Abstract
In recent years, the consumption of dietary supplements (DS) has increased worldwide. In Argentina, approximately 14 million DS units were sold between 2015 and 2017. The adulteration of DS with active pharmaceutical ingredients or their analogues has been reported. This represents an alarming emerging risk to public health. The aim of this work was to detect the possible adulteration of a DS marketed in Argentina for the treatment of erectile dysfunction. Initially, thin layer chromatography analysis of the DS capsules content suggested the presence of a major compound. For the isolation and purification of this compound, an easy method consisted of a liquid-liquid extraction (water/CH2Cl2) followed by re-crystallisation from ethanol, is reported. Spectroscopic techniques such as mono- and bidimensional nuclear magnetic resonance, Fourier transform infrared spectroscopy and mass spectrometry allowed its identification as tadalafil. A rapid and reliable method was developed for the quantification of tadalafil in this DS by high performance liquid chromatography-mass spectrometry (HPLC-MS/MS). The mean content of tadalafil per capsule was 21.2 mg which represents a slightly higher value than that found in approved products in Argentina (5 or 20 mg per tablet). In addition, an undeclared alga was identified in the DS by microscopic techniques.
Author Contributions
Academic Editor: Fatma Mady, Minia University Faculty of pharmacy, Egypt.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2018 Flavia Redko, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Since 1998, the use of dietary supplements (DS) in Argentina has been regulated in the “Código Alimentario Argentino” (CAA) (Law no. 18284). DS are defined as “products used to increase the regular dietary intake, supplementing the incorporation of nutrients in the diet of healthy people who, in the absence of pathological conditions, present an unmet dietary need”. In terms of composition, DS should provide nutrients such as proteins, vitamins, minerals, lipids, carbohydrates, and fibres. Some botanicals or extracts thereof can also be used 1. Initially, DS were mainly products based on vitamins, minerals, proteins and fibre, but over the last years, and mainly in response to consumer demands, the profile of these products has changed, and other constituents such as herbal products have been incorporated. In 2001, ANMAT, the food and drug regulatory agency of Argentina, authorised a total of 35 herbs that can be incorporated in DS (ANMAT Res. no. 1637/2001). These herbs are Astragalus membranaceus, Ginkgo biloba, Panax ginseng, Passifloraincarnata, Valeriana officinalis, and Equisetum arvense, among others. These herbs are only permitted if accompanied by vitamins, minerals, carbohydrates, proteins and fibres. The use of DS has increased worldwide as a result of the demand for sexual enhancement, sports performance enhancement and weight loss products. As reported by Harris 2 more than 60 % of people do not disclose the use of DS to their physicians. People obtain information on DS from friends and family, magazines, food stores and the Internet and, they often take them without obtaining adequate information about the products. An important problem arises from the adulteration of these DS with either synthetic drugs or drug analogues 3, 4, which represents an alarming emerging risk to public health. A wide array of DS adulterants has been reported. These substances are added to enhance the properties of the product 5, 6.
In Argentina, approximately 14 million DS units were sold between 2015 and 2017 according to IMS 7 and only one adulteration has been reported 8. According to Vaclavik et al. 9, the products most frequently adulterated with synthetic drugs are those for the treatment of erectile dysfunction (ED), obesity/overweight, diabetes mellitus, hypertension and arthritis.
ED is a widespread health problem defined as the inability to get or maintain an erection during sexual intercourse. It affects ca.150 million men worldwide and this number is expected to increase to about 322 million by 2025 10. Tadalafil, sildenafil and vardenafil are selective inhibitors of the type 5 cGMP-specific phosphodiesterase (PDE-5) approved for the treatment of this condition. These drugs enhance the relaxation of the cavernosal smooth muscle, thus enhancing erection 8. Even though PDE-5 inhibitors have a good safety profile, they are contraindicated in men taking nitrates and they should be administered carefully in subjects under multiple antihypertensive medications, as they can cause a dramatic decrease of the blood pressure 11.
