright signal intensity on T2W images, findings due to the presence of submucosal edema. However, the mucosal and serosal layers in noninflamed obstructed bowel are thin and enhance normally(unlike those in acute inflamed segments), and the bowel lumen is dilated. In patients with Crohn’s disease, not all small bowel obstructions are the result of fibrotic strictures, and not all dilated small-bowel segments are obstructed. Peritoneal adhesions are common in Crohn’s disease and may lead to obstruction. Radiologists should look for acutely angled or tethered bowel loops, an abrupt transition in luminal diameter, and an absence of mural thickening. Collapsed bowel segments may appear thickened,with an avidly enhancing appearance which mimic that of active inflammation 3, 6,14, 15, 16,26, 29.
Conclusion
Crohn’s disease is characterized by structural bowel wall damage with progression from inflammation to fibrosis and stricture over time2, 3, 9. MRE already plays an important role in the management,diagnosis and staging of Crohn’s disease of small-bowel. MRE demonstrates active small-bowel inflammation and complications such as bowel obstruction, penetrating disease, and abscess formation without ionizing radiation. At present, MRE is most useful for the assessment of symptomatic patients with known Crohn’s disease. The utility of MRE for the evaluation of mural and transmural involvement of inflammatory bowel disease, aids to discover and consider the pathophysiology of Crohn’s disease, also aids to clinicians in the optimal therapeutic procedures. However, in the future as imaging and interpretation techniques getting improved and undergo further standardization of several diagnostic methods, indications for MRE may expand to include assessment of response to therapy in clinical trials which fulfills and courages radiologists and gastroenterologists to work together for the management of this life-long stayable disease.To my experience, MRE will be a new horizon for the diagnosis of inflammatory bowel diseases and will aid in the routine follow-up of patients during the remission and therapy periods.
Acknowledgements
I really appreciate Dr. Oktay Algin for his help about the style of manuscript and his contributions for the design of all figures.
References
- 1.Mendoza J L, González-Lama Y, Taxonera C, Suárez-Ferrer C, Matute F et al. (2012) Using of magnetic resonance enterography in the management of Crohn’s disease of the small intestine: First year of experience. , Rev Esp Enferm Dig 104, 578-583.
- 2.Belinda L, Tom S, Arian L, Oliver H. (2012) . Small Bowel Crohn’s disease MRI pictorial essay.Journal of Medical Imaging and Radiation Oncology 56: 310-317.
- 3.Markova I, Kluchova K, Zboril R, Mashlan M, Herma M. (2010) Small bowel imaging still a radiologıc approach?. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 154(2), 123-132.
- 4.Grand D J, Beland J T, Machan W W, Mayo-Smith. (2012) Detection of Crohn’s disease: Comparison of CT and MR enterography without anti-peristaltic agents performed on the same day. , European Journal of Radiology 81, 1735-1741.
- 5.Algin O, Evrimler S, Ozmen E, Metin M R, Ocakoglu G et al. (2013) A novel biphasic oral contrast solution for enterographic studies. , J Comput Assist Tomogr 37(1), 65-74.
- 6.Maglinte D T, Gourtsoyiannis N, Rex D, Howard T J, Kelvin F M. (2003) Classification of small bowel Crohn’s subtypes based on multimodality imaging. , Radiol Clin N Am 41, 285-303.
- 7.Martin D R, Kalb B, Sauer C G, Alazraki A, Goldschmid S. (2012) Magnetic resonance enterography in Crohn's disease: techniques, interpretation, and utilization for clinical management. , Diagn Interv Radiol 18(4), 374-86.
- 8.Fallis S A, Murphy P, Sinha R, Hawker P, Gladman L et al.et al.Magnetic resonance enterography in Crohn's disease: A comparison with the findings at surgery. Colorectal Dis.2013Jul19. doi: 10.1111/codi.12361. [Epub ahead of print].
- 9.Makanyanga J C, Taylor S A. (2013) Current and Future role of MR Enterography in the management of Crohn’s disease. , AJR 201, 56-64.
