Abstract
Introduction:
Organophosphate (OP) pesticide poisoning is a major challenging public-health problem in developing countries. Vitamin D deficiency is pandemic, yet it is the most under-diagnosed and under-treated nutritional deficiency in the world and it has been reported to be clinically correlated with psychiatric illness and manifestation of severe systemic inflammatory response syndrome like ARDS. Thus vitamin D deficiency may affect clinical course and outcome in cases of OPP.
Aim:
To evaluate status of 25 hydroxyvitamin D (25(OH)D) level in OP poisoning and its correlation with outcome of such patients.
Materials and Methods:
Serum 25(OH)D levels were measured at the time of hospitalization by electro-chemiluminescent Assay in 96 patients (76 male and 20 female) suffering from OP poisoning. Diagnosis of OP poisoning was made by history of poisoning including container of the poison brought by patient’s relative, clinical examination and measurement of serum butyrylcholinesterase activity. All patients were evaluated as per Performa and follow up till discharge.
Results:
Mean level of 25(OH)D in our cases was 24.57±9.91ng/ml and 66.7% had low levels of 25(OH)D. Our study shows linear relationship between 25(OH)D level and duration of hospital stay. All cases of OP poisoning who developed severe manifestations like ARDS, Intermediate syndrome (IMS) were having significant 25(OH)D deficiency. Our study also shows lower levels of 25(OH)D were associated with poor outcome (11.27±3.21vs 27.02±8.54, p<0.001).
Conclusion:
Vitamin D deficiency in OP poisoning is associated with longer hospital stay, more requirement of ventilator support and high prevalence of complication (ARDS and IMS) and poor outcome. Awareness of 25(OH)D level in patients with OP poisoning may be important to improve outcome.
Author Contributions
Academic Editor: Christopher Ochner, Icahn School of Medicine at Mount Sinai, United states.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2018 Bal Kishan Gupta, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Organophosphorus (OP) pesticide poisoning is a major challenging public-health problem in developing countries.1OP compounds are being used most commonly for suicidal purpose because of low cost and easy availability in India and they are the most common cause of self poisoning deaths in India.2The incidence is higher in young, economically active group with a case fatality ratio of 4-30%.3
Vitamin D (25(OH)D) deficiency is pandemic, yet it is the most under-diagnosed and under-treated nutritional deficiency in the world.4 25(OH)D deficiency is widespread in individuals irrespective of their age, gender, race and geography. Low serum levels of 25(OH)D have been linked with mental health issues and are involved in numerous brain processes including neuroimmunomodulation, regulation of neurotrophic factors, neuro-protection, neuroplasticity and brain development5 and might be associated with some psychological disorders and play an important role in the treatment of psychological disorders.6
OP poisoning is associated with early and late neurological manifestation and severe systemic inflammatory response syndrome like Adult Respiratory Distress Syndrome (ARDS) Intermediate Syndrome (IMS).7Primary cause of death in most of the cases is acute respiratory failure caused by various mechanisms central and peripheral like local pulmonary muscarinic effects causing bronchoconstriction, bronchorrhea, and alveolar edema, central depression of the respiratory centre by direct effect on medulla/hindbrain, Glial cell inflammation , seizure, flaccid paralysis and fasciculation of the muscles of respiration through depolarizing block, aspiration pneumonia, ARDS, paralysis of proximal muscles particularly affecting the muscles of respiration after resolution of acute cholinergic syndrome (Type II paralysis) causing intermediate syndrome.8,9,10 25(OH)D deficiency have been reported to be clinically correlated with psychiatric illness and manifestation of severe systemic inflammatory response syndrome like ARDS.11 Thus 25(OH)D deficiency may affect clinical course and outcome in cases of OP poisoning. We do not find any study regarding status of 25(OH)D level and its role in outcome of OP poisoning in the world literature. Therefore this prospective observational cross-sectional analytic study was planned to evaluate status of 25(OH)D level in OP poisoning and its correlation with outcome of such patients.
Materials and Methods
The study was conducted on 96 consecutive cases of OP poisoning admitted in medical wards & ICU during the period from July 2017 to March 2018. Diagnosis of OP poisoning was made by history of poisoning including container of the poison brought by patient’s relative, clinical examination and measurement of serum butyrylcholinesterase activity. INCLUSION CRITERIA: (1) Patients suffering from OP poisoning.(2) Patients given consent to participate in the study. EXCLUSION CRITERIA: (1) Patient admitted with other type of poisoning (other than OP) or multiple poisoning. (2) Patient suffering from any co-morbid conditions likediabetes mellitus, Ischemic heart disease, malignancy or other chronic conditions. (3) Patient already on vitamin D therapy. (4) Patients who are not giving consent for the study.
All patients were evaluated thoroughly by clinical history and physical examination as per Performa. Laboratory investigations were done in all patients at the time of admission including Complete blood count, Renal function test, Liver function test, Butyrylcholinesteraselevel, Acid Base Gas analysis, Serum 25-hydroxyvitamin D, Serum electrolytes (Na+, K+, Ca++). Other necessary special investigations like X-ray Chest PA view, Ultrasonography, ECG, CT scan, MRI scan etc were done as per requirement. Measurement of serum butyrylcholinesterasewas done by spectrophotometry from Lal PathLabs (Bio. Ref. Interval 4.62-11.50 kU/L). All patients were treated12 and followed up during hospital stay as per protocol.
25-hydroxyvitamin D estimation was done by electro-chemiluminescent Assay13 using Elecsys 25(OH)D Assay (k060755) manufactured by Roche Diagnostics, Germany. Levels of 25(OH)D were classified into three categories as per US Endocrine Society (2011)14 criteria that is:
(1) Deficient :- ≤20 ng/ml, (2) Insufficient :- 21-30 ng/ml and (3) Sufficient :- >30 ng/ml.
