Abstract
Objective:
Individuals diagnosed with Down syndrome (DS) are predisposed to obstructive sleep apnea (OSA). The aim of this study is to assess the prevalence of OSA and factors associated with OSA in this population.
Study Design:
Systematic literature review and meta-analysis.
Methods:
Studies of DS and OSA in the English language through May 2017 were reviewed. Since parental report of symptoms has limited validity related to the diagnosis of OSA, only studies using in laboratory polysomnography to diagnose OSA were included.
Results:
Twenty three studies examining OSA among 1,469 people with DS were found. Among ten studies using community referred samples, 71.5% of people with DS had OSA, compared to 69.6% in referred community samples suspected of having respiratory events. There was an inverse relationship between apnea hypopnea index (AHI) cutoffs and OSA prevalence as higher cutoffs were associated with somewhat lower prevalence. Examining age groups, adults had a higher prevalence of OSA (90.0%) compared to infants (66.5%) and children between 2-21 years of age (69.9%). Oxygen desaturation and gender did not affect prevalence. Although surgery had less effect on successfully treating OSA among DS individuals compared to those without DS in prior studies, lingual tonsillectomy had the greatest effect (mean AHI decrease of 9.0).
Conclusion:
OSA appears to occur frequently in children and adults with DS. Untreated, OSA may contribute to health problems and premature death, highlighting the potential importance of identifying OSA among people with DS. Even after traditional surgeries (i.e., adenotonsillectomy, tonsillectomy, adenoidectomy), repeating PSG is highly recommended as residual OSA can persist
Author Contributions
Academic Editor: He Jinbo, University of Macau
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2017 Sharanah Ridore, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Down syndrome (DS), also known as trisomy 21, is a common genetic disorder with an incidence of 1 per 660 live births. DS is caused by a full or partial extra copy of chromosome 21 (~95% of DS individuals have trisomy 21 compared to 3-4% that have an unbalanced translocation). It is the most common chromosomal cause of intellectual disabilities. Clinical signs and symptoms of DS include low muscle tone, a flattened facial profile, an upward slant palpebral fissures, low-set auricles, prominent tongue, a single deep palmar crease, other physical symptoms (e.g., gastrointestinal reflux, thyroid problems, cardiovascular disease and pulmonary hypertension), and intellectual disability. To confirm the diagnosis, a chromosomal analysis via karyotype is necessary. There is an increased risk of sleep-disordered breathing (SDB), including obstructive sleep apnea (OSA), in children with DS. The American Academy of Pediatrics recommends polysomnography (PSG) in children 4 years of age or younger with suspected OSA 1. Predisposing anatomic factors for OSA in children with DS include the low muscle tone in the upper airways (i.e., laryngomalacia and subglottic stenosis), poor airway movement coordination leading to gastroesophageal reflux, craniofacial abnormalities (i.e., midface hypoplasia and micrognathia) leading to naso- and oropharyngeal stenosis, relative macroglossia, central apnea, and hypertrophy of adenoid and tonsillar tissues. Other risk factors include increased upper airway infections, nasal secretions, hypothyroidism, and a higher incidence of obesity 2. In a study including 303 children with DS, 47.8% were obese compared to 12.1% of age- and gender-matched controls in the general population 3. However, although obesity presents a risk factor for developing OSA in non-genetically affected individuals, it is not consistently associated with increased prevalence of SDB 4. Studies also suggest a higher prevalence of sleep issues among males with DS compared to females. Similarly, there are sporadic case reports on adults with DS noting a higher prevalence of OSA, hypoxemia, hypoventilation, and sleep fragmentation 5. Examining surgical treatment, although adenotonsillar hyperplasia plays an important role in children with OSA, studies of children with DS suggest only partial improvements in their breathing after adenotonsillectomy 6.
