Abstract
Pyruvate holds superior biomedical properties in increase of hypoxia tolerance, correction of severe acidosis, exertion of anti-oxidative stress and protection of mitochondria against apoptosis, so that it improves multi-organ function in various pathogenic insults. Particularly, pyruvate preserves key enzyme: pyruvate dehydrogenase (PDH) activity through direct inhibition of pyruvate dehydrogenase kinas (PDK), as a PDH activator, in hypoxia. Therefore, pyruvate is robustly beneficial for cell/organ function over citrate, acetate, lactate, bicarbonate and chloride as anions in current medical fluids. Pyruvate-enriched oral rehydration salt/solution (Pyr-ORS) and pyruvate-based intravenous (IV) fluids would be more beneficial than WHO-ORS and current IV fluids in both crystalloids and colloids, respectively. Pyruvate-containing fluids as the new generation would be not only a volume expander, but also a therapeutic agent simultaneously in fluid resuscitation in critical care patients. Pyruvate may be also beneficial in prevent and treatment of diabetes, aging and even cancer. Pyruvate clinical applications indicates a new revolutionary medical advance, following the WHO-ORS prevalence, this century.
Author Contributions
Academic Editor: Godwin Ajayi, Prenatal Diagnosis and Therapy Centre, College of Medicine, University of Lagos, Nigeria.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2020 Fang-Qiang Zhou
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Pyruvate in Oral Rehydration Salt
On the 50th anniversary of clinical application with the World Health Organization (WHO)-guided oral rehydration salt/solution (ORS) and oral rehydration therapy (ORT), prestigious journals, Lancet and JAMA, published articles for the memory in 2018 1, 2. In 1978, the Lancet hailed the ORS for oral treatment of diarrhea and cholera worldwide as a most medical advance in the past century because of the survival of a couple of million lives by the ORT per year 3.
The findings in intestinal physiology, in 1950s, that Sodium-Glucose linked transporters (SGLT1) exist in intestinal epithelium in mammalian are the base of ORS theory, by which sodium easily with glucose is actively and rapidly absorbed and water is passively and massively passed through the intestinal barrier. WHO-ORS consists of powders of sodium bicarbonate or citrate, sodium chloride, potassium chloride and glucose, which is called WHO-ORS I or II. Citrate-based reduced osmolarity one with less sodium chloride and glucose is known as WHO-ORS III, which may be more effective for non-cholera patients with diarrhea 4. In last decades, due to its superiority of sodium and water absorption, the ORS has been promoted to rescue young patients with burns for vast rehydration with or without intravenous (IV) infusion. Currently, the ORT has been one of guidelines in burn shock resuscitation and it is also effective in resuscitation of burns in adults 5, 6.
Since 2012, pyruvate-enriched ORS (Pyr-ORS) has been innovated by equimolar pyruvate of sodium salt to replace bicarbonate or citrate in WHO-ORS (I or II) and so do in reduced osmolarity WHO-ORS (III) also 7, 8, 9, 10. Intriguing findings are that Pyr-ORS reveals marked superiority in resuscitation of hemorrhagic and burn shock in animal models, particularly in severe acidosis correction, visceral blood flow preservation, organ and intestinal barrier protection and survival improvement, compared with WHO-ORSs. Opposite to bicarbonate or citrate, pyruvate of sodium salt holds specific biological and pharmacological properties that benefit critically ill patients: increase of anoxia/hypoxia tolerance, reversal of hypoxic lactic acidosis, anti-oxidative stress and inflammation, protection of mitochondria and anti-apoptosis and so on 11, 12, 13, 14, 15, 16. Of all beneficial advantages above, it may be critical that exogenous pyruvate acts as a stimulator like dichloroacetate (DCA) of the key rate-limited glucose metabolic enzyme, pyruvate dehydrogenase (PDH) activity through the direct inhibition of pyruvate dehydrogenase kinase (PDK), probably via the phosphoinositide 3-kinase(PI3K) pathway, so that exogenous pyruvate increases the active form of non-phosphorylated PDH (PDHa/PDHt: active/total), as substantiated with many research reports in last decades (Figure 1) 17, 18, 19, 20, 21. Therefore, pyruvate is a modulator of glucometabolic disorders, such as Warburg effect, and a protector of multi-organ (particularly brain, heart, liver, kidney and intestine) function in numerous pathogenic insults like hypoxia, trauma/burn and sepsis in critical illnesses, diabetes and aging (see below).