Given that the adulteration of DS is a major problem, we have been screening for the detection of illegal substances in order to discover products that may pose a health risk 8. In this article we report the isolation and the identification, by spectroscopic techniques, of tadalafil as an adulterant in a DS marketed in Argentina for the treatment of ED. A rapid and reliable method was developed for the quantification of tadalafil in this DS by high performance liquid chromatography-mass spectrometry (HPLC-MS/MS). An undeclared alga was also identified in the DS by microscopic techniques.
Material and Methods
Chemical and Reagents
Analytical grade solvents were purchased from Sintorgan (Buenos Aires, Argentina). LiChrosolv® methanol (MeOH) and dichloromethane (CH2Cl2) were supplied by Merck (Darmstadt, Germany). Formic acid was purchased from Baker (New Jersey, USA). Ultrapure water was generated in-house with a Barnstead Thermo Scientific™ purification system (resistance ≥18.2Ω). Analytical thin layer chromatography (TLC) was performed on silicagel 60-coated F254 aluminium plates (Merck KGaA, Germany). Tadalafil working standard was isolated from tablets of CialisTM (20 mg) and its identity and purity was evaluated by nuclear magnetic resonance (NMR), Fourier transform infrared spectroscopy (FT-IR) and mass spectroscopy (MS) and compared to those obtained from tadalafil pharmaceutical secondary standard from Sigma Aldrich. Dimethylsulfoxide-d6 (DMSO-d6, 99.8%) (Sigma, St. Louis, MO, USA) was used as solvent in nuclear magnetic resonance (NMR) experiments.
Instrumentation
Fourier transform infrared spectroscopy (FT-IR) experiments were performed on an Alpha FT-IR Spectrometer (Bruker, Madison WI, USA). FT-IR data were processed with a Bruker OPUS/IR 7.5.18 software and recorded over a spectral range spanning from 4,000 to 400 cm−1 with an optical resolution of 4 cm−1. NMR spectra were recorded on a Bruker Avance III 600 MHz equipped with a cryoprobe. Mono- and bidimensional NMR experiments were run using the programs from the Bruker software. Chemical shift values (δ) were expressed in parts per million (ppm). The HPLC-MS/MS system consisted of an UltiMate 3000 HPLC device coupled to a TSQ Quantum Access MAX Triple Quadrupole Mass Spectrometer with electrospray ionization (Massachusetts, USA). A Thermo Scientific™ Acclaim™ Mixed-Mode HILIC-1 (150 x 4 mm, 5 µm particle size) column was used. Microscopic analysis was performed in a Zeiss Axioscop 2 plus microscope equipped with a Moticam X Wifi camera.
Thin Layer Chromatography (TLC) Analysis
DS samples were obtained in the local market. The powder contained in 3 DS capsules was extracted with CH2Cl2 (3 x 50 mL) with ultrasonic shaking at 25°C for 1 h. The extract was filtered, and the solvent evaporated under vacuum at 40°C yielding the dried extract. The marc was then extracted with MeOH in the same way. Both extracts were analysed by TLC on silicagel 60 F254 using ethylacetate: water: n-butanol (25:50:100, v/v/v) (upper layer) (system I) and chloroform: MeOH: diethylamine (9:1:0.1, v/v/v) (lower layer) (system II) as mobile phases. The detection of spots was done with UV light (254 and 366 nm) and by derivatisation with Draggendorff and anisaldehyde/sulfuric acid reagents.
Isolation of a Major Component of the CH2Cl2 Extract
To isolate the major compound present in the CH2Cl2 extract, the powder contained in 4 capsules (1.42 g) was extracted with water (100 mL), with ultrasonic shaking at 25°C for 15 min. The filtrate was alkalinised with 0.1N NaOH to pH=10 and extracted with CH2Cl2 (3 x 100 mL). The organic phase was evaporated to dryness under vacuum at 40°C. This extract was dissolved in ethanol at room temperature. The insoluble material was collected and re-crystallised from ethanol to yield the target compound.