The high prevalence of SDB in people with DS is unquestioned. In a study involving 16 adults, an estimated 88% of subjects with DS had an Apnea Hypopnea Index (AHI) ≥ 15/h compared to only 9% of non-DS adults in a population-based study, while 75% of subjects with DS had a saturation nadir < 85% compared to 8% of the non-DS population 5. Since untreated OSA could increase the risk for cardiovascular and neurological complications, early diagnosis and treatment of OSA can significantly improve quality of life.
PSG remains the gold standard in diagnosing OSA. With greater awareness of OSA in recent years, however, the OSA-18 questionnaire has been increasingly utilized to screen and diagnose OSA 7. Though often administered, the OSA-18 was found to have poor sensitivity in identifying OSA among a sample of 225 children, as most children with severe OSA were not correctly diagnosed 8. In a study examining 56 children with DS, the positive predictive value for parental-based diagnoses of OSA was 36.4% (4/11), while the negative predictive value was 45.8% (11/24) 4. In addition, while the use of in-home sleep studies and overnight pulse oximetry has been rising, especially when there is a lack of or inability to conduct PSG, their accuracy in diagnosing OSA remains questionable 9. In a review assessing the accuracy of oximetry in 25 published studies involving normal children, oximetry use accurately identified those individuals with moderate to severe OSA 10. However, less is known about its efficacy in identifying OSA in children with DS. An underestimation of the prevalence of OSA was found in study comparing laboratory PSG (n = 8) to in home PSG (n = 36), a lower prevalence of OSA was found in the home PSG (56%) compared to the in laboratory method (88%) 11.
Since the prevalence of OSA may vary depending on the assessment method used, our present review aims to identify the prevalence of OSA in the DS population using the gold-standard method of laboratory PSG. In non-genetically affected individuals, OSA occurs in 1-3% of the general population; however, a much higher prevalence of OSA is suggested in the population diagnosed with DS. A better estimate as to how high the prevalence of OSA is among people with DS would aid clinicians and policy makers in making assessment decisions, including whether universal screening or evaluation might be indicated.
Potential risk factors of higher prevalence are also analyzed, and OSA severity described, to better understand OSA in this population. Although there is no gender difference in non-genetically affected children with OSA, a higher prevalence occurs in adult males and postmenopausal women. Exploration of gender differences was thus conducted in this study. Additionally, younger children tend to have narrower airways and thus may be at higher risk for OSA. Thus, we conducted an analysis of prevalence of OSA in different age groups (<2 years old, 2-21 years of age and adults). Among the published data there is great diversity in the rate of OSA among individuals with DS, possibly highlighting differences in age, body habitus and associated medical consequences across studies. Additionally, over the years researchers used different grading criteria for AHI (e.g., the cutoff number for OSA severity, and oxygen desaturation to diagnose hypopnea), potentially affecting OSA prevalence in the DS literature. Thus, the aim of this study was to estimate the overall point prevalence of OSA among people with DS, while examining factors that may explain the heterogeneity in prevalence across studies.
Methods
Study Selection and Data Extraction
A literature search was conducted using the following search engines: PubMed, Google Scholar, PLOS and Ovid. All English language published articles were identified by searching for “Down syndrome” crossed by “obstructive sleep apnea” and “polysomnography”. Conventionally, PSG has been used as the reference standard for the diagnosis of OSA, especially in individuals with DS. Thus, studies utilizing actigraphy, pulse oximetry or parental reports were excluded. Similarly, parental reports (e.g., studies using the OSA-18) were omitted given the only modest to moderate correlation between such reports and diagnosis of OSA using PSG. All studies reporting PSG (in-laboratory only) were included.
Analysis of the prevalence of OSA was assessed among people with DS while examining the role of risk factors that included age group (infant, children/adolescents, and adults), gender (male vs female), and study geographical location (i.e., country or region). Additionally, while adenotonsillectomy (AT) is effective in up to 75% of children in general, it is effective in only 25-45% of children with DS and those with other craniofacial abnormalities older than 7 years of age 12. The second half of the study examines the effect of AT, both collective surgeries combined and each individually (i.e., tonsillectomy, adenoidectomy and combined adenotonsillectomy and lingual tonsillar removal), in addition to the lingual tonsillectomy effect on OSA in children with DS.