In our study low level of 25(OH)D means levels ≤30 ng/ml and severe deficiency defined as levels ≤10 ng/ml.15 Severity and outcome of OP poisoning was also assessed at the time of admission by Peradeniya organophosphorus poisoning (POP) scale16and by GCS score.17
Results
Out of 96 cases of OP poisoning (age ranging 14-55 years, mean age 26±8, 85.4% were below age 30 years) 70 were males (72.9%, age ranging 14-55, mean age 28±8) and 26 females (27.1%, age ranging 17-35, mean age 22±5). The epidemiologic profile and distribution of 25(OH)D levels is shown in Table 1.The mean level of 25(OH)D in our study subjects was 24.57 ± 9.91 and lowest levels were found in high age group >40 years (p<0.009). Although statistically there was not much difference in 25(OH)D levels in males and females but we found more numbers of females were having low levels of 25(OH)D as compared to males (84.7% vs 60%). We also found significant numbers of rural subjects (65.9%) were having low levels of 25(OH)D, but there was no statistically significant difference in various groups of socioeconomic status and education status. 25(OH)D levels were low in higher BMI group although statistically not significant.
Table 1. The epidemiologic profile and Distribution of 25-hydroxyvitamin D level| Parameter | No of cases(%) | Mean±SD | 25-hydroxyvitamin D Levels (ng/ml) No (%) | p-value | ||
| ≤20 | 21-30 | >30 | ||||
| Total | 96(100) | 24.57±9.91 | 36(37.5) | 28(29.2) | 32(33.3) | |
| Age Group | ||||||
| ≤20 | 25(26) | 24.32±9.34 | 11(44) | 7(28) | 7(28) | 0.009* |
| 21-30 | 57(59.4) | 25.90±9.57 | 16(28.1) | 18(31.6) | 23(40.4) | |
| 31-40 | 8(8.3) | 25.43±11.84 | 3(37.5) | 3(37.5) | 2(25) | |
| >40 | 6(6.3) | 11.79±2.44 | 6(100) | 0 | 0 | |
| Sex | ||||||
| Male | 70(72.9) | 25.63±10.90 | 24(34.3) | 18(25.7) | 28(40) | 0.084 |
| Female | 26(27.1) | 21.70±5.76 | 12(46.2) | 10(38.5) | 4(15.4) | |
| Residence | ||||||
| Rural | 94(97.9) | 24.78±9.85 | 35(37.2) | 27(28.7) | 32(34) | 0.143 |
| Urban | 2(2.1) | 14.40±8.91 | 1(50) | 1(50) | 0 | |
| Socioeconomic status | ||||||
| High | 1(1) | 23.20±0.00 | 0 | 1(100) | 0 | 0.895 |
| Middle | 3(3.1) | 27.14±13.47 | 1(33.3) | 1(33.3) | 1(33.3) | |
| Low | 92(95.8) | 24.50±9.91 | 35(38) | 26(28.3) | 31(33.7) | |
| Educational status | ||||||
| Illiterate | 42(43.8) | 24.65±8.66 | 15(35.7) | 14(33.3) | 13(31) | 0.814 |
| <Graduate | 51(53.1) | 24.29±10.83 | 20(39.2) | 13(25.5) | 18(35.3) | |
| ≥Graduate | 3(3.1) | 28.08±12.89 | 1(33.3) | 1(33.3) | 1(33.3) | |
| Occupation | ||||||
| Farmer | 79(82.3) | 24.74±10.31 | 29(36.7) | 22(27.8) | 28(35.4) | 0.892 |
| Labourer | 4(4.2) | 20.78±10.08 | 3(75.0) | 0 | 1(25) | |
| House Wife | 3(3.1) | 22.02±2.44 | 1(33.3) | 2(66.7) | 0 | |
| Driver | 1(01) | 20.70±0.00 | 0 | 1 | 0 | |
| Student | 9(9.4) | 25.99±8.57 | 3(33.3) | 3(33.3) | 3(33.3) | |
| BMI | ||||||
| ≤18.5 | 19(19.8) | 27.45±8.88 | 5(26.3) | 6(31.6) | 8(42.1) | 0.212 |
| 18.51-24.99 | 75(78.1) | 24.05±10.13 | 29(38.7) | 22(29.3) | 24(32) | |
| 25-29.99 | 2(2.1) | 16.55±1.77 | 2(100) | 0 | 0 | |
| ≥30 | 0 | 0 | 0 | 0 | 0 | |
The most common compound of OP poisoning was chlorpyrifos 20%followed by monocrotophos 36%, phorate10%, quinalphos 25%, dimethoate 30%, acephate 75%, malathion 50%, oxydemeton-methyl 25%and triazophos 40%. Distribution of cases according to type of OP poison is shown in Table 2. All the 96 cases were having isolated OP compound poisoning, cases with mixed poisoning were not taken in the study like two patients of mixed poisoning one each with CYMAX (chlorpyriphos 50% plus cypermethrin 5%) and pendimethalin plus chlorpyrifos poisoning were excluded from the study. We did not found any statistically significant difference of 25(OH)D levels in cases with different compounds of OP poisoning but the difference was highly significant (p<0.001) with regards to cause of poisoning (accidental or suicidal). There were 56 cases of suicidal poisoning out of which 45 (80.4%) had low levels of 25(OH)D as compared to 19 (47.5%) out of 40 cases with accidental poisoning. Suicidal poisoning was more commonly seen in females (65.38%) as compare to males (55.71%) and all were due to ingestion of poison.