Identification of the Target Compound by Spectroscopic Techniques
The structure of the isolated compound was determined by 1D (1H- and 13C-) and 2D NMR experiments: distortionless enhancement by polarization transfer (DEPT 135) and heteronuclear single quantum coherence (HSQC), FT-IR and mass spectrometry (MS). FT-IR experiments were performed on solid state using KBr pellets. The crystals obtained from the compound were dissolved in dimethylsulfoxide-d6 (DMSO-d6) for NMR experiments.
HPLC-MS/MS Quantification of the Target Compound
Quantification of tadalafil in capsules was carried out by a HPLC-MS/MS method previously validated according to ICH guidelines 12. A high-efficiency, silica-based, mixed-mode stationary phase HPLC column, consisting of a hydrophobic alkyl chain with a diol group at the terminus, was used. A mixture of 0.1% formic acid in MeOH and 0.1% formic acid in water (95:5 v/v) was used as mobile phase for the isocratic elution. The flow rate was 0.5 mL/min. Temperatures of the oven and the sampler were set at 25°C and 10°C, respectively. The injection volume was 5 µL. MS/MS optimization conditions were made with a working standard solution of tadalafil. Tuning conditions were as follows: spray voltage, 3.0 kV; vaporizer temperature, 193°C; capillary temperature, 266°C; sheath gas pressure and aux gas pressure were set at 10 and 5 units, respectively. Three scan events were performed: single ion monitoring (SIM), selective reaction monitoring (SRM) and data dependent experiments (table 1).
A calibration curve (seven concentration levels) was prepared from a 1.45 mg/mL stock solution of tadalafil working standard in methanolic 0.1% formic acid. The final concentrations ranged from 0.001 µg/mL to 1.5 µg/mL. Each level was analysed by triplicate.
DS samples were prepared as follows: thirty-two capsules were emptied, and their content weighed. The average powder content per capsule was determined. The content of the capsules was then pooled and homogenised (sample powder). Samples were prepared in sextuplicate. An amount of 400±20 mg of sample powder was accurately weighed and transferred to a 25 ml volumetric flask. Around 15 ml of HPLC quality MeOH were added to each sample followed by sonication for 30 min at 25ºC. After allowing samples to reach room temperature, sufficient HPLC MeOH was added to make a final volume of 25 ml. Samples were then filtered through nylon membranes and the filtrates diluted. Two 1/100 serial dilutions in methanolic 0.1% formic acid were made to yield the sample solutions that were injected in duplicate. The content of tadalafil per capsule was calculated by extrapolating values from the calibration curve and referring it to the average capsule content. Results were expressed as mean value ± standard deviation.
Microscopic Analysis of the Capsule Content
The capsule content (dried powder) was analysed by light microscopy at 100X, 200X, 400X and 1000X magnification. Images were captured with a built-in camera.
Results and Discussion
Among the many reasons why DS are popular worldwide include their accessibility, since they are readily acquirable in pharmacies as over-the-counter products, in supermarkets, through internet websites, in natural food stores, in herbal shops and at gyms. People believe that these products are 100 % natural, and therefore, innocuous and safer than pharmaceutical drugs 10.
Adulteration of DS by the addition of synthetic drugs represents a great problem and a major area of concern globally 13. Synthetic compounds are increasingly being detected in DS, turning the detection of these adulterants into an important field of active research. In this context, we have previously described the identification of aminotadalafil, a non-approved tadalafil analogue, as an adulterant in a DS marketed in Argentina for the treatment of ED 8. To the best of our knowledge, this was the first report of an adulteration of a DS in Argentina. In this study we report the presence of tadalafil as an adulterant in another DS also employed for ED and marketed in our country and labeled to contain only herbs and vitamins.
