Abstract
Syndrome of inappropriate antidiuretic hormone secretion (SIADH) is a known side effect of several oncology drugs, but it is rarely seen secondary to vinblastine with only a few cases reported worldwide. Herein we present a patient with nodular lymphocyte-predominant Hodgkin disease that developed a severe acute hyponatremia seven days after her first cycle of chemotherapy with R-ABVD. After fluid restriction, symptoms and concentration of blood sodium were restored. After a comprehensive review of the literature vinblastine was thought to be the cause and avoided in the second infusion of chemotherapy without recurrence of the SIADH.
Author Contributions
Academic Editor: Cesar A. Perez, James Graham Brown Cancer Center, University of Louisville.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2015 Ivonne Salcedo, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Hyponatremia is a common electrolyte disturbance that is usually detected in patients suffering from both solid and hematological malignancies. It is defined by serum sodium concentrations to less than 135 mEq/L. Although often asymptomatic, some patients may develop neurological symptoms such as headache, vomiting, muscle cramps, disorientation, and lethargy which sometimes can be more severe, evolving to seizures, respiratory depression or coma. The severity of these symptoms is related to how fast hyponatremia establishes.1
Syndrome of inappropriate secretion of a diuretic hormone (SIADH) is the most common cause of hyponatremia in cancer patients with a reported incidence of about 30%2. Most of the time, it is a consequence of an ectopic secretion of antidiuretic hormone (ADH) by the tumor cells. Also, some drugs used to treat cancer may increase the release of ADH. Among those, cisplatin, carboplatin, cyclophosphamide, ifosfamide, melphalan, methotrexate and vinca alkaloids are the usual suspects. With respect to vinca alkaloids, most of the reported cases of SIADH have been associated with vincristine, but the relationship of SIADH to vinblastine is rare .3, 4, 5
Vinblastine causes a clinical spectrum of neurotoxicity characterized by peripheral neuropathy and autonomic neuropathy at the level of the cranial nerves but, unlike vincristine myelosuppression is more severe and usually precedes neurotoxicity.6
It has been hypothesized that the increased ADH release caused by vincristine and vinblastine is related to interactions with normal microtubule assembly causing interference of cell division in the metaphase and thus causing the rupture of the cell membrane at the level of the cells of the posterior hypothalamus where ADH is produced and stored. Neurotoxicity is thought to be responsible for causing a disruption on the volume receptors on the periphery that leads to erroneous signals of hypovolemia to the neurohypophisis in the setting of a normal blood volume. So far there is insufficient evidence to attribute the direct kidney damage to cause salt-loss nephropathy but, in animal models has been described the production of autophagic vacuoles from lysosomes to increase damage to the tubular epithelial cells.7, 8, 9, 10, 11, 12, 13
Herein we present the case of a patient with acute severe hyponatremia after administration of combined chemotherapy that included vinblastine.
Case Report.