Figure 1.Exogenous and glycolytic pyruvate metabolic pathways and intracellular hydrogen (H+) consumption in various injuries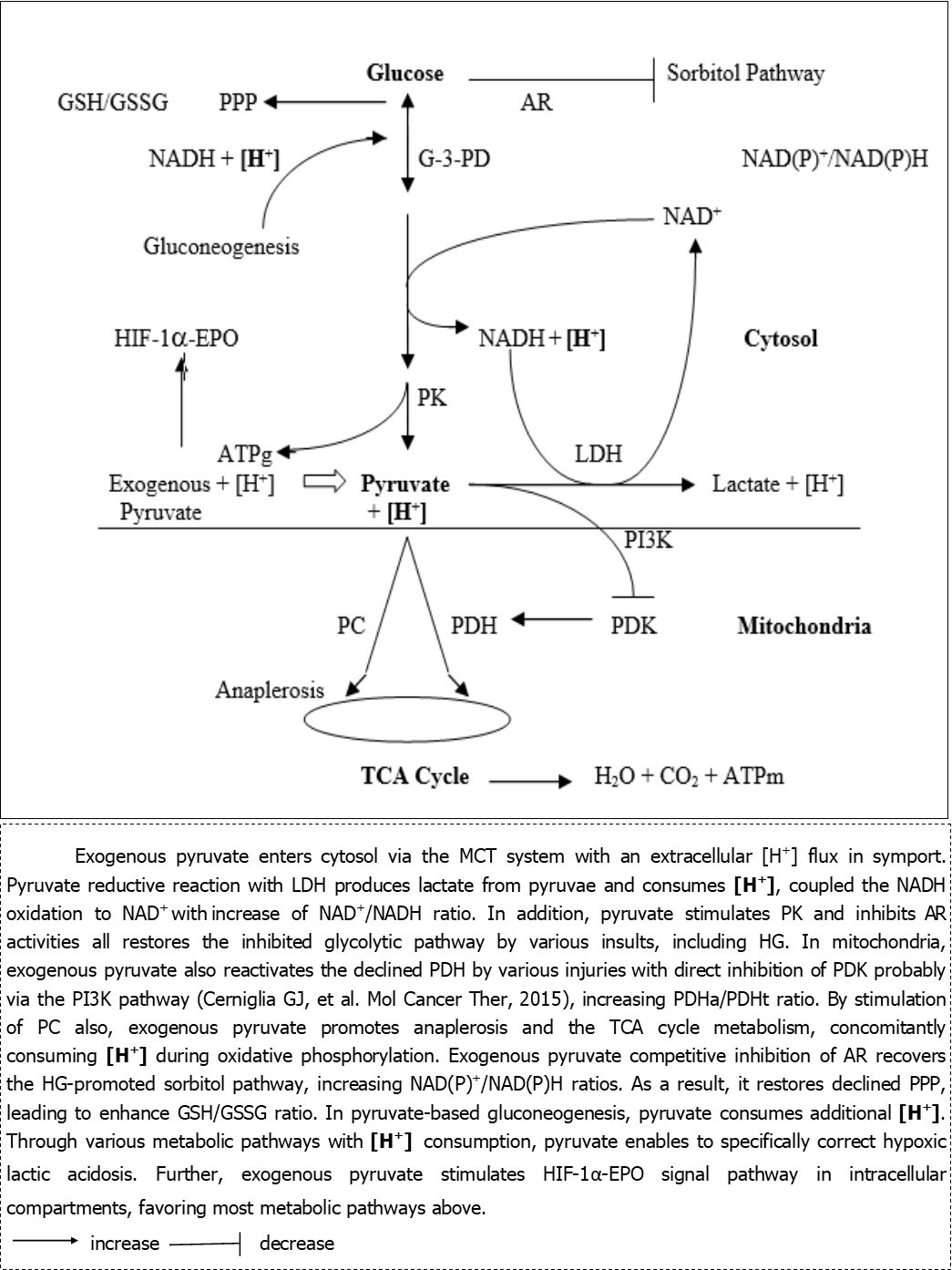
As illustrated with IV or peritoneal pyruvate in severe injured animals 20, 21, it was recently first demonstrated with diabetic kidney tissues that oral pyruvate in Pyr-ORS can mostly reactivate the depressed PDH, stimulating glucose oxidative phosphorylation; it also can enhance nicotinamide adenine dinucleotide oxidized/reduced form (NAD+/NADH) ratio simultaneously induced in the pyruvate reductive reaction, free of energy, by lactate dehydrogenase (LDH) coupled with the NADH oxidative reaction, promoting the regular glycolysis pathway, as indicated in retinal tissues 22. Thereby, oral pyruvate with its stimulation of hypoxia-inducible factor-1α-erythropoietin (HIF-1α-EPO) signal pathway in both hypoxia and normoxia enables to improve glycolytic and glucose oxidative metabolisms to preserve cell function in various injuries 7, 8, 9, 10.