Thin Layer Chromatography (TLC) Analysis
As a first approach, a TLC analysis of CH2Cl2 and MeOH extracts of the capsule content was carried out to analyse the presence of the herbal drugs declared on the label. The TLC analysis of plant extracts normally renders chromatograms with numerous spots corresponding to a wide variety of plant metabolites. Many sources including pharmacopeias such as the US Pharmacopeia-National Formulary and the European Pharmacopoeia, and reference bibliography 14 detail the TLC techniques that can be used to analyse and identify different plant drugs. Normally, when herbal products containing many plant organs are analysed by TLC after an extraction procedure, complex chromatograms are obtained, especially those obtained with methanolic extracts. The TLC chromatograms obtained with the CH2Cl2 and MeOH extracts of the DS showed a small number of spots blurred by a greenish colour. The simplicity of the chromatograms was suspicious, together with the finding that the CH2Cl2 extract presented a major compound. However, the purification of such compound was not easy due to sample matrix complexity, which is known to affect the performance of analytical methods, e.g. co-extraction phenomena may occur 13. Knowing that the majority of the adulterated DS used as sexual enhancers contain approved synthetic PDE-5 inhibitors or their analogues and considering that most of those inhibitors contain nitrogen atoms in their structure 13, 15, 16 we developed a rapid and easy purification method.
Isolation an Identification of the Major Component of the CH2Cl2 Extract
The isolation consisted of a liquid-liquid extraction (water/CH2Cl2) profiting from the acid-base properties of such compounds, followed by re-crystallisation from ethanol, to afford the target compound as white crystals. In our previous work, the isolation of the adulterant was accomplished by a single column chromatography process that yielded the pure compound. In this case, the sample matrix seemed less complex as shown by the TLC and HPLC profiles 8. Spectroscopic data (Figure 1 and Figure 2) allowed the identification of the major compound as tadalafil (Figure 3) 17, 18.
Figure 1.1H- and 13C- NMR spectra of tadalafil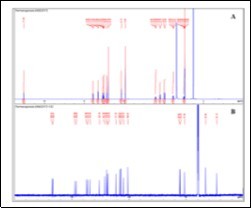
Figure 2.DEPT 135 and HSQC NMR spectra of tadalafil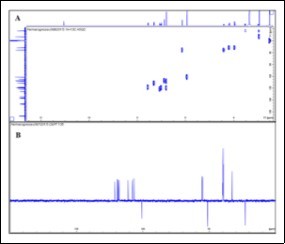
HPLC-MS/MS Quantification of Tadalafil in the DS Capsules
For the quantification of tadalafil in the DS, the HPLC chromatographic conditions needed optimisation. Several methods using liquid chromatography have been previously described for the identification of tadalafil and related compounds 19, 20, 21. Given the chemical characteristics of tadalafil, several additives, such as triethanolamine, trifluoroacetic acid and formic acid have been used to avoid tailing. As shown in figure 3, the tadalafil molecule presents several nitrogen atoms, which interact with the stationary phase resulting in an irregular peak shape. This has been partially improved by the addition of mobile phase modifiers. In our method, we replaced the typical reverse phase by one that combines both reverse-phase and hydrophilic interactions. The Acclaim® Mixed-Mode HILIC-1 column provided by Thermo Scientific was employed. The particular properties of this stationary phase are given by the covalent functionalisation of high-purity spherical silica with a silyl ligand consisting of both an alkyl chain and a diol group. The use of this stationary phase not only avoids peak tailing, thus providing an improved symmetry and sensitivity, but also prevents ionic suppression given by the addition of mobile phase additives and the detriment of the column performance.
For the optimisation of MS/MS conditions, a standard solution of 1.0 µg/mL tadalafil was first infused directly into the spectrometer at a flow rate on 10 µL/min. In a full scan spectrum, the main peak of the standard corresponded to a m/z=390.4, representing the M+H+. Therefore, this was chosen as the precursor ion. Secondly, the most abundant product ions were determined, and their collision energies optimised. These product ions correspond to m/z=268.0 and m/z=240.0 (Table 1). Once the tuning conditions were optimised, the method settings were established. Three scan events were set, which comprised a full scan, a single ion monitoring (SIM) and a data dependent experiment. While the SRM experiment was used for the quantification of tadalafil, the data dependant experiment was employed for the identification of tadalafil by comparing the full spectrum of the peak with that of the standard. Chromatograms and spectra obtained from the standard and sample are shown in figure 4.
