Abstract
Objective:
To evaluate the efficacy and safety of a novel tomato-based food supplement on the lower urinary tract symptoms (LUTS) of patients with benign prostatic hyperplasia (BPH).
Methods:
Twenty patients with BPH were enrolled in this observational study. They were assigned to consume daily a sachet of Lycoprozen® (5 grams) dissolved in water for two months.
Results:
All patients successfully completed the Lycoprozen scheduled regimen and the IPSS (International Prostatic Symptom Score) questionnaire before and after treatment. No side effects due to treatment were noticed. In this preliminary study, we have found that Lycoprozen® significantly reduced the LUTS severity (paired t-test, two-tailed p value < 0.0001). The IPSS mean values before and after the treatment were 16.95+6.0 SD (range 31-6) and 12.2+4.9 SD (range 20-2), respectively.
Conclusions:
Based on these data, Lycoprozen® may represent a suitable alternative option for the treatment of symptomatic BPH patients which worth of further testing in a phase 2 prospective randomized double blind placebo controlled study. The treatment was without side effects and acceptance among patients was high.
Author Contributions
Academic Editor: María López, Universidad Francisco de Vitoria, Spain.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2018 Alberto Cellini, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
PGN, MP, VF and SI are shareholders at Janus Pharma srl.
Citation:
Introduction
Benign prostatic hyperplasia BPH affects aging men and represents the most common urologic disease among elderly men1. BPH is a growth of both epithelial and stromal cells from both the transition zone and periurethral areas, and typically develops after the age of 40 years, ranging in prevalence from >50% at 60 years to as high as 90% by 85 years of age2.
The diagnosis of BPH is often in response to the development of lower urinary tract obstructive, irritative, and post-micturition symptoms (LUTS), including urinary hesitancy, urgency, frequency and post void dribble. Medical treatment options include α-adrenergic antagonists or 5-α reductase inhibitors, however about one-third of men with LUTS do not respond to either treatment approach3. Patients who are resistant to treatment, or who become resistant to treatment over time, will become candidates for surgical intervention to reduce LUTS severity.
Further understanding the causes of LUTS will guide interventions to prevent LUTS or increase sensitivity to medical treatment. To date, there are multiple theories on the cellular and molecular processes underlying the pathogenesis of BPH leading to a symptomatic disease. In addition to androgens, both chronic and acute inflammation can lead to events that can cause proliferation within prostatic tissue through a variety of mechanisms, notably oxidative stress 4, 5. At present, non-steroidal anti-inflammatory drugs are used to improve urinary symptoms and flow measures, but their long-term effectiveness and safety are not known6.
BPH is also associated with obesity and related pathologies. However, the biological pathways linking obesity and BPH are poorly understood. Centralized adipose deposition was associated with the severity of prostate tissue inflammation and LUTS and an approach to minimize centralized fat deposition may reduce LUTS severity in BPH patients7.
A large number of anti-inflammatory compounds has been identified in tomato extracts8;moreover, tomato consumption reduces inflammation by decreasing inflammatory cytokines in overweight and obese humans 9, 10.
We have also observed that a feed enriched with a 10% whole tomato preparation versus a tomato-free control feed is able to increase the anti-oxidant serum activity and to reduce serum levels of inflammatory biomarkers in the TRAMP mouse model of prostate carcinogenesis, even before the appearance of cancer11.
Recently, a novel food supplement named Lycoprozen® was developed by Janus Pharma, Roma, Italy. It consists of whole tomatoes specially processed for make their antioxidant and anti-inflammatory micronutrients highly bioavailable, and a small percentage of olive’s vegetation water. In the present study, we have evaluated the effects of Lycoprozen® on the LUTS severity in subjects affected by BPH.
Materials and Methods
Lycoprozen® is available as food supplement on the Italian market since 2017 with the Ministry of Health registration number 68843. The patented multi-step production process results into an increased bioavailability of antioxidant and anti-inflammatory tomato micronutrients, which include carotenoids (mainly lycopene), flavonoids, and ketosamines12. Lycoprozen® is made of two components, a whole tomato powder and an olive water waste12.
The compositions of the tomato powder and polyphenolic extract from olive vegetation water are reported in Table 1 and in Table 2, respectively.