Table 2. Distribution of cases according to type of OP poison| Type of OP Poison | Total No of cases (%) | 25-hydroxyvitamin D Level | Outcome | |||
| <20 (%) | 21-30 (%) | >30 (%) | Expired | Recovered | ||
| Chlorpyrifos 20% | 32(33.33%) | 15(46.88) | 10(31.25) | 7(21.87) | 2(6.25) | 30(93.75) |
| Monocrotophos 36% | 18(18.75%) | 8(44.44) | 6(33.33) | 4(22.22) | 3(16.67) | 15(83.33) |
| Phorate 10% | 16(16.67%) | 7(43.75) | 2(12.5) | 7(43.75) | 2(12.5) | 14(87.5) |
| Quinalphos 25% | 13(13.54%) | 3(23.08) | 3(23.08) | 7(53.84) | 1(7.69) | 12(92.31) |
| Dimethoate 30% | 13(13.54%) | 3(23.08) | 5(38.46) | 5(38.46) | 1(7.69) | 12(92.31) |
| Acephate 75% | 1(1.04%) | 0 | 0 | 1(100) | 0 | 1(100) |
| Malathion 50% | 1(1.04%) | 0 | 1(100) | 0 | 0 | 1(100) |
| Oxydemeton 25% | 1(1.04%) | 0 | 0 | 1(100) | 0 | 1(100) |
| Triazophos 40% | 1(1.04%) | 0 | 1(100) | 0 | 0 | 1(100) |
| Total | 96(100) | 36(37.5) | 28(29.2) | 32(33.3) | 9(9.38) | 87(90.62) |
Clinical correlation of 25(OH)D levels and course and outcome of OP poisoning is shown in Table 3. Severity of poisoning at the time of admission assessed by POP sore and GCS scale was found to be associated with significantly lower levels of 25(OH)D. We found linear correlation between 25(OH)D levels and duration of hospital stay (Figure 1A and Figure 1B); cases with 25(OH)D deficiency had 10±3.70 days stay before fit to discharge as compared to 8±2.40 days with 25(OH)D insufficiency and 6.43±1.58 days with sufficient 25(OH)D levels (p<0.001). 76.2% cases out of 42 who required >10 days of hospitalization for recovery had low levels of 25(OH)D while low levels were found only in 51.1% cases out of 55 who recovered in ≤10 days of hospitalization (p=0.002). We also found that cases with lower levels of 25(OH)D required more amount of atropine (atropine requirement 188.67±109.29, 149.64±87.66 and 127.75±60.77 respectively for 25(OH)D deficient, insufficient and sufficient levels; p=0.009).
Figure 1A and 1B.25(OH)D levels and duration of hospital stay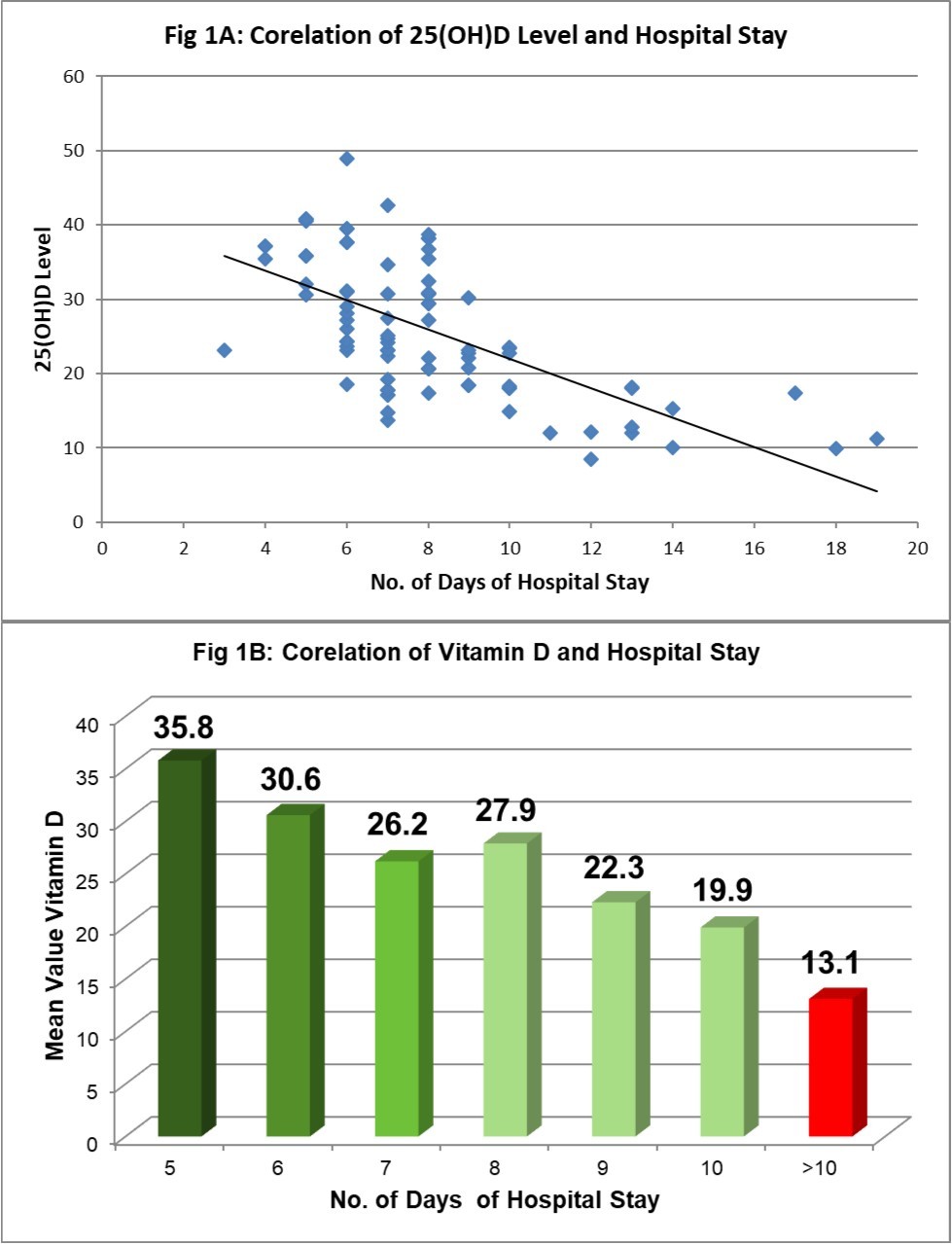
| Parameter | No of cases (%) | Mean±SD | 25-hydroxyvitamin D Levels (ng/ml) No (%) | p-value | ||
| ≤20 | 21-30 | >30 | ||||
| Cause of poisoning | ||||||
| Accidental | 40(41.7) | 29.12±8.64 | 7(17.5) | 12(30) | 21(52.5) | <0.001* |
| Suicidal | 56(58.3) | 21.32±9.52 | 29(51.8) | 16(28.6) | 11(19.6) | |
| POP Score | ||||||
| 0-3 | 8(8.3) | 28.54±6.32 | 0 | 6(75) | 2(25) | 0.002* |