Table 1. SIM and SRM parameters for tadalafil| Compound | Parent ion (m/z) | Product ion (m/z) | CE (eV) |
| 240.0 | 59 | ||
| Tadalafil | 390.2 | ||
| 268.0 | 13 |
Figure 4.HPLC chromatograms corresponding to tadalafil standard (a y b) and sample solutions (c y d) in SIM and SRM mode respectively.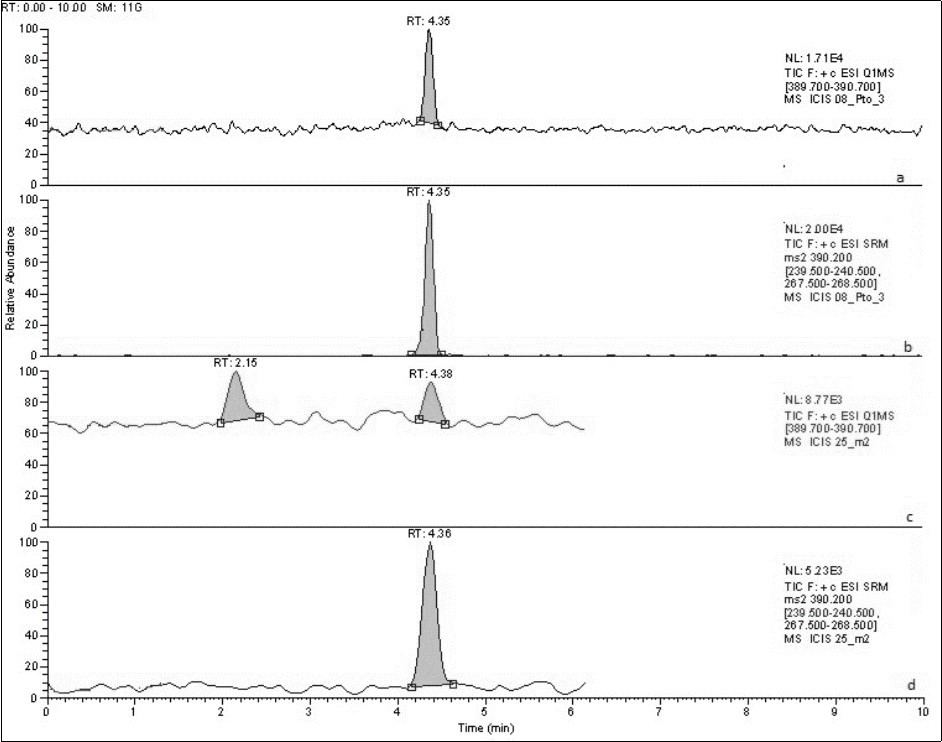
The content of tadalafil in the DS was determined by HPLC-MS/MS. The mean content per capsule was 21.2 ± 2.6 mg which represents a slightly higher value than that found in approved products in Argentina, i.e., 5 or 20 mg per tablet 22. Taking these results into account, it can be concluded that, unlike pharmaceuticals, certain DS are not subjected to a complete quality control process. This entails a substantial variability in the tadalafil content among capsules. Even though the amount of tadalafil found is similar to that found in approved marketed products, the presence of this drug in DS poses a serious health risk. As stated above, most patients consuming DS are unaware of potential adulterations and many consider DS as safe natural products. The common side effects associated with PDE-5 inhibitors include flushing, headache, dyspepsia, back pain, myalgia, dizziness, and rhinitis, but these effects are self-limited. However, some other more serious drug-related problems may occur, such as visual and auditory changes, cardiovascular effects, and alterations of renal and hepatic functions 11. Adulteration with synthetic drugs and their analogues pose a pharmacological and toxicological health risk but also the presence of impurities and degradation products may be of relevance 23.