Notably, a recent finding displayed that oral Pyr-ORS improved diabetic status: significantly reduced body weight and fasting blood sugar level and robustly raised blood insulin level in diabetic db/db mice in the setting of diabetes-established disease. Surprisingly, oral pyruvate in Pyr-ORS reversed the high glucose (HG)-declined pyruvate kinase (PK) and PDH activities, concomitantly with inhibition of the HG-promoted pyruvate dehydrogenase kinase (PDK), in diabetic mice. The restoration of enzyme activities, in vivo, was confirmed in the investigation with HK-2 cell line (human proximal tubule epithelial cell line) in HG by pyruvate addition, in vitro. Also, the stimulated aldose reductase (AR) levels by HG were fallen in both, in vivo and in vitro, tests, leading to recover the promoted sorbitol pathway (Figure 1). As a result, the typical glycolysis inhibition and glucometabolic Warburg effect-like disorder in diabetic db/db mice was mostly corrected with exogenous pyruvate 23. Another discovery in the same diabetic model was that oral Pyr-ORS significantly decreased the advanced glycation end products (AGEs) in renal tissues, as demonstrated previously 24, 25, and protected kidney function: declines of 24-hour urine protein, urine neutrophil gelatinase associated lipocalin and plasma cystatin C levels (data submitted for publication).
Prior studies discover that oral pyruvate in large doses (about 0.3-1.0g/kg/d) can improve diabetic status, even resulting in hypoglycemia in patients with type 1 diabetes, pancreatic insulin secretion and mitochondrial disorders in adults and children 26, 27, 28, 29. However, single pyruvate is malabsorption and a large dose of pyruvate is gastrointestinal irritative, but a regular dose less than 25 g as a single ingestion is not functional well and no blood pyruvate is raised if only 7.0g/d is orally taken for 7 days 30, 31. The Pyr-ORS is, thus, created by replacement of alkalizers in ORS with equimolar pyruvate; consequently, a regular amount of pyruvate can be sufficiently absorbed from intestine with enough glucose in Pyr-ORS via SGLT1 located in intestinal mucosa to compellingly increase pyruvate levels in blood and tissues to function as anticipated 9, 32. Therefore, oral pyruvate in Pyr-ORS would prevent from multi-organ dysfunction and reverse disorders of glucometabolic pathways and acid-base balance in critical care patients subjected to various injuries 8, 9, 10.
Pyruvate in Intravenous Fluid Resuscitation
Generally, sodium pyruvate powders in aqueous solutions are not stable at pH over 5.0 at room temperature 33. However, pyruvate fluids for clinical uses (from 28 mM in pyruvate Ringer’s solution to 154 mM in pyruvate saline, etc.) are long-term stable, if the pH of solutions is adjusted to lower than pH 5.0, at room temperature 34. Pyruvate systemic protection of cells/tissues in either anaerobic or aerobic condition includes glucose metabolic pathways and function in red blood cells (RBCs) that may play a critical role in improvement from hypoxia in tissues of critically ill patients 11. Particularly, pyruvate, as a PDH activator, preserves PDH activity and corrects hypoxic lactic acidosis 12, 18, 19, 20, 21, so that it would be more superior than citrate, acetate, lactate, bicarbonate and chloride as current anions in medical fluids in protection of cell function against various injures in clinical settings 7, 8, 9, 10, 11, 12, 13, 14. Although malate might be suitable to correct lactic acidosis in critical care patients 37, it has no protection of RBCs because of its inability to be metabolized as pyruvate under anaerobic condition.