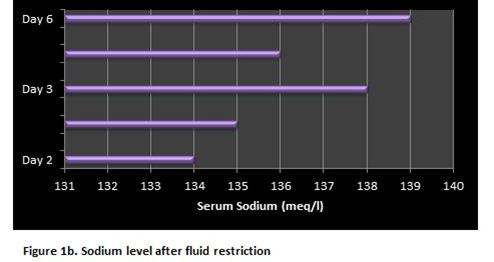
A 57-years old female patient with a past medical history relevant for several years of rheumatoid arthritis with good control of symptoms under specific pharmacological treatment was seen in the Hematology-Oncology service complaining of lymphadenopathy predominantly on the left side of her neck. After excisional biopsy of a cervical lymph node, Nodular Lymphocytic-Predominant Hodgkin Lymphoma (NLPHL), Ann Arbor Stage III-A. A decision was made by starting chemotherapy with rituximab 375 mg/m2/d, doxorrubicin 25 mg/m2/d, bleomycin 10UI/m2/d, vinblastine 6 mg/m2/d and dacarbazine 375 mg/m2/d days 1 and 15 of a 28-day cycle (R-ABVD). During day 1 of her first cycle of chemotherapy no major complications were seen. Relevant blood test of control on the morning of the 7th day after chemotherapy showed WBC: 2.6x109/L, ANC: 1.2x109/L, Hemoglobin: 12.8 g/dl, platelet count: 92x109/L, serum Na: 136 mEq/L, K: 4.3 mEq/L, Cl: 103 mEq/L, CO2: 28.2 mEq/L. At about 12 hr after blood test were drawn the patient suddenly developed headache, nausea, vomiting, generalized weakness, muscle pain and drowsiness that lead her into the emergency room. On the physical examination, she was found with mild hypertension with other vital signs within the normal range. No edema or dehydration was documented. The new set of blood test revealed serum Na 115 mEq/L, BUN: 9.1 mg/dl, creatinine 0.6 mg/dl, serum osmolality 242 mOsm/kg, urine osmolality 474 mOsm/kg, urinary Na 140 mEq/L, and uric acid: 1.0 mg/dl. The clinical picture was an euvolemic hyponatremia. Fluids with 2 L of normal saline IV was started as an initial approach. The control Na came back in 116 mEq/L. Thyroid function tests and serum cortisol were found within normal limits and a kidney consult was requested. A decision was made to continue with hypertonic fluids because neurologic symptoms persisted.The measurement of serum and urinary sodium, and urine osmolality continued every 4 hours. After the first bolus of hypertonic saline the patient had scarce improvement of symptoms, hence a second bolus of hypertonic saline was given, and stopped. Neurological symptoms improved. Eight hours after the second bolus of hypertonic saline the patient was asymptomatic. Serum Na increased up to 129 mEq/L 4 hours after this second bolus of hypertonic saline. Interestingly, 8 hours after initial normal IV saline was started, polyuria developed associated with appropriate water diuresis in accordance with apparent inhibition of the action of ADH. After the second bolus of hypertonic saline, isotonic fluids with 25% human albumin were initiated in an attempt to expand the intracellular volume and to assess adequate (a renal salt wasting syndrome was also suspected) or inadequate secretion of ADH. After 6 hours of transient adequate polyuria, urine volume decreased, and sustained high urinary sodium with a high urinary osmolality was seen despite the low serum sodium concentration. The behavior of the serum and urinary Na, and urinary osmolality were consistent with SIADH and the restriction of fluids was decided. Serum Na progressively increased and maintained at around 136 mEq/L for about 24 hours. The patient was discharged to home. Two days after discharge from hospital the serum Na was 139 mEq/L, the CBC recovered completely. With a serum NA of 139 mEq/L decision was made to resume day 15 of the first cycle of R-ABVD without vinblastine. The patient had neither clinical nor laboratory evidence of relapsed SIADH after 3 complete cycles of chemo-immunotherapy. (Table, Figure 1a and Figure 1b).
Table 1. Patient's clinical course and management| Day | Management | Time | Serum Sodium(mEq/l) | Urinary Osmolarity(mOsm/Kg) | Urinay Sodium(mEq/l) |
| 0 | ER Arrival | 115 | 474 | 140 | |
| 1 | Hydration | 116 | |||
| Hypertonic Fluids | 4 hours | 122 | 97 | 32 | |
| 8 hours | 129 | 74 | |||
| 12 hours | 131 | 74 | |||
| 2 | Re-challenge of fluids | 135 | 113 | 99 | |
| 134 | 310 | 103 | |||
| 133 | 331 | 105 | |||
| Restriction of fluids | 134 | 315 | 174 | ||
| 135 | 327 | 49 | |||
| 3 | Without fluids | 138 | 663 | 181 | |
| 136 | 430 | 165 | |||
| 6 | Outpatient | 139 | 730 | 141 |
Figure 1a.Patient’s clinical course and management
Discussion :
Herein we described a middle age patient that meets the current clinical and laboratory criteria for SIADH. She presented with neurological symptoms, a normal kidney, heart, adrenal and thyroid function associated with severe acute normovolemic hyponatremia. Low serum Na, low osmolality and high urine osmolality, along with low urea nitrogen and uric acid supported our clinical impression. However, we always took into account the possibility of a temporary renal salt wasting syndrome or a transient appropriate inhibition of ADH.