Microscopic Analysis of the Capsule Content
Microscopic methods are used as part of the identification tests when the DS contains either intact plant material or its powder 4. Microscopic methods can detect other herbal drugs as adulterants as well as the presence of undeclared plant parts. In order to verify the presence of the herbal drugs declared in the DS label, we analysed the capsules content by light microscopy and polarized light microscopy. The analysis showed mainly the presence of the alga Arthrospirasp. and numerous simple starch grains (Figure 5). The presence of Arthrospirawas confirmed by comparison of their powder characteristics with those found in the literature 24. Arthrospirais a genus of cyanobacteria that constitutes dietary supplements commonly known as spirulina. This alga has long been used as food, and although most alga are innocuous, a significant proportion of cyanobacterial species are harmful due to the production of cyanotoxins 25. The possible presence of these toxins in these DS reinforces the need for a better-quality control 26.
Conclusions
From a regulatory perspective, it is necessary to develop rapid and sensitive methods for detecting adulterants in DS. In this paper we report the presence of tadalafil, a drug employed for the treatment of ED, as an adulterant in a DS. For the quantification of tadalafil, a sensitive and reliable HPLC-MS/MS method was developed. This method could be suitable for the analysis of DS in various forms, including tablets, powders, liquids and pills. The presence of spirulina, which was not declared in the label, was also identified by microscopic techniques. As DS are considered foods, they do not require any safety assessment prior to their commercialisation. The methods described for the isolation, purification, identification and quantification of tadalafil in the DS would be useful for the detection of this drug and other related compounds in commercial DS formulations.
Acknowledgments
Authors wish to thank Cátedra de Farmacognosia, Facultad de Farmacia y Bioquímica, Universidad de Buenos Aires and Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) for the use of equipment and facilities.
Compliance with Ethical Standards
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent Not applicable
References
- 1.ANMAT. (2018) Nacional de Medicamentos, Alimentos y Tecnología Médica. Available from: http://www.anmat.gov.ar/Alimentos/Suplementos_Dietarios-Hierbas.pdf.
- 2.I M Harris. (2000) Regulatory and ethical issues with dietary supplements. , Pharmacotherapy 20, 1295-1302.
- 3.da Justa Neves, B D, E D Caldas. (2015) Dietary supplements: International legal framework and adulteration profiles, and characteristics of products on the Brazilian clandestine market. , Regul. Toxicol. Pharmacol 73, 93-104.
- 4.Sarma N, Giancaspro G, Venema J. (2016) Dietary supplements quality analysis tools from the United States Pharmacopeia. Drug Test Anal.8(3-4): 418–423.https://doi: 10.1002/dta.1940.
- 5.M R Cole, C W Fetrow. (2003) Adulteration of Dietary Supplements. , Am. J. Health Syst. Pharm 60, 1576-1580.
- 6.Peddi P, Agrawal S S. (2014) Detection and estimation of synthetic PDE-5 inhibitors viz. Sildenafil and tadalafil in marketed herbal aphrodisiacs. New Trends Pharm. , Sci 1, 1-9.
- 7. (2017) IMS Health. Vitaminas y Suplementos Dietarios. http://noticias.imshealth.com/article.php?q=11&a=2080. [Accessed 12July2018]
- 8.Ulloa J, Sambrotta L, Redko F, O N Mazza, Garrido G et al. (2015) Detection of a tadalafil analogue as an adulterant in a dietary supplement for erectile dysfunction. , J. Sex. Med 12, 152-7.
- 9.Vaclavik L, A J Krynitsky, J I Rader. (2014) Mass spectrometric analysis of pharmaceutical adulterants in products labeled as botanical dietary supplements or herbal remedies: a review. , Anal. Bioanal. Chem 406, 6767-90.
- 10.F El Amrawy, ElAgouri G, Elnoweam O, Aboelazayem S, Farouk E et al. (2016) Adulterated and counterfeit male enhancement nutraceuticals and dietary supplements pose a real threat to the management of erectile dysfunction: A global perspective. , J. Diet. Suppl 13, 660-693.
- 11.F A Yafi, I D Sharlip, E F Becher. (2018) Update on the safety of phosphodiesterase type 5 inhibitors for the treatment of erectile dysfunction. , Sex. Med. Rev 6, 242-252.