Table 1. Tomato powder composition (for 100 g)| Carbohydrates (g) | 64,5 |
| Proteins (g) | 10.2 |
| Lipids (g) | 3.4 |
| Carotenoids (mg) | 500 |
| Lycopene isomers (mg) | 190 |
| Alpha-tocopherol (mg) | 2.3 |
| Total flavonoids (mg) | 200 |
| Ketosamines (mg) | 8 |
| Fibers (g) | 15.9 |
| Humidity (g) | < 5 |
| Oleuropeinaglycon | 6% |
| Ligtrosideaglycon | 2% |
| Oleuropeindialdialdehydeaglycon | 6% |
| Ligtrosidedialdehydeaglycon | 7% |
| Verbascoside | 6% |
| Pinoresinolo and Deacetoxypinoresinolo | 5% |
| Tyrosol | 3% |
| Hydroxytyrosol | 10% |
| Undentified polyphenols | 8% |
Patients
Twenty patients with BPH were enrolled in this observational study. All patients underwent clinical and instrumental examinations and completed the IPSS (International Prostatic Symptom Score) questionnaire13. Furthermore, patients were informed both orally and by means of a written informed consent form. They were assigned to receive an oral sachet of Lycoprozen® (5 grams) every 24 hours, for two months. The main outcome measures were a reduction of LUTS and improved quality of life at the end of the study period.
Results
All patients successfully completed treatment with no side effects.
The IPSS is based on the answer to seven questions concerning urinary symptoms, and is the only questionnaire validated by WHO in Italian language. Each question is assigned points from 0 to 5, indicating increasing severity of a particular symptom. The total score can therefore range from 0 to 35 (asymptomatic to very symptomatic). Although there are presently no standard recommendations into grading patients with mild, moderate or severe symptoms, patients can be tentatively classified as follows: 0 - 7 = mildly symptomatic; 8 - 19 = moderately symptomatic; 20 - 35 = severely symptomatic.
In this preliminary study, we have found that Lycoprozen® significantly reduced the LUTS severity (paired t-test, two-tailed p value < 0.0001). Following the treatment, IPSS value decreased in 17 out of 20 patients, independently of the starting level, while remained unchanged in the remaining three cases (Figure 1). The IPSS mean values before and after the treatment were 16.95+6.0 SD (range 31-6) and 12.2+4.9 SD (range 20-2), respectively.
Figure 1.Urinary symptoms, evaluated according the IPSS* score, in patients with benign prostatic hyperplasia before(blue) and after (red) Lycoprozen®treatment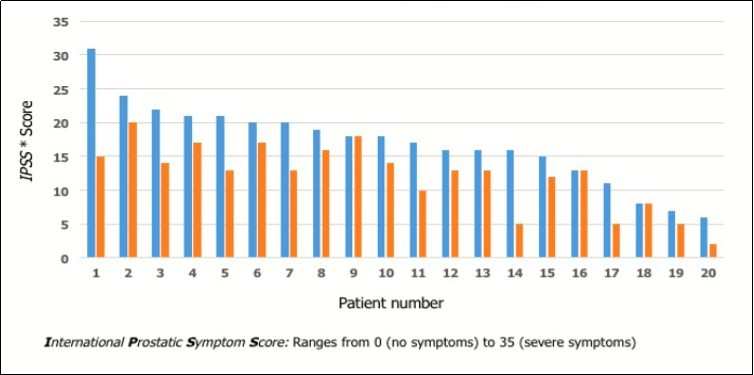
Discussion
As reported above, Lycoprozen® is a mixture of two components, tomato powder and a polyphenolic extract from olives.
The original tomato powder was firstly developed to produce a food for special medical purposes (FSMP) and investigated as an adjuvant therapy in patients with chronic hepatitis C infection. Our results showed that this FSMP was effective in improving carotenoid status in healthy subjects, whereas in patients with hepatitis, it prevented carotenoid serum depletion and improved the oxidative status during antiviral therapy14.
Next, the anti-neoplastic activity of tomato preparation was analyzed in the transgenic adenocarcinoma of the mouse prostate (TRAMP) model11. The TRAMP mouse is a model for progressive prostate cancer that mirrors the stages of the human form. Tomato diet significantly increased overall survival, delayed progression from prostatic intraepithelial neoplasia to adenocarcinoma, and decreased the incidence of poorly differentiated carcinoma. Biochemical data disclosed an increase in serum antioxidant activity and a reduction of serum inflammation/angiogenesis biomarkers of particular relevance in prostate carcinogenesis.
The patented method to produce Lycoprozen® leads to a final product enriched in bio-active compounds which have been found more active in reducing serum levels of IL-6 and VEGF in TRAMP mice12.
The present observational study revealed that a once-daily intake of Lycoprozen® for two months significantly improved urinary symptoms and quality of life in patients with BPH.
BPH has been shown to be associated with an inflammatory environment, which is known to sustain proliferative programs resulting in nodules of BPH 4, 5, 15 and the anti-angiogenic/anti-inflammatory activities of tomato could represent the basis of its beneficial effect on BPH 8, 9, 10, 11.
Conclusion
Based on these preliminary data, Lycoprozen® may be a suitable alternative option for the treatment of symptomatic BPH patients. The treatment was without side effects and acceptance among patients was high.