| 4-7 | 69(71.9) | 27.03±6.08 | 20(28.9) | 20(28.9) | 29(42.0) | |
| 8-11 | 19(19.8) | 14.37±5.96 | 16(84.2) | 2(10.5) | 1(5.3) | |
| GCS Score | ||||||
| 3-8 | 16(16.67) | 12.06±5.14 | 15(93.8) | 1(6.2) | 0 | 0.03* |
| 9-13 | 66(68.75) | 22.73±6.38 | 21(31.8) | 27(40.9) | 18(27.3) | |
| 14-15 | 14(14.58) | 34.28±7.01 | 0 | 0 | 14(100) | |
| Duration of Hospital stay (days) (Total No of cases=87, Excluding expired patients) | ||||||
| ≤10 | 45(51.7) | 28.79±9.23 | 9(20) | 14(31.1) | 22(48.9) | 0.002* |
| >10 | 42(48.3) | 22.89±8.52 | 17(40.5) | 15(35.7) | 10(23.8) | |
| Total Atropine Requirement (mg) | ||||||
| ≤150 | 55(57.3) | 26.78±9.86 | 18(32.7) | 14(25.5) | 23(41.8) | 0.009* |
| >150 | 41(42.7) | 21.59±9.27 | 18(43.9) | 14(34.1) | 9(22) | |
| Requirement for Ventilator | ||||||
| Yes | 19(19.8) | 13.29±5.21 | 17(89.5) | 2(10.5) | 0 | <0.001* |
| No | 77(80.2) | 27.35±8.75 | 19(24.7) | 26(33.8) | 32(41.6) | |
| Complications—(1)ARDS | ||||||
| Yes | 10(10.4) | 12.06±4.09 | 10(100) | 0 | 0 | <0.001* |
| No | 86(89.6) | 27.14±8.69 | 26(25) | 28(35) | 32(40) | |
| Complications—(2)IMS | ||||||
| Yes | 6(6.25) | 11.08±2.14 | 6(100) | 0 | 0 | <0.001* |
| No | 90(93.75) | 27.14±8.69 | 30(25) | 28(35) | 32(40) | |
| Outcome | ||||||
| Expired | 9(9.4) | 11.27±3.21 | 9(100) | 0 | 0 | <0.001* |
| Recovered | 87(90.6) | 25.94±9.33 | 27(31) | 28(32.2) | 32(36.8) | |
Clinical significance of 25(OH)D levels in relation to complications and outcome of OP poisoning is illustrated in Figure 2. All the cases who developed serious complications like ARDS or IMS or who required ventilator support during hospital stay had deficiency of 25(OH)D (p<0.001). 47.22% of the 36 cases who had 25(OH)D deficiency (<20ng/ml) required ventilator support and none with sufficient levels. 10 cases (10.42%) out of 96 developed ARDS during course of illness and all had significantly low levels of 25(OH)D (mean 12.06±4.09; ranging 5.09-18.21) while 6 (6.25%) developed IMS had mean 25(OH)D level 11.08±2.14 (ranging 8.50-14.58) (p<0.001).
Figure 2.25(OH)D levels and complications and outcome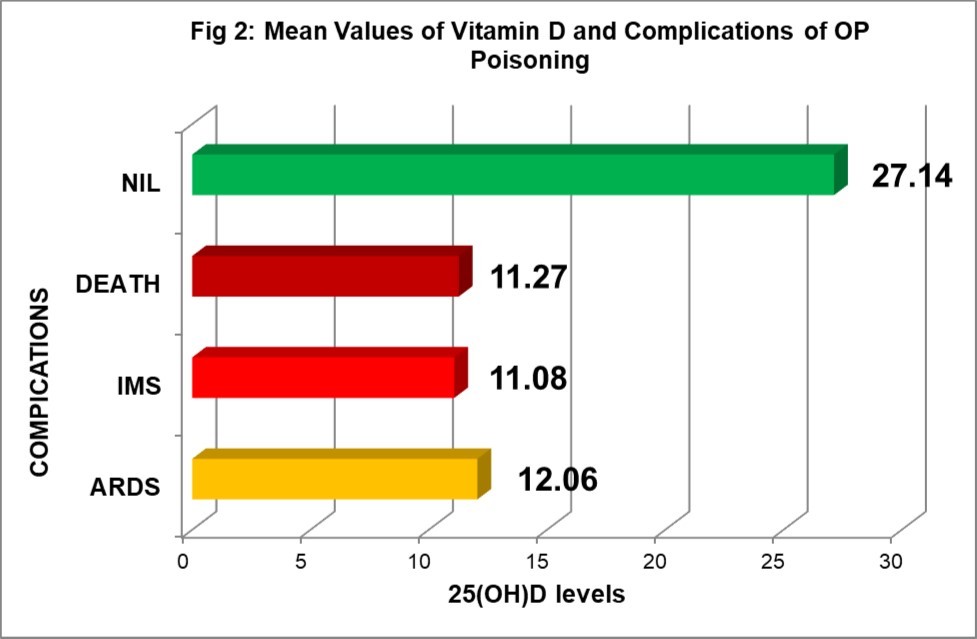
Out of 96 cases, 87(90.63%) were discharged on recovery while 9(9.37%) succumbed to poisoning. All the cases who died had moderate to severe deficiency 25(OH)D (mean level 11.27 ±3.21 ranging 5.09 -14.58) as compared to those who recovered (25.94±9.33 ranging 8.50- 48.91). This shows lower levels of 25(OH)D level is associated with poor outcome (p<0.001).