In addition to pyruvate specific benefits in IV resuscitation, experimental pyruvate-based peritoneal dialysis solutions also showed the merits in peritoneal dialysis and peritoneal resuscitation from shock in animal studies 38, 39, 40, the similarity as demonstrated with pyruvate-enriched priming solution in experimental bypass surgery 11. The advantages of pyruvate resuscitation mainly are rapid correction of hypoxic lactic acidosis, distinct multi-organ protection, specific preservation of visceral blood flow and intestinal barrier and profound increment of survival. In addition, as a carrier solution of colloids, pyruvate may eliminate cytotoxicity of hydroxyethyl starch (HES) 130/0.4 on kidney in fluid resuscitation 41. Accordingly, IV (crystalloids and colloids) or oral pyruvate is not only a volume expander, but also a therapeutic agent to protect against multi-organ dysfunction and metabolic disturbances simultaneously in fluid resuscitation. Due to its stability, superiority and safety without clinical toxicity (LD50 > 10g/kg oral pyruvate in rats 42) 26, 29, 34, 42, 43, it is highly possible to manufacture pyruvate-enriched fluids, IV or oral solutions (Pyr-ORS), for dealing with critical care patients from perioperative fluid management to prehospital rescue to win a golden-window time in a large scale, particularly in resource-poor settings like earthquake, in the near future 12, 14, 44, 45.
Pyruvate in Anti-Aging and Beyond
NAD+, a star molecule for anti-aging, is well recognized in health and diseases 46. However, pyruvate may be theoretically more beneficial than NAD+ in protection against aging: 1) exogenous pyruvate anaerobically generates NAD+ spontaneously by the LDH reduction on the 1:1 basis in all tissues, thus, one pyruvate molecule administered basically equals to one NAD+ intake; 2) pyruvate has additional beneficial properties over NAD+, among which the following pyruvate actions play significantly critical roles in protection of cell function: reactivation of the PDH activity, correction of lactic acidosis, stimulation of HIF-1α-EPO pathway, exertion of anti-oxidative/nitrosative stress and inhibition of AGEs formation; cellular PDH inhibition, oxidative stress, acidosis and AGEs deposition are involved in pathogenesis of degenerative nervous diseases, including Alzheimer’s Disease. Therefore, pyruvate shows robust neuroprotection in many animal studies 32, 47. A recent report supported that pyruvate was equimolarly more beneficial than NAD+ in cell function, in vitro48. Besides, it is worthwhile to note that pyruvate may inhibit cancer and improve the effectiveness of chemotherapeutic agents in certain conditions, as several studies substantiated 49, 50, 51, 52. Further, oral pyruvate may protect normal tissues aside from cancer/tumor, including the protection of skin against radiation injury 53. However, further studies and clinical trials are warranted to verify these hypotheses.
Although ethyl pyruvate (EP) has been extensively investigated with even better benefits than sodium pyruvate (SP) in cell protection with animal models of various diseases, which further strengthened clinical values with SP. EP has distinct differences from SP. It is required for EP with carboyxlesterase to be hydrolyzed into pyruvate in the plasma and intracellular environment, however, the enzyme is abundant in animals, but absent in humans. EP activation with carboxylesterase in intracellular spaces produces pyruvate, which may, however, rapidly be metabolized, resulting in little impact on peroxides, like H2O254, 55. The EP phase II multicenter clinical trial failed ten years ago may support the hypothesis above, in comparison with effective clinical trials with SP 26, 27, 28, 29, basically making the EP, per se, clinical prospect hopelessly 56.