Statistical Analysis
Data were analyzed using MetaXL 5.3 (EpiGear.com). Summary estimates of prevalence were calculated using a random effects model with double arcsin transformation 13. To quantify the amount of dispersion between studies, I2 was used, with 25%, 50% and 75% representing small, moderate and high levels of heterogeneity respectively. However, since some authors question the absolute function of I2 in detecting heterogeneity, Cochran’s Q value was also reported. Prevalence estimates were stratified by age (under 2 years; 2 through 21 years; over 21 years), OSA severity (AHI = 1 vs. AHI = 1.5 vs. AHI = 2), and country or region of the study sample (e.g., United States; Europe; Asia) to examine whether each of these factors affected prevalence.
Results
Included Studies
A literature search yielded 73 publications in the English language that involved discussion about the prevalence or incidence of OSA in the DS population. However, since PSG was the reference standard to accurately diagnose OSA, 13 studies were excluded due to their use of parental questionnaire 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26 or endoscope 27, 28, 29; three studies were excluded due to their use of pulse oximetry, a less accurate indicator of OSA 9, 30, 31 and/or in-home sleep studies 11; 24 studies were eliminated because only partial or incomplete information was provided 2, 32, 33, 34, 35, 36, 37, 38, 39, 40, 27, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53; three were excluded due to imaging of the airway 54, 55, 56; another three that used endoscopy 27, 28, 29; two other studies were excluded due to their focus on adenotonsillectomy outcome which held a selection bias 57, 58;. and another study was eliminated as it contained data already included in a larger study 59. Due to largely overlapping data, two more studies were eliminated 24, 60. Finally, one study used primarily in home PSG, with a small subgroup using in laboratory PSG; however, separate statistics were not provided for the laboratory PSG subgroup so this study was eliminated 61. A final set of 10 studies were included that examined non-referred (e.g., who were not referred for a sleep study due to concern about sleep problems, upper airway obstruction, or other potentially comorbid problems) community samples of people with DS, containing 443 individuals 4, 5, 6, 59, 62, 63, 64, 65, 66, 67. In addition, 13 studies of 1,026 individuals were included to examine the prevalence of OSA among referred samples (see Table 1 for a summary of included studies) 3, 11, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78.
Table 1. Study Characteristics and Prevalence of OSA among Individuals with Down Syndrome| Author | N | Age Range (Mean Age) | Gender (Male%) | Average AHI | % OSA | Sample |
| Austeng et al. (2014). | 29 | 8 yrs | 48.3% | 10±8.8 | 96.6% | Non-referred |
| Banjar et al. (2013). | 23 | -- | 65.0% | 12.3±13.4 | 83.0% | Referred, as some participants were recruited while attending an appointment while sick (respiratory problems), others while attending a well-child visit. |