The term acute hyponatremia was established under the criteria of a decrease serum Na in a period of less than 24 hours. Because of an acute presentation of neurological symptoms associated with hyponatremia the use of hypertonic fluids with improvement of symptoms and then fluid restriction was considered an acceptable emergency approach to this patient with serum Na completely recovered on day 10 after administration of chemotherapy14. Without evidence of drugs used for the treatment of AR for many years as causes of SIADH and a recent history of chemotherapy with conventional R-ABVD, the vinca alkaloid vinblastine came out as the main suspect in this case.
Drugs included in the R-ABVD regimen where explored as the cause of SIADH in our patient. Doxorubicin is known to be nephrotoxic at doses higher than 5 mg/kg however, when present, it occurs late (6 months) and is primarily due to a direct glomerular damage rather than tubular injury 15, 16, 17, 18, 19. Neither dacarbazine nor bleomycin are nephrotoxic.
There are case reports of SIADH after the administration of vinca alkaloids. Vincristine is the most common agent associated with this rare side effect, however case reports describing SIADH as a side effect after the use of vinblastine at standard doses are scattered in the medical literature. There is insufficient evidence to support one specific mechanism of action of vinblastine in pathophysiology or clinical manifestations of SIADH. One of the proposed mechanisms is its neurotoxic effect through the disruption of the normal microtubule assembling and stabilization causing an arrest in metaphase of the cells of the posterior hypothalamus causing rupture of the cell membrane and release of inappropriately high concentrations of antidiuretic hormone. Another hypothesized mechanism is a direct tubular damage in the proximal convoluted tubule with impairment of the tubular reabsorption of phosphorus and chloride causing a drop in the concentration of these electrolytes in the blood associated with the hyponatremia of SIADH.
Finally the reversibility of the tubular injury may be secondary to a rapid turnover of the phospholipids layer of the luminal membrane of the renal-tubule cells 11. SIADH may be secondary to a dual effect: dilutional and secondary to a sudden renal loss of sodium after both high and therapeutic doses of vinblastine7, 8, 9, 10, 11, 12, 13. To the best of our knowledge eleven patients with secondary SIADH after the administration of vinblastin have been reported so far3, 5, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25. Table 2 summarizes the clinical features of these patients. The gender distribution was 50% for male and females and the median age was 39.5 years (range 5 months to 68 years), so gender and age do not appear to be predisposing factors. The testicular germ cell tumor represented 40% of the cases, followed by breast cancer, immunoblastic lymphoma, metastatic melanoma and neuroendocrine tumor in adulthood, while histiocytosis was mainly found in childhood. Because a greater proportion of patients were treated for a testicular germ cell tumor, the chemotherapy included bleomycin and cisplatin. Although they are nephrotoxic there is no clinical evidence to be the cause of SIADH at therapeutic doses. In the initially described patients SIADH developed after high-dose, but then SIADH was documented with therapeutic doses of vinblastine
Table 2. Summary clinical features of patients with SIADH after exposure to vinblastine.| Author | Yr | No. | A/G | Diagnostic | Tx | Dose of Vinblastine | DOSAC | Clinical Features | No. CT | SS | US | SO | UO | M | No. DNS | Vinblastine again | Status |
| (mEq/I) | (mOsm/Kg) | ||||||||||||||||
| Ginsberg | 1977 | 1 | NS/NS | Tumor Germinal metastasic | Cis | 0.4 mg/Kg D1,D2 (OD) | NS | NS | 1 | 112 | NS | 239 | 743 | NS | NS | Y/Dose NS | Alive |