Multi-logistic regression analysis of hospital stay and outcome for various factors like butyrylcholinesteraseactivity on presentation, age, sex, GCS on admission, POP score, 25(OH)D concentration , POP score and GCS score shows that 25(OH)D concentration is an independent prognostic marker for OP insecticide poisoning (Table 4A, Table 4B and Figure 3A, Figure 3B).
Table 4A. Multi-logistic regression analysis of various factors for hospital stay and outcome| Effect | Value | F | Hypothesis df | Error df | Sig. | |
| age | Pillai's Trace | .070 | 3.334a | 2.000 | 88.000 | .040 |
| Wilks' Lambda | .930 | 3.334a | 2.000 | 88.000 | .040 | |
| Hotelling's Trace | .076 | 3.334a | 2.000 | 88.000 | .040 | |
| Roy's Largest Root | .076 | 3.334a | 2.000 | 88.000 | .040 | |
| sex1 | Pillai's Trace | .055 | 2.572a | 2.000 | 88.000 | .082 |
| Wilks' Lambda | .945 | 2.572a | 2.000 | 88.000 | .082 | |
| Hotelling's Trace | .058 | 2.572a | 2.000 | 88.000 | .082 | |
| Roy's Largest Root | .058 | 2.572a | 2.000 | 88.000 | .082 | |
| vitd3 | Pillai's Trace | .280 | 17.081a | 2.000 | 88.000 | .000 |
| Wilks' Lambda | .720 | 17.081a | 2.000 | 88.000 | .000 | |
| Hotelling's Trace | .388 | 17.081a | 2.000 | 88.000 | .000 | |
| Roy's Largest Root | .388 | 17.081a | 2.000 | 88.000 | .000 | |
| pop | Pillai's Trace | .139 | 7.107a | 2.000 | 88.000 | .001 |
| Wilks' Lambda | .861 | 7.107a | 2.000 | 88.000 | .001 | |
| Hotelling's Trace | .162 | 7.107a | 2.000 | 88.000 | .001 | |
| Roy's Largest Root | .162 | 7.107a | 2.000 | 88.000 | .001 | |
| gcs | Pillai's Trace | .103 | 5.045a | 2.000 | 88.000 | .008 |
| Wilks' Lambda | .897 | 5.045a | 2.000 | 88.000 | .008 | |
| Hotelling's Trace | .115 | 5.045a | 2.000 | 88.000 | .008 | |
| Roy's Largest Root | .115 | 5.045a | 2.000 | 88.000 | .008 | |
| che | Pillai's Trace | .088 | 4.252a | 2.000 | 88.000 | .017 |
| Wilks' Lambda | .912 | 4.252a | 2.000 | 88.000 | .017 | |
| Hotelling's Trace | .097 | 4.252a | 2.000 | 88.000 | .017 | |
| Roy's Largest Root | .097 | 4.252a | 2.000 | 88.000 | .017 | |
| Source | Dependent Variable | Type III Sum of Squares | df | Mean Square | F | Sig. |
| Corrected Model | hs | 284.620a | 6 | 47.437 | 6.281 | .000 |
| outcome1 | 4.729b | 6 | .788 | 20.466 | .000 | |
| Intercept | hs | 3.317 | 1 | 3.317 | .439 | .509 |
| outcome1 | 1.488 | 1 | 1.488 | 38.638 | .000 | |
| age | hs | 50.297 | 1 | 50.297 | 6.660 | .011 |
| outcome1 | .106 | 1 | .106 | 2.762 | .100 | |
| sex1 | hs | 34.082 | 1 | 34.082 | 4.513 | .036 |
| outcome1 | .134 | 1 | .134 | 3.469 | .066 | |
| vitd3 | hs | 63.960 | 1 | 63.960 | 8.469 | .005 |
| outcome1 | .273 | 1 | .273 | 7.091 | .009 | |
| pop | hs | 11.742 | 1 | 11.742 | 1.555 | .216 |
| outcome1 | .204 | 1 | .204 | 5.306 | .024 | |
| gcs | hs | 26.656 | 1 | 26.656 | 3.530 | .064 |
| outcome1 | .392 | 1 | .392 | 10.183 | .002 | |
| che | hs | 33.525 | 1 | 33.525 | 4.439 | .038 |
| outcome1 | .011 | 1 | .011 | .296 | .588 |
Figure 3A and 3B.Multi-logistic regression analysis of various factors for hospital stay and outcome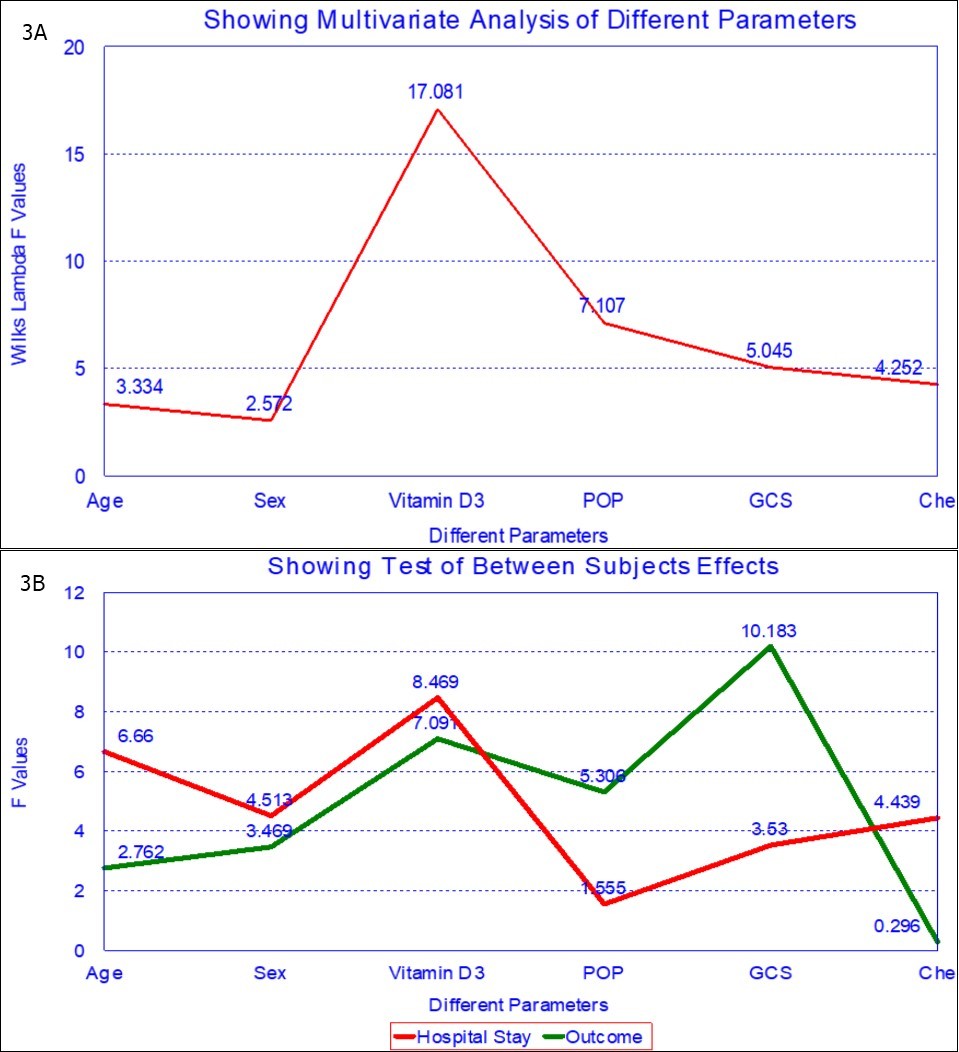
Discussion
Vitamin D deficiency is increasingly recognized as pandemic but its clinical significance is yet to be established properly and therefore it is mostly under treated in most part of the world.18,19,20 The overall prevalence of hypovitaminosis D in our cases of OP poisoning was 66.7%. We did not find any study in the world literature done on such cases. The prevalence of 25(OH)D deficiency has been reported to vary from 33% to 95% from all over the world.18,19 Most of the cases of our study were from rural area (97.9%) having ample exposure to sunlight despite that we found high prevalence of 25(OH)D deficiency, this may be because of change in life style, with modernization the number of hours spent indoor have increased21,cultural and traditional habits like "Burqa" and the "pardah" system,22, increased pollution23, poor nutrition and lack of awareness of the features, benefits and necessity of balanced nutrition, vegetarianism24,25,26high melanin content in the skin27 and increasing use of sun screen cream and lotion.28