| Basil et al. (2016). | 177 | 2.1-19.1 yrs (10.6) | 55.0% | 74.0% | Referred (some participants were referred due to concerns over sleep problems, others participated in routine screening). | |
| Breslin et al. (2014). | 31 | 7-12 yrs (9.6) | 39.5% | 5.8±7.2 | 61.3% | Non-referred |
| Brockmann et al. (2016). | 8 | 0.8-6.6 yrs (3.4) | 50% | 4.9 | 87.5% | Referred due to snoring, etc. |
| Brooks et al. (2015). | 25 | 7.2-18.7 yrs (10.1) | 56% | 76% | Non-referred | |
| Capone et al. (2013). | 37 | 14-30 yrs | 51.4% | 75.7% | Referred (in a program for co-morbid medical or mental health problems). | |
| Dyken et al. (2003). | 19 | 3-18 yrs (9.1) | 47.4% | 9.7±20.6 | 63.2% | Non-referred |
| Esbensen et al. (2016). | 455 | 5-21 yrs (12.58) | 55% | 63.1% | Referred for sleep problems | |
| Fitzgerald et al. (2007). | 33 | 0.2-19 yrs (4.9) | 60.6% | 12.9±12.0 | 87.9% | Referred for snoring |
| Goffinski et al. (2015). | 59 | 0-0.5 yrs (0.09) | 54.2% | 19.0±14.0 | 95% | Referred for sleep problems |
| Levanon et al. (1999). | 23 | 1.7-8 yrs (4.8) | 60.9% | 2.8±2.3 | 43.5% | Referred for breathing difficulties while asleep |
| Lin et al. (2014). | 49 | 0.3-16.9 yrs (6.2) | 53% | 6.1 | 71% | Referred for suspected OSA |
| Linz et al. (2013). | 51 | 0.1-11.2 months (2.7 m) | 52.9% | 52.9% | Referred for an oral appliance | |
| Maris et al. (2016). | 122 | 2.8-10.7 yrs (5.2) | 56.6% | 4.3 | 66.4% | Non-referred |
| Miguel-Diaz et al. (2003) | 108 | 1 – 18 yrs (7.9) | 63.9% | 6.2±6.8 | 64.8% | Non-referred |
| Mitchell et al. (2003). | 23 | 1-10.2 yr (1.8) | 56.5% | 48.0% | Referred for upper airway obstruction | |
| Ng et al. (2006). | 22 | <18 yrs (10.8) | 68.2% | 45.6% | Non-referred | |
| Resta et al. (2003). | 6 | 28-53 yrs (38.7) | 50.0% | 19±9.2 | 83.3% | Non-referred |
| Rosen (2010). | 29 | 0-2 yrs | 55.2% | Referred for OSA evaluation | ||
| Shete et al. (2010). | 59 | (8.5) | 64.0% | 15.3±12.6 | 72.9% | Referred (some participants were referred for tonsillectomy). |
| Shott et al. (2006). | 65 | 1.7-5.3 yrs (3.5) | 57.0% | Non-referred | ||
| Trois et al. (2009). | 16 | 19-56 yrs | 50.0% | 94.0% | Non-referred |
I – Prevalence of OSA and Potential Moderators
Overall Prevalence of OSA among People with Down Syndrome
The pooled prevalence of OSA across the 10 studies using non-referred samples was 71.5% (95% CI: 62.6 to 79.6). The I² statistic was 69.8% (95% CI: 42.0 to 84.3) and Cochran’s Q was 29.8 (p < 0.001), suggesting heterogeneity in prevalence across studies. The presence of heterogeneity is further supported visually by the forest plot (see Table 2). To address heterogeneity across studies, subgroup analyses were conducted.
Table 2.Prevalence of obstructive sleep apnea (OSA) among people with Down Syndrome, non-referred samples
Examining the 13 studies of referred samples, a similar pooled prevalence of OSA was found at 69.6% (95% CI: 61.6 to 77.1). The I² statistic was 81.9% (95% CI: 70.3 to 89.0) and Cochran’s Q was 66.5 (p < 0.001), suggesting high level of heterogeneity in prevalence across studies. The presence of heterogeneity is again further supported visually by the forest plot (see Table 3).