Given iatrogenic drawbacks of normal saline and lactate Ringer’s solution in fluid resuscitation from critical care patients, the novel pyruvate solutions, such as pyruvate/chloride saline ([Na+] 154 mmol/L, [Pyr-] 50 mmol/L, [Cl-] 104 mmol/L) and pyruvate Ringer’s solution ([Pyr-] 28 mmol/L) in crystalloids and colloids11, 12, 34, 36, 41 may be the new generation of resuscitation fluids. Pyr-ORS as an alternative to IV-fluid can be used as the first-line medicine for fluid therapy, not only in emergency departments, but also in whole hospitals57. With this respect, Pyr-ORS would further prompt ORT prevalence in critical care and pre-hospital rescue; of note, it may also prevent and improve diabetes and aging as a function drink in a large population. Pyruvate as the metabolic core of three major substances: glucose, lipid and protein would improve overall treatment outcomes of various diseases and social health care quality amongst acute critical illnesses and chronic diabetes, aging and cancer58,59. The prospect of pyruvate applications may be another most important medical advance this century.
Abbreviations
AR: aldose reductase; ATPg: glycolytic ATP; ATPm: mitochondrial ATP; G-3-DP: glyceraldehyde-3-phosphate dehydrogenase; GSH/GSSG: glutathione (reduced/oxidized); [H+]: metabolic hydrogen; [H+]: consumed hydrogen; HG: high glucose; HIF-1α-EPO: hypoxia inducible factor-1α-erythropoietin; LDH: lactate dehydrogenase; MCT: monocarboxylate transporter; NAD(P)+/NADH(P): nicotinamide adenine dinucleotide (phosphate): (oxidized/reduced form); PDHa/PDHt: pyruvate dehydrogenase (active/total part); PDK: pyruvate dehydrogenase kinase); PI3K: phosphoinositide 3-kinase;PK: pyruvate kinase; PPP: pentose phosphate pathway; PC: pyruvate carboxylase; TCA cycle: tricarboxylic acid cycle
Funding
No funds supported.
References
- 1.Nalin D R, Cash R A. (2018) 50 years of oral rehydration therapy: the solution is still simple. , Lancet 392, 536-538.
- 2.Glass R I, Stoll B J. (2018) Oral rehydration therapy for diarrheal diseases: A 50-year perspective. , JAMA 320, 865-866.
- 4.Pulungsih S P, Punjabi N H, Rafli K, Rifajati A, Kumala S et al. (2006) Standard WHO-ORS versus reduced-osmolarity ORS in the management of cholera patients. J Health Popul Nutr. 24(1), 107-112.
- 5.Pham T N, Cancio L C. (2008) Gibran NS; American Burn Association. American Burn Association practice guidelines burn shock resuscitation. J Burn Care Res. 29(1), 257-266.
- 6.Milner S M, Greenough WB 3rd, Asuku M E, Feldman M, Makam R et al. (2011) From cholera to burns: a role for oral rehydration therapy. J Health Popul Nutr. 29(6), 648-651.
- 7.Hu S, Liu W W, Zhao Y, Lin Z L, Luo H M et al. (2014) Pyruvate-enriched oral rehydration solution improved intestinal absorption of water and sodium during enteral resuscitation in burns. , Burns 40(4), 693-701.
- 8.Yu W, Hu S, Xie Z Y, He Z J, Luo H M et al. (2015) Pyruvate oral rehydration solution improved visceral function and survival in shock rats. J Surg Res. 193, 344-354.
- 9.Liu R, Hu X H, Wang S M, Guo S J, Li Z Y et al. (2016) Pyruvate in oral rehydration salt improves hemodynamics, vasopermeability and survival after burns in dogs. Burns. 42, 797-806.
- 10.Liu R, Wang S M, Li Z Y, Yu W, Zhang H P et al. (2018) Pyruvate in reduced osmolarity oral rehydration salt corrected lactic acidosis in sever scald rats. , J Surg Res 226, 173-180.
- 11.Gou D, Tan H, Cai H, Zhou F. (2012) Pyruvate effects on red blood cells during in vitro cardiopulmonary bypass with dogs' blood. Artif Organs. 36, 988-991.
- 12.Hu S, Bai X D, Liu X Q, Wang H B, Zhong Y X et al. (2013) Pyruvate Ringer's solution corrects lactic acidosis and prolongs survival during hemorrhagic shock in rats. , J Emerg Med 45(6), 885-893.