In our study we found 58.3% cases of poisoning were due to suicidal attempt and out of which 80.4% were having hypovitaminosis D. High prevalence of 25(OH)D deficiency in subjects with suicidal attempt compared to healthy controls and non suicidal depressed patients has also been found by previous workers.29,30,31Low 25(OH)D levels are associated with higher levels of the inflammatory cytokines IL-6 and IL-18 in the blood. Peripheral and central inflammation is increased in suicidal patients; low level of 25(OH)D could be a contributing cause of this inflammation as inflammation is suggested to directly be part of neural mechanism underlying depressive and suicidal behavior.29
Our study shows that 25(OH)D deficiency is associated with longer hospital stay, more requirement of ventilator support and high prevalence of complications like ARDS and IMS associated with OP poisoning. We did not found any study in the world literature to compare our findings.
We observed lower the 25(OH)D level longer the hospital stay for recovery. Quraishi et al(2014) showed that 25(OH)D level were inversely associated with length of hospital stay in surgical ICU patients.32 Han et al(2016) showed that high dose vitamin D supplement safely increase plasma 25 (OH) D concentration into the sufficient range and was associated with decrease hospital length of stay without altering other clinical outcomes.33But in contrast Amrein et al (2014) in a randomized clinical trial (The VITdAL-ICU) found that among critically ill patients with 25(OH)D deficiency, administration of high dose 25(OH)D compared with placebo did not reduce hospital length of stay, hospital mortality, or 6 month mortality.34
In our study we observed severe deficiency of 25(OH)D in all the cases who developed IMS (11.08±2.14 vs 27.50±8.49; p<0.001). IMS is probably due to combined pre and postsynaptic impairment of neuromuscular transmission as evidenced by electromyographic observations, and that it occur after prolonged and severe acetylcholinestrase inhibition.35 vitamin D affects muscle strength, and in vitamin D deficiency muscle weakness particularly proximal myopathy, gait disturbance has been reported.36,37 The vitamin D receptor (VDR) is expressed in the cell nuclei of muscle cells and vitamin D has been shown to affected muscle cell contractility.38 Vitamin D deficiency causes secondary hyper-parathyroidism which may also impair muscle function.39
Vitamin D also has an immunomodulatory role through its anti inflammatory and anti autoimmune actions. In the nervous system vitamin D is involved in the regulation of calcium mediated neuronal excitotoxicity, in the reduction of oxidative stress and in the induction of synaptic structural proteins, neurotrophic factors and deficient neurotransmitters.40In the absence of vitamin D an important dysfunction is failure of the neuromuscular junction which results in hypocalcemic tetany. In the presence of low calcium levels in the plasma, the neuromuscular junction fails to operate properly giving rise to continual excitation of muscle by nerve. 25(OH)D together with parathyroid hormone plays an important role in the maintenance of plasma calcium concentration at a level which provides for normal function of nerve and muscle.41 The important function of 25(OH)D is to elevate plasma calcium to prevent the failure of the neuromuscular junction. Thus 25(OH)D deficiency may play an important role in the pathogenesis of IMS in patients with OP poisoning.