- 10.Amzallag-Bellenger E, Oudjit A, Ruiz A, Cadiot G, Soyer P A et al. (2012) Effectiveness of MR enterography for the assessment of small-bowel diseases beyond Crohn disease. , Radiographics 32(5), 1423-1444.
- 11.Rimola J, Ordás I, Rodriguez S, García-Bosch O, Aceituno M et al. (2011) Magnetic resonance imaging for evaluation of Crohn's disease: validation of parameters of severity and quantitative index of activity. Inflamm Bowel Dis. 17(8), 1759-1768.
- 12.Kayhan A, Oommen J, Dahi F, Oto A. (2010) Magnetic resonance enterography in Crohn’s disease: Standard and advanced techniques.World. , J Radiol 2(4), 113-21.
- 13.Siddiki H, Fidler J. (2009) MR imaging of the small bowel in Crohn's disease. , Eur J Radiol 69(3), 409-17.
- 14.Algin O, Evrimler S, Arslan H. (2013) Advances in radiologic evaluation of small bowel diseases.J. , Comp. Assist.Tomogr 37(6), 862-71.
- 15.Pariente B, Peyrin-Biroulet L, Cohen L, Zagdanski A M, Colombel J F. (2011) Gastroenterology review and perspective: the role of cross-sectional imaging in evaluating bowel damage in Crohn disease. , AJR:AmJ.Roentgenol. 197(1), 42-49.
- 16.Hafeez R, Punwani S, Boulos P, Bloom S, McCartney S et al. (2011) . Diagnostic and therapeutic impact of MR enterography in Crohn’s disease.Clin Radiol 66(12), 1148-1158.
- 17.Messaris E, Chandolias N, Grand D, Pricolo V.(May2010) Role of magnetic resonance enterography in the management of Crohn disease. doi: 10.1001/archsurg.2010.68. Arch Surg. 145(5), 471-5.
- 18.Sinha R, Verma R, Verma S, Rajesh A. (2011) MR enterography of Crohn disease: part 2, imaging and pathologic findings.AJR;AmJ.Roentgenol.197(1):. 80-85.
- 20.Leyendecker J R, Bloomfeld R S, DiSantis D J, Waters G S, Mott R et al. (2009) . MR Enterography in the Management of Patients with Crohn Disease. RadioGraphics 29, 1827-1846.
- 21.Koh D M, Miao Y, Chinn R J, Amin Z, Zeegen R et al. (2001) MR imaging evaluation of the activity of Crohn’s disease. , AJR Am J Roentgenol 177(6), 1325-1332.
- 22.Korman U, Kuruoglu S, Ogut G. (2005) Conventional enteroclysis with complementary MR Enteroclysis: a combination of small bowel imaging. , Abdom Imaging 30, 1-13.
- 24.Nolan D J. (2000) Enteroclysis of non-neoplastic disorders of small intestine. , Eur Radiol 10, 342-353.
- 25.Engin G, Balk E. (2005) Imaging findings of intestinal tuberculosis. , J Comp. Assist Tomog 29, 37-41.
- 26.Maglinte D D. (2006) Small bowel imaging:a rapid changing field and a challange to radiology.Eur Radiol. 16, 967-971.
- 27.Tuohy A M, O’Gorman M, Byington C, Reid B, Jackson W D. (1999) Yersinia enterocolitis mimicking Crohn's disease in a toddler. , Pediatrics 104(3), 36.
- 28.Korman U, Cantasdemir M, Kurugoglu S, Mihmanli I, Soylu N et al. (2003) Enteroclysis findings of intestinal Behcet disease: a comparative study with Crohn disease. , Abdom Imaging 28(3), 308-312.
- 29.Villa C, Pompili G, Franceschelli G, Munari A, Radaelli G et al. (2012) Role of magnetic resonance imaging in evaluation of the activity of perianal Crohn’s disease. , European Journal of Radiology 81, 616-622.