- 13.Liu R, Wang S M, Liu X Q, Guo S J, Wang H B et al. (2016) Erratum to "Pyruvate alleviates lipid peroxidation and multiple organ dysfunction in rats with hemorrhagic shock". , Am J Emerg Med 34(3), 525-530.
- 14.Hu S, Lin Z L, Zhao Z K, Liu R, Ma L et al. (2016) Pyruvate is superior to citrate in oral rehydration solution in the protection of intestine via hypoxia-inducible factor-1 activation in rats with burn injury. , JPEN J Parenter Enteral Nutr 40(7), 924-933.
- 15.Kim J Y, Lee S H, Bae I H, Shin D W, Min D et al. (2018) Pyruvate protects against cellular senescence through the control of mitochondrial and lysosomal function in dermal fibroblasts. , J Invest Dermatol 138(12), 2522-2530.
- 16.Varma S D, Chandrasekaran K. (2015) High sugar-induced repression of antioxidant and anti-apoptotic genes in lens: reversal by pyruvate. , Mol Cell Biochem403(1-2): 149-158.
- 17.Kerbey A L, Randle P J, Cooper R H, Whitehouse S, Pask H T et al. (1976) Regulation of pyruvate dehydrogenase in rat heart. Mechanism of regulation of proportions of dephosphorylated and phosphorylated enzyme by oxidation of fatty acids and ketone bodies and of effects of diabetes: role of coenzyme A, acetyl-coenzyme A and reduced and oxidized nicotinamide-adenine dinucleotide. , Biochem 154(2), 327-348.
- 18.Priestman D A, Orfali K A, Sugden M C. (1996) Pyruvate inhibition of pyruvate dehydrogenase kinase. Effects of progressive starvation and hyperthyroidism in vivo, and of dibutyryl cyclic AMP and fatty acids in cultured cardiac myocytes. , FEBS Lett.393(2-3): 174-178.
- 19.Saiki Y, Lopaschuk G D, Dodge K, Yamaya K, Morgan C et al. (1998) Pyruvate augments mechanical function via activation of the pyruvate dehydrogenase complex in reperfused ischemic immature rabbit hearts. J Surg Res. 79(2), 164-169.
- 20.Sharma P, Walsh K T, Kerr-Knott K A, Karaian J E, Mongan P D. (2005) Pyruvate modulates hepatic mitochondrial functions and reduces apoptosis indicators during hemorrhagic shock in rats. Anesthesiology. 103(1), 65-73.
- 21.Sharma P, Benford B, Li Z Z, Ling G S. (2009) Role of pyruvate dehydrogenase complex in traumatic brain injury and Measurement of pyruvate dehydrogenase enzyme by dipstick test. J Emerg Trauma Shock. 2(2), 67-72.
- 22.Hegde K R, Kovtun S, Varma S D. (2010) Inhibition of glycolysis in the retina by oxidative stress: prevention by pyruvate. , Mol Cell Biochem.343(1-2): 101-105.
- 23.Zhang G, Darshi M, Sharma K. (2018) The Warburg Effect in Diabetic Kidney Disease. Semin Nephrol. 38(2), 111-120.
- 24.Hegde K, Kovtun S, Varma S. (2011) Prevention of cataract in diabetic mice by topical pyruvate. Clin Ophthalmol. 5, 1141-1145.
- 25.Scott G F, Nguyen A Q, Cherry B H, Hollrah R A, Salinas I et al. (2017) Featured Article: Pyruvate preserves antiglycation defenses in porcine brain after cardiac arrest. Exp Biol Med (Maywood). 242(10), 1095-1103.
- 26.Petkova I, Hristov V, Petrov K, Thorn W. (2007) Oral application of sodium pyruvate in healthy persons and patients with diabetes mellitus type 1. Medecine Clinique. 60, 579-584.
- 27.Inoue T, Murakami N, Ayabe T, Oto Y, Nishino I et al. (2016) Pyruvate improved insulin secretion status in a mitochondrial diabetes mellitus patient. J Clin Endocrinol Metab. 101, 1924-1926.
- 28.Nagasaka H, Komatsu H, Inui A, Nakacho M, Morioka I et al. (2017) Circulating tricarboxylic acid cycle metabolite levels in citrin-deficient children with metabolic adaptation, with and without sodium pyruvate treatment. Mol Genet Metab. 120(3), 207-212.