We also observed significant deficiency of 25(OH)D in all the cases who developed ARDS (12.24±3.76 vs 27.50±8.49; p<0.001). Although there is no study available in the world literature to compare our observation but we have found some studies indicating association of 25(OH)D deficiency and ARDS because of sepsis. Barnet et al (2014) found that 88% of patients with severe sepsis had 25(OH)D deficiency or insufficiency.42 Dancer et al(2015) concluded that 25(OH)D deficiency is nearly universal in the development of ARDS and mechanistically related to exaggerated lung alveolar inflammation and alveolar epithelial cell injury and hypoxia.8
Vitamin D hinders immune cell differentiation, restrains macrophage and monocyte interaction, and down regulates lymphocyte activity.43 In several inflammatory diseases, higher serum 25(OH)D levels or vitamin D supplementation has been associated with reduced levels of C-reactive protein, erythrocyte sedimentation rate and inflammatory cytokines.44 Vitamin D also enhances the function of the innate immune system by stimulating formation of the macrophage associated cathelicidin antimicrobial peptide.45Thus our study indicate clinically that vitamin D may have important role in the pathogenesis of ARDS in OP poisoning.
In our study out of 96 patients, 9 patients (9.4%) expired all of them were having 25(OH)D deficiency (mean 25(OH)D 11.27 ± 03.21; p<0.001). Moraes et al (2015) observed high mortality rate in critically ill patients with 25(OH)D level < 12ng/ml (32.2%) as compared to 25(OH)D levels > 12ng/ml (13.2%)(p<0.05).They found that 25(OH)D level on ICU admission are an independent risk factor for mortality in critically ill patients.46 Low 25(OH)D levels at ICU admission may have a causal relationship with mortality and may serve as an indicator for vitamin D replacement among critically ill patients. In our study multi-logistic regression analysis of various factors for hospital stay and outcome has shown statistically high significance of 25(OH)D levels and as independent prognostic indicator. This may be because of role of vitamin D in the pathogenesis of organ dysfunction, perhaps mediated by its effects on immunity and on cardiovascular system.47
Conclusion
We are reporting study on clinical significance of 25(OH)D level in patients with OP poisoning for the first time in the world. We also observed that vitamin D deficiency is highly prevalent. 25(OH)D level is having linear correlation with length of hospital stay. Severe 25(OH)D deficiency is associated with more requirement for ventilator support, development of serious complication like ARDS, IMS and poor outcome. Thus our study concluded that vitamin D may play an important role in the pathogenesis, clinical course and outcome in cases of OP poisoning. Further studies in the form of intervention with vitamin D supplementation in such cases are required to document our observation.
Declaration
Authors Contribution
Designed the study: BKG. Drafted the manuscript: BKG. Approved the final version to be published: BKG. Carried out clinical assessment, data collection and review of literature: BKG, AKR, SLM, RKS and JG. Evaluated and analyzed laboratory data and their interpretation: BKG, AKR, SLM, RKS and JG. All authors read and approved the final manuscript. Guarantors of the paper: BKG, AKR.
Funding
None
Competing Interest
None declared
Ethical Approval
A prior approval has been taken from the Institutional Ethics Committee to carry out this work, and an informed consent was obtained from the subjects enrolled in this study.
References
- 1.Mew E J, Padmanathan P, Konradsen F, Eddleston M, Chang S-S et al. (2017) The global burden of fatal self- poisoning with pesticides 2006-15: Systematic review. , Journal of Affective Disorders; 219, 93-104.
- 2.Chendake M B, Mohite V R. (2013) Study of Organophosphorus Poisoning In Hospitalized Subject. , Indian J.Sci.Res.; 4(2), 49-59.
- 3.Cherian M A, Roshini C, Visalakshi J, Jeyaseelan L, cherian A M. (2005) . Biochemical and Clinical Profile After Organophosphorus Poisonning – A Placebo – Controlled Trial using Pralidoxime. JAPI; 53, 427-430.
- 4.Van Schoor NM, Lips P. (2011) Worldwide Vitamin D Status. , Best Pract Res Clin Endocrinol Metab; 25, 671-680.
- 5.DA Fernandes de Abreu, Eyles D, Feron F. (2009) Vitamin D, a neuroimmunomodulator: implications for neurodegenerative and autoimmune diseases. Psychoneuroendocrinology;34(suppl1): S265- 77.
- 6.Chan R, Chan D, Woo J, Ohlsson C, Mellstrom D et al. (2011) Association between serum 25-hydroxyvitamin D and psychological health in older Chinese men in a cohort study. , J Affect Disord; 130, 251-9.
- 7.Akgur S, Veral A, Ege B. (2008) Adult respiratory distress syndrome in human organophosphate poisoning cases. , Toxicol Environ Chem; 90, 493-499.
- 8.Hulse E J, JOJ Davies, Simpson A J, Sciuto A M, Eddleston M. (2014) Respiratory Complications of Organophosphorus Nerve Agent and Insecticide Poisoning Implications for Respiratory and Critical Care. , Am J Respir Crit Care Med; 190(12), 1342-1354.
- 9.Senanayake N, Karalliedde L. (1987) Neurotoxic effects of organophosphorus insecticides. An intermediate syndrome. , N Engl J Med; 316, 761-763.
- 10.Indira M, Andrews M A, Rakesh T P. (2013) Incidence, predictors, and outcome of intermediate syndrome in cholinergic insecticide poisoning: a prospective observational cohort study. , Clin Toxicol (Phila); 51, 838-845.
- 11.RCA Dancer, Parekh D, Lax S, D’Souza V, Zheng S et al. (2015) Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS). , Thorax; 70, 617-624.
- 13.Holick M F. (2009) Vitamin D status: measurement, interpretation and clinical application. , Ann Epidemiol; 19(2), 73-8.
- 14.Holick M F, Binkley N C, Bischoff-Ferrari H A, Gordon C M, Hanley D A. (2011) Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. , J Clin Endocrinol Metab; 96, 1911-1930.
- 15.Grundmann M, F von Versen-Höynck. (2011) Vitamin D - roles in women’s reproductive health?. http://www.rbej.com/content/9/1/146. Reproductive Biology and Endocrinology; 9, 146.
- 16.Pradeep V Vernekar1.Dr Kiran Shivaraj (2017). Peradeniya organophosphorus poisoning scale (POP) as a predictor of respiratory failure and mortality in organophosphorus poisoning. , Sch J App Med Sci;5(5B): 1841-1844.
- 17.JOJ Davies, Eddleston M, Buckley N A. (2008) Predicting Outcome in Acute Organophosphorus Poisoning with a Poison Severity Score or the Glasgow Coma Scale. , QJM; 101(5), 371-379.