Limitations of the Study and Ongoing Activities
The aim of the present study was to assess the short-term efficacy of Lycoprozen®. However, its value is limited by the absence of a control group. This should therefore be considered as a starting point for future studies. Currently, we are actively recruiting BPH patients in a phase II prospective, randomized, double-blinded, placebo-controlled study (participating Institutions: National Cancer Institute Regina Elena, Roma, Italy and Foggia’s University, Foggia, Italy). This study is aimed at investigating the effects of Lycoprozen® on biomarkers of lipid peroxidation, inflammation and angiogenesis, serum levels of PSA and carotenoids, mainly lycopene.
Acknowledgement
All patients signed an informed consent (agreement) before the study.
References
- 2.Roehrborn C G. (2008) BPH progression: concept and key learning from. , MTOPS, ALTESS, COMBAT, and ALF-ONE. BJU Int 101, 17-21.
- 3.McConnell J D, Roehrborn C G, Bautista O M, Andriole GL Jr, Dixon C M. (2003) The Long- Term Effect. of Doxazosin, Finasteride, and Combination Therapy on the Clinical Progression of Benign Prostatic Hyperplasia. New EnglandJ Med 349, 2387-2398.
- 4.Chughtai B, Lee R, Te A, Kaplan S. (2011) . Role of Inflammation in Benign Prostatic Hyperplasia. Rev Urol 13, 147-150.
- 5.Ficarra V, Rossanese M, Zazzara M, Giannarini G, Abbinante M. (2014) The role of inflammation in lower urinary tract symptoms (LUTS) due to benign prostatic hyperplasia (BPH) and its potential impact on medical therapy. , Curr Urol Rep 15, 463-469.
- 6.Kahokehr A, Vather R, Nixon A, Hill A G. (2013) Non-steroidal anti-inflammatory drugs for lower urinary tract symptoms in benign prostatic hyperplasia: Systematic review and meta-analysis of randomized controlled trials. , BJU Int 111, 304-311.
- 7.Fowke J H, Koyama T, Fadare O, Clark P E. (2016) Does inflammation mediate the obesity and BPH relationship? An epidemiologic analysis of body composition and inflammatory markers in blood, urine, and prostate tissue, and the relationship with prostate enlargement and lower urinary tract symptomssPloS. One.11: 6-0156918.
- 8.Mohri S, Takahashi H, Sakai M, Takahashi S, Waki N. (2018) Wide-range screening of anti-inflammatory compounds in tomato using LC-MS and elucidating the mechanism of their functions. , PLoS One 12, 13-0191203.
- 9.Ghavipour M, Saedisomeolia A, Dialali M, Sotoudeh G, M R Eshraghyan. (2013) Tomato juice consumption reduces systemic inflammation in overweight and obese females. , BrJ Nutr109 2031-2035.
- 10.Li Y F, Chang Y Y, Huang H C, Y C Wu, Yang M D. (2015) Tomato juice supplementation in young women reduces inflammatory adipokine levels independently of body fat reduction. , Nutrition 31, 691-696.
- 11.Pannellini T, Iezzi M, Liberatore M, Sabatini F, Iacobelli S. (2010) . , Cancer Prev Res 10, 1284-1291.
- 12.Fogliano V, Iacobelli S, Piantelli M. (2016) Tomato powder-based composition”. US Patent App.15/024,165
- 13.Badia X, Garcia-Losa M, Dal-Re R. (1997) Ten-lenguage translation and harmonization of the International Prostate Symptom Score: developing methodology for multinational clinical trials. , Eur Urol 31, 129-140.
Cited by (3)
- 1.Quiros-Roldan Eugenia, Carriero Canio, Paghera Simone, Degli Antoni Melania, Fiorini Chiara, et al, 2021, Symptoms and quality of life in HIV-infected patients with benign prostatic hyperplasia are improved by the consumption of a newly developed whole tomato-based food supplement. A phase II prospective, randomized double-blinded, placebo-controlled study, Journal of Functional Foods, 82(), 104495, 10.1016/j.jff.2021.104495
- 2.Natali Pier Giorgio, Piantelli Mauro, Minacori Marco, Eufemi Margherita, Imberti Luisa, 2023, Improving Whole Tomato Transformation for Prostate Health: Benign Prostate Hypertrophy as an Exploratory Model, International Journal of Molecular Sciences, 24(6), 5795, 10.3390/ijms24065795
- 3.Cormio Luigi, Calò Beppe, Falagario Ugo, Iezzi Manuela, Lamolinara Alessia, et al, 2021, Improvement of urinary tract symptoms and quality of life in benign prostate hyperplasia patients associated with consumption of a newly developed whole tomato-based food supplement: a phase II prospective, randomized double-blinded, placebo-controlled study, Journal of Translational Medicine, 19(1), 10.1186/s12967-020-02684-3