- 29.Koga Y, Povalko N, Inoue E, Nashiki K, Tanaka M. (2019) Biomarkers and clinical rating scales for sodium pyruvate therapy in patients with mitochondrial disease. Mitochondrion. 48, 11-15.
- 30.Morrison M A, Spriet L L, Dyck D J. (1985) Pyruvate ingestion for 7 days does not improve aerobic performance in well-trained individuals. , J Appl Physiol 89(2), 549-556.
- 31.Olek R A, Kujach S, Wnuk D, Laskowski R. (2014) Single sodium pyruvate ingestion modifies blood acid-base status and post-exercise lactate concentration in humans. , Nutrients 6(5), 1981-1992.
- 32.Bai W P, Li J, Han R L, Gu Y, Sun X D et al. (2017) Effects of hypotonic pyruvate oral rehydration solution on brain injury in rats subjected to asphyxial cardiac arrest. Chin J Injury Repair and Would Healing. 12(5), 326-330.
- 33.Margolis S A, Coxon B. (1986) Identification and quantitation of the impurities in sodium pyruvate. Anal Chem. 58(12), 2504-2510.
- 34.Zhou F Q.Stable aqueous solution containing sodium pyruvate and the preparation and use thereof. US patent: 2014; No. US8,835,508 B2 .
- 35.Jaimes R 3rd, Kuzmiak-Glancy S, Brooks D M, Swift L M, Posnack N G et al. (2016) Functional response of the isolated, perfused normoxic heart to pyruvate dehydrogenase activation by dichloroacetate and pyruvate. Pflugers Arch. 468(1), 131-142.
- 36.Wang Y, Huang Y, Yang J, Zhou F Q, Zhao L et al. (2018) Pyruvate is a prospective alkalizer to correct hypoxic lactic acidosis. , Mil Med Res 5(1), 13.
- 37.Dai Z L, Wu J, Meng C, Zeng F, Yang Y et al. (2012) Ringer's malate solution protects against the multiple organ injury and dysfunction caused by hemorrhagic shock in rats. , Shock 38(3), 268-274.
- 38.R van Westrhenen, Vlijm A, Hiralall J K, Krediet R T. (2008) Experimental study on long-term exposure to a biocompatible, hypertonic, pyruvate-buffered dialysis solution. Perit Dial Int.28Suppl5:. 43-47.
- 39.Hu S, Ma L, Luo H M, Lin Z L, Wang X Q et al. (2014) Pyruvate is superior to reverse visceral hypoperfusion in peritoneal resuscitation from hemorrhagic shock in rats. , Shock 41(4), 355-361.
- 40.Lu X G, Kang X, Zhou F Q, Wang X Z, Guo S et al. (2015) Effects of pyruvate-enriched peritoneal dialysis solution on intestinal barrier in peritoneal resuscitation from hemorrhagic shock in rats. J Surg Res. 368-376.
- 41.Hu S, Dai Y L, Gao M J, Wang X N, Wang H B et al. (2018) Pyruvate as a novel carrier of hydroxyethyl starch 130/0.4 may protect kidney in rats subjected to severe burns. , J Surg Res 225, 166-174.
- 42.Mateva L, Petkova I, Petrov K, Beniozef D, Bojilova M et al. (1996) Ten-Day course of sodium pyruvate infusions in patients with chronic liver diseases (CLD). Jpn Pharmacol Ther. 24, 2629-2639.
- 43.Schillinger W, Hünlich M, Sossalla S, Hermann H P, Hasenfuss G. (2011) Intracoronary pyruvate in cardiogenic shock as an adjunctive therapy to catecholamines and intra-aortic balloon pump shows beneficial effects on hemodynamics. , Clin Res Cardiol 100, 433-438.
- 44.Taniguchi H, Sasaki T, Fujita H. (2011) Oral rehydration therapy for preoperative fluid and electrolyte management. , Int J Med Sci 8(6), 501-509.
- 45.Gupta R, Gan T J. (2016) Peri-operative fluid management to enhance recovery. , Anaesthesia.71(Suppl 1, 40-45.