- 18.Ritu G, Gupta Ajay. (2014) Vitamin D Deficiency in India: Prevalence, Causalities and Interventions. , Nutrients; 6, 729-775.
- 19.Michael F Holick, Tai C Chen. (2008) Vitamin D deficiency: a worldwide problem with health consequences. , Am J Clin Nutr; 87, 1080-1086.
- 20.Suchanda Gadre, Yadav K S, Gomes Merlyn W. (2016) Vitamin D Status in Indian Population: Major Health Concern.World. , Journal of Pharmaceutical Research; 5(1), 362-378.
- 22.Ford L, Graham V, Wall A, Beng J. (2006) Vitamin D concentrations in an UK inner city multicultural outpatient population. , Ann Clin Biochem ; 43, 468-473.
- 23.Agarwal K S, Mughal M Z, Upadhyay P, Berry J L, Marwer E B et al. (2002) The impact of atmospheric pollution on vitamin D status of infants and toddlers. in Delhi, India. Arch Dis Child;87: 111-113.
- 24.Holick M F, Chen T C. (2008) Vitamin D deficiency: A worldwide problem with health consequences. , Am J Clin Nutr; 87, 1080-1085.
- 25.Harinarayan C V, Ramalakshmi T, Venkataprasad U. (2004) High prevalence of low dietary calcium and low vitamin D status in healthy south Indians. Asia Pac. , J. Clin. Nutr 13, 359-364.
- 26.Beaudoin M S, Graham T E. (2011) Methylxanthines and human health: Epidemiological and experimental evidence. , Handb. Exp. Pharmacol 200, 509-548.
- 27.Matsuoka L Y, Wortsman J, Haddad J G, Kolm P, Hollis B W. (1991) Racial pigmentation and the cutaneous synthesis of vitamin D. Arch Dermatology;. 127, 536-538.
- 28.Aggrawal M, Jain A, Meena R C, Yadav L, Qureshi P et al. (2017) Study on vitamin D deficiency and its associating factors in tertiary care center. , Rajasthan. IOSR-JDMS; 16(4), 1-7.
- 29.Grudet C, Malm J, Westrin A, Lena B. (2014) Suicidal patients are deficient in vitamin D, associated with a pro-inflammatory status in the blood. , Psychoneuroendocrinology; 50, 210-219.
- 30.Umhau J C, George D T, Heaney R P, Lewis M D, Ursano R J. (2013) Low vitamin D status and suidice : a case control study of active duty military service members. PLOs One;8:e51543.
- 31.Tariq M M, Streeten E A, Smith H A, Sleemi A, Khabazghazvini B. (2011) Vitamin D: a potential role in reducing suicide risk?. , Int J Adolesc Med Health; 23, 157-165.
- 32.Quraishi S A, Bittner E A, Blum L, McCarthy C M, Bhan I et al. (2014) Prospective study of vitamin D status at initiation of care in critical ill surgical patients and risk of 90 day mortality. Crit Care Med;. 42(6), 1365-1371.
- 33.Han J E, Jones J L, Tangpricha V, Brown M A, Hao L et al. (2016) High dose vitamin D administration in ventilated intensive care unit patients: A pilot double blind randomized controlled trial. , J Clin Transl Endocrin; 4, 59-65.
- 34.Amrein K, Schnedl C, Holl A, Riedl R, Christopher K B. (2014) Effect of high dose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency. , JAMA; 312(15), 1520-1530.
- 35.Yang C C, Deng J F. (2007) Intermediate syndrome following organophosphate insecticide poisoning. , J Chin Med Assoc; 70(11), 467-472.
- 36.Rajnmark L. (2011) Effects of vitamin D on muscle function and performance: a review of evidence from randomized controlled trials. Therapeutics advances in Chr Dis;. 2(1), 25-37.
- 37.Pfeifer M, Begerow B, Minne H, Suppan K, Fahrleitner-Pammer A et al. (2009) Effects of a long term vitamin D and calcium supplementation of falls and parameters of muscle function in community dwelling older individuals Osteophoros Int;. 20, 315-322.
- 38.Bouillon R, Carmeliet G, Verlinden L. (2008) Vitamin D and human health: Lessons from vitamin D receptor null mice. , Endocrine Reviews; 29, 726-776.
- 39.Baczynski R, Massry S G, Magott M, S el Belbessi, Kohan R. (1985) Effect of parathyroid hormone on energy metabolism of skeletal muscle. , Kidney Int; 28, 722-727.
- 40.Mpandzou G, E Ait Ben Haddou, Regragui W, Benomar A, Yahyaoui M. (2016) Vitamin D deficiency and its role in neurological conditions: A review. Rev Neurol (Paris);. 172(1), 109-22.
- 41.Rasmussen H, DeLuca H, Arnaud C, Hawker C, M von Stedingk. (1963) The relationship between vitamin D and parathyroid hormone. , J Clin Invest; 42, 1940-1946.
- 42.Barnett N, Zhao Z, Koyama T, Janz D R, Wang C Y. (2014) Vitamin D deficiency and risk of acute lung injury in severe sepsis and severe trauma: a case control study. Annals of Intensive care;. 4, 5-4.
- 44.Mahon B D, Gordon S A, cruz J, Cosman F, Contorna M T. (2003) Cytokine profile in patients with multiple sclerosis following vitamin D supplementation. , J Neuroimmunol; 134, 128-132.
- 45.Liu P T, Stenger S, Li H, Wenzel L, Tan B H et al. (2006) Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. , Science; 311, 1770-1773.
Cited by (1)
- 1.Gupta Bal Kishan, Narula Kashish, Saini Makhan Lal, Baberwal Rakesh Kumar, Gupta Jigyasa, et al, 2020, Clinical Evaluation of Significance of 25(Oh)D (Vitamin D) Status in Swine Flu (H1N1), International Journal of Nutrition, 6(2), 1, 10.14302/issn.2379-7835.ijn-20-3369