| Winter | 1977 | 1 | 3 /M | Histiocytosis | -- | 0.8 mg/kg D1 (OD) | 5 | NS | NS | 128 | 70 | NS | NS | RF | 11 | N | Alive |
| Antony | 1980 | 1 | 53/M | Teratocarcinoma of testis | B | 0.8 mg/kg D1,D2 (OD) | 9 | insomnio, hallucinations | 1 | 113 | 142 | 254 | 877 | HS | 14 | NS | Alive |
| Stahel | 1982 | 1 | 35/M | Testicular carcinoma | B,Cis | 1 mg/Kg (OD) | 6 | severe muscle pain, delirium | 1 | 120 | NS | 251 | NS | RF | 10 | N | Alive |
| Ravikumar | 1983 | 2 | 68/F | Metastasic melanoma | B.Cis | 6mg/m2 D1,D2 | 7 | pharyngeal pain and nausea | 1 | 112 | 126 | 237 | 760 | RF | NS | Y/Dose 50% | Alive |
| 22/M | Neuroendocrine tumor | B,Cis | 6 mg/m2 D1,D2 | 7 | asymptomatic | 1 | NS | NS | 261 | 680 | RF | NS | Y/Dose 50% | Alive | |||
| Fraschini | 1987 | 3 | 69/F | Mestastasic breast cancer | -- | 2 mg/m2 D1 to D5 (IH) | 5 | lethargy, ileus | 1 | 116 | NS | 248 | 393 | RF,HS,D | NS | NS | Alive |
| 57/F | Metastasic breast cancer | .. | 2 mg/m2 D1 to D5 (IH) | 10 | weakness, mental confusion and drowsiness | 1 | 128 | 153 | 264 | 334 | RF,HS,D | 10 | Y/Dose 80% | Alive | |||
| 44/F | Metastasic breast cancer | Cis | 2 mg/m2 D1 to D5 (IH) | 3 | weakness | 1 | 124 | 127 | 253 | 637 | RL,HS,D | 15 | NS | Alive | |||
| Zavagli | 1988 | 1 | 28/F | Inmunoblastic lymphoma | ABDa | 6 mg/m2 D1 | 10 | impaired consciousness | 1 | 104 | NS | 226 | 680 | HS | 2 | NS | Alive |
| Park | 1998 | 1 | 5m/M | Histiocytosis | .. | 0.2 mg/Kg D1 | NS | NS | NS | 119 | NS | 240 | 433 | NS | NS | Y/Dose 50% | Alive |
In our review of literature, the emergence of this syndrome with therapeutic doses is confirmed with most of the patients having vinblastine as a single agent. Detection of hyponatremia was detected on the average seven days after the first administration of vinblastine in 90% of patients. Fifty percent of cases the decrease in serum sodium was sudden, leading mainly to neurological symptoms that needed an initial treatment with hypertonic solutions with subsequent restriction of fluids and, in some cases the administration of demeclocycline .With the exception of one of the reported cases, most of them had moderate to severe hyponatremia. Fifty percent of the patients were re-challenge with vinblastine in subsequent cycles with reduced dose down to 50%. None of the patients repeated SIADH except for one case which serum Na drop to 133 mEq/L without symptoms, however dose reduction in that particular patient was not provided in the text. In ten patients the management was appropriate (a patient does not specify the outcome), and no deaths have been reported in this set of patients.
Internist and medical oncologists should keep in mind this rare complication of vinblastine that may develop at both therapeutic and higher doses in patients with hematological or oncological diseases in need of a chemotherapy combination including vinblastine. Proper approach with only restriction of liquids will resolve this complication in most cases, with the exception of severe acute hyponatremia in which management with hypertonic fluids may be adequate for the patient's life as was the case that we present in this report.
References
- 1.Peri A, Giuliani C. (2014) Management of euvolemic hyponatremia attributed to SIADH in the hospital setting. Minerva Endocrinol. 39(1), 33-41.
- 2.Castillo J J, Vincent M, Justice E. (2012) Diagnosis and management of hyponatremia in cancer patients. Oncologist. 17(6), 756-65.
- 3.Ginsberg S J, Comis R L, Fitzpatrick A V. (1977) Vinblastine and inappropriate ADH secretion. , N Engl J Med 296-16.
- 5.Winter S C. (1977) Arbus GS. Syndrome of inappropriate secretion of antidiuretic hormone secondary to vinblastine overdose. , Can Med Assoc J 117(10), 1134.