- 46.Aman Y, Frank J, Lautrup S H, Matysek A, Niu Z et al. (2019) The NAD+-mitophagy axis in healthy longevity and in artificial intelligence-based clinical applications. Mech Ageing Dev. 111194.
- 47.Koivisto H, Leinonen H, Puurula M, Hafez H S, Alquicer Barrera G et al. (2017) Corrigendum: Chronic pyruvate supplementation increases exploratory activity and brain energy reserves in young and middle-aged mice. Front Aging Neurosci. 9-67.
- 48.Iannetti E F, JAM Smeitink, PHGM Willems, Beyrath J, WJH Koopman. (2018) Rescue from galactose-induced death of Leigh syndrome patient cells by pyruvate and NAD+. Cell Death Dis. 9(11), 1135.
- 49.Wojtkowiak J W, Cornnell H C, Matsumoto S, Saito K, Takakusagi Y et al. (2015) Pyruvate sensitizes pancreatic tumors to hypoxia-activated prodrug TH-302. Cancer Metab. 3(1), 2.
- 50.Ma R, Wu Y, Zhai Y, Hu B, Ma W et al. (2019) Exogenous pyruvate represses histone gene expression and inhibits cancer cell proliferation via the NAMPT-NAD+-SIRT1 pathway. Nucleic Acids Res. 47(21), 11132-11150.
- 51.Tornin J, Mateu-Sanz M, Rodríguez A, Labay C, Rodríguez R et al. (2019) Pyruvate plays a main role in the antitumoral selectivity of cold atmospheric plasma in osteosarcoma. Sci Rep. 9(1), 10681.
- 52.Mungo E, Bergandi L, Salaroglio I C, Doublier S. (2018) Pyruvate treatment restores the effectiveness of chemotherapeutic agents in human colon adenocarcinoma and pleural mesothelioma cells. , Int J Mol 19(11), 3550.
- 53.Yoo H, Kang J W, Lee D W, Oh S H, Lee Y S et al. (2015) Pyruvate metabolism: A therapeutic opportunity in radiation-induced skin injury. Biochem Biophys Res Commun. 460(3), 504-510.
- 54.Bahar F G, Ohura K, Ogihara T, Imai T. (2012) Species difference of esterase expression and hydrolase activity in plasma. , J Pharm Sci 101(10), 3979-3988.
- 55.Guarino V A, Oldham W M, Loscalzo J, Zhang Y Y. (2019) Reaction rate of pyruvate and hydrogen peroxide: assessing antioxidant capacity of pyruvate under biological conditions. Sci Rep. 9(1), 19568.
- 56.Bennett-Guerrero E, Swaminathan M, Grigore A M, Roach G W, Aberle L G et al. (2009) A phase II multicenter double-blind placebo-controlled study of ethyl pyruvate in high-risk patients undergoing cardiac surgery with cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 23(3), 324-329.
- 57.Patiño A M, Marsh R H, Nilles E J, Baugh C W, Rouhani S A et al. (2018) Facing the Shortage of IV Fluids - A Hospital-Based Oral Rehydration Strategy. , N Engl 378(16), 1475-1477.
Cited by (4)
- 1.Zhang Xiao Meng, Wang Yi Zhen, Tong Jin Dong, Ning Xu Chao, Zhou Fang Qiang, et al, 2020, Pyruvate alleviates high glucose‐induced endoplasmic reticulum stress and apoptosis in HK‐2 cells, FEBS Open Bio, 10(5), 827, 10.1002/2211-5463.12834
- 2.Zhang Xiao Meng, Deng Hao, Tong Jin Dong, Wang Yi Zhen, Ning Xu Chao, et al, 2020, Pyruvate-Enriched Oral Rehydration Solution Improves Glucometabolic Disorders in the Kidneys of Diabetic db/db Mice, Journal of Diabetes Research, 2020(), 1, 10.1155/2020/2817972
- 3.Zhou Fang-Qiang, 2021, NAD+, Senolytics, or Pyruvate for Healthy Aging?, Nutrition and Metabolic Insights, 14(), 117863882110534, 10.1177/11786388211053407
- 4.Zhou Fang-Qiang, 2022, Advantages of pyruvate-based fluids in preclinical shock resuscitation-A narrative review, Frontiers in Physiology, 13(), 10.3389/fphys.2022.1027440