- 12. (2005) Food and Drug Administration. ICH Harmonized Tripartite Guideline Validation of Analytical Procedures:. ICH Harmon Tripart Guidel Valid Anal Proced TEXT Methodol Q2(R1) step4.Doi: 10-1017.
- 13.Rocha T, J S Amaral, Oliveira M B P P. (2016) adulteration of dietary supplements by the illegal addition of synthetic drugs: A review. , Compr. Rev. Food Sci. Food Saf 15, 43-62.
- 14.Wagner H, Bladt S. (1996) Plant drug analysis: A thin layer chromatography atlas.Springer Verlag,Berlin.
- 15.Fejôs I, Neumajer G, Béni S, Jankovics P. (2014) Qualitative and quantitative analysis of PDE-5 inhibitors in counterfeit medicines and dietary supplements by HPLC-UV using sildenafil as a sole reference. , J. Pharm. Biomed. Anal 98, 327-333.
- 16.Strano-Rossi S, Odoardi S, Castrignanò E, Serpelloni G, Chiarotti M. (2015) Liquid chromatography-high resolution mass spectrometry (LC-HRMS) determination of stimulants, anorectic drugs and phosphodiesterase 5 inhibitors (PDE5I) in food supplements. , J. Pharm. Biomed. Anal 106, 144-152.
- 17.Mei-Chih L, Yi-Chu L, Yun-Lian L, Jer-Huei L. (2009) Identification of a tadalafil analogue adulterated in a dietary supplement. , J. Food Drug Anal 17, 451-458.
- 18.Abdel-Aziz AA-, Asiri Y A, El-Azab A S, Al-Omar M A, Kunieda T. (2011) . , Tadalafil. Profiles Drug Subst. Excip. Relat. Methodol 36, 287-329.
- 19.Zhu X, Xiao S, Chen B, Zhang F, Yao S. (2005) Simultaneous determination of sildenafil, vardenafil and tadalafil as forbidden components in natural dietary supplements for male sexual potency by high-performance liquid chromatography-electrospray ionization mass spectrometry. , J. Chromatogr. A 1066, 89-95.
- 20.Zhang Y, Huang Z, Ding L, Yan H, Wang M et al. (2010) Simultaneous determination of yohimbine, sildenafil, vardenafil and tadalafil in dietary supplements using high-performance liquid chromatography-tandem mass spectrometry. , J. Sep. Sci 33, 2109-2114.
- 21.Schreiber L, Halko R, Hutta M. (2017) Fast ultra-high-performance liquid chromatography with diode array and mass spectrometry method for determination of tadalafil drug substance and its impurities. , Biomed. Chromatogr 31, 1-8.
- 22. (2018) . Vademecum ANMAT Available from: http://anmatvademecum.servicios.pami.org.ar/index.html. [AccessedJuly2018]
- 23.B J Venhuis, D de Kaste. (2012) Towards a decade of detecting new analogues of sildenafil, tadalafil and vardenafil in food supplements: A history, analytical aspects and health risks. , J. Pharm. Biomed. Anal 69, 196-208.
- 24.Wan D, Wu Q, Kuĉa K. (2016) Spirulina. In: Gupta RC (ed) Nutraceuticals. Efficacy, Safety and Toxicity, Academic Press,London. 556-583.
Cited by (3)
- 1.Ichim Mihael Cristin, Häser Annette, Nick Peter, 2020, Microscopic Authentication of Commercial Herbal Products in the Globalized Market: Potential and Limitations, Frontiers in Pharmacology, 11(), 10.3389/fphar.2020.00876
- 2.Gheorghiu Oana Ramona Cătălina, Ciobanu Anne Marie, Guțu Claudia Maria, Chițescu Carmen Lidia, Costea Giorgiana Valentina, et al, 2023, Determination of Phosphodiesterase Type-5 Inhibitors (PDE-5) in Dietary Supplements, Molecules, 28(10), 4116, 10.3390/molecules28104116
- 3.Muschietti Liliana, Redko Flavia, Ulloa Jerónimo, 2020, Adulterants in selected dietary supplements and their detection methods, Drug Testing and Analysis, 12(7), 861, 10.1002/dta.2806