- 6.Carbone P P, Bono V, Frei E 3rd, Brindley C O. (1963) Clinical studies with vincristine. Blood. 21, 640-7.
- 7.Slater L M, R A Wainer. (1969) Serpick A A. Vincristine neurotoxicity with hyponatremia. Cancer. 23(1), 122-5.
- 8.Cutting H O. (1971) Inappropriate secretion of antidiuretic hormone secondary to vincristine therapy. , Am J Med 51(2), 269-71.
- 9.Stuart M J, Cuaso C, Miller M, Osky F A. (1975) Syndrome of recurrent increased secretion of anti-diuretic hormone following multiple doses of vincristine. Blood. 45(3), 315-20.
- 10.Schwartz W B, Bennet W, Curelop S, Barlter F C. (1957) A syndrome of renal sodium loss and hyponatremia probably resulting from inappropriate secretion of antidiuretic hormone. , Am J Med 23(4), 529-42.
- 11.Weiss H D, Walker M D, Wiernik P H. (1974) Neurotoxicity of commonly used anti-neoplastic agents. , N Engl J Med 291(3), 127-33.
- 12.Robertson G L, Bhoopalam N, Zelkowitz L J. (1973) Vincristine neurotoxicity and abnormal secretion of antidiuretic hormone. Arch intern Med. 132(5), 717-20.
- 13.Rassat J, Robenek H, Themann H. (1981) Paracrystalline inclusions in lysosomes of mouse kidney tubule cells induced by vinblastine and vincristine. Virchows Arch B Cell Pathol Incl Mol Pathol. 37(2), 199-205.
- 15.Stoll R, Kinne R, Murer H. (1979) Efect of dietary phosphate intake on phosphate transport by isolated rat renal brush-border vesicles. , Biochem J 180(3), 465-70.
- 16.Antony A, Robinson W A, Roy C, Pelander W, Donohue R.Inappropriate antidiuretic hormone secretion after high dose. 123(5), 783-4.
- 18.Oliverio V T.Derivates of triazenes and hydrazine. In:Cancer Medicine 1982.Holland JF, Frei E III editors. Philadelphia: Lea and Febiger 806-17.
- 19.F S Philips, Gilladoga A, Marquardt H, S, P M Vidal. (1975) Some observations on the toxicity of adriamycin (NSC-123127).CancerChemotherRep. 6-177.
- 20.Mizutani M, Nakamori Y, Sakaguchi H, Kageyama Y, Oya E et al. (2013) Development of syndrome of inappropriate secretion of ADH and reversible posterior leukoencephalopathy during initial rituximab-CHOP therapy in a patient with diffuse large B-cell lymphoma. Rinsho Ketsueki. 54(3), 269-72.
- 21.Sthael R A, Oelz O. (1982) Syndrome of inappropriate ADH Secretion Secondary to vinblastine. Cancer Chemother Pharmacol. 8(2), 253-4.
- 22.Ravikumar T S, Grage T B. (1983) The syndrome of inappropriate ADH secretion secondary to vinblastine-bleomycin therapy. J Surg Onc. 24(3), 242-5.
- 23.Fraschini G, Recchia F, Holmes F. (1987) Syndrome of inappropriate antidiuretic hormone secretion associated with hepatic arterial infusion of vinblastine in three patients with breast cancer. Tumori. 73(5), 513-6.
Cited by (2)
- 1.Cordova Ezequiel, Morganti Laura, Odzak Andrea, Arcondo Florencia, Silva Mariana, et al, 2017, Severe hypokalemia due to a possible drug–drug interaction between vinblastine and antiretrovirals in a HIV-infected patient with Hodgkin’s lymphoma, International Journal of STD & AIDS, 28(12), 1259, 10.1177/0956462417703026
- 2.Verzicco Ignazio, Regolisti Giuseppe, Quaini Federico, Bocchi Pietro, Brusasco Irene, et al, 2020, Electrolyte Disorders Induced by Antineoplastic Drugs, Frontiers in Oncology, 10(), 10.3389/fonc.2020.00779