Table 3.Prevalence of obstructive sleep apnea (OSA) among people with Down Syndrome, referred samples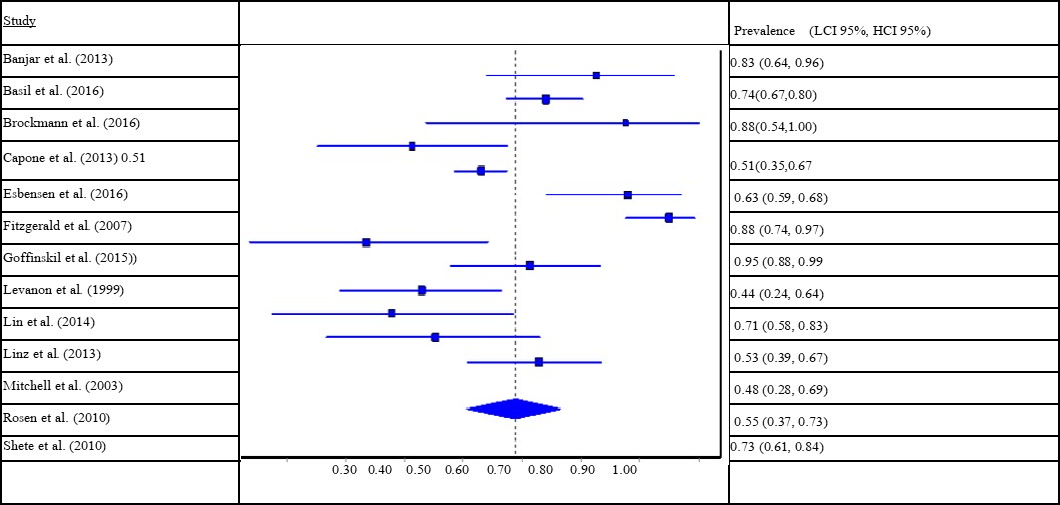
To address heterogeneity across studies, subgroup analyses were conducted. Given the high degree of similarity in prevalence rate across non-referred and referred samples, the non-referred and referred samples were pooled into one data set of 23 studies and 1,469 individuals for the subgroup analyses in order to enhance statistical power.
Prevalence of OSA as a Function of Severity Criterion
The Apnea Hypopnea Index (AHI) is a sleep measure commonly used to diagnose the severity of OSA; it is the sum of apneas (≥ 90% reduction of airflow for ≥ 10 seconds or equivalent to two breaths in children) and hypopneas (≥ 50% reduction in respiratory effort with ≥ 3 or 4% oxygen desaturation or followed by arousals) per hour of sleep 79. Studies use variable AHI cutoff points (≥ 1, ≥ 1.5, or ≥ 2) to diagnose and categorize the severity of OSA 79. We identified eight studies with 235 individuals using an AHI ≥ 1.0 cutoff for OSA among people with DS; these studies indicate an OSA prevalence of 78.7% (95% CI: 64.0 to 90.6) 11, 62, 65, 68, 71, 74, 75, 76. Among studies using an AHI cutoff of ≥ 1.5, six studies with 149 individuals were found, indicating an OSA prevalence of 76.8% (95% CI: 63.2 to 88.2) 62, 63, 64, 65, 66, 74. Finally, nine studies with 629 individuals were found using an AHI cutoff ≥ 2.0, indicating an OSA prevalence of 74.2% (95% CI: 65.1 to 82.4) 3, 6, 59, 62, 65, 68, 70, 72, 77. Thus, as would be expected, OSA prevalence decreases somewhat as more restrictive AHI cutoffs are utilized.
To identify the severity of OSA prevalence among children and adolescents with DS, analysis of 11 studies including 666 youth age 21 and younger that included a measure of OSA severity were included. For the purpose of examining prevalence of different OSA severities, we defined an AHI cutoff of 2.0 or greater as indicating the presence of OSA. A cutoff AHI of ˂ 2/hr was utilized in most of these studies to define the absence of OSA, while 2.0-4.9/hr is often used to define mild OSA, 5.0-9.9 to define moderate OSA, and ˃10/hr to define severe OSA. Descriptively, across these child studies 21.5% of children had mild OSA (95% CI: 16.7 to 26.7), 20.3% moderate OSA (17.3 to 23.5), and 30.4% had severe OSA (20.5 to 41.2) 3, 6, 59, 62, 63, 65, 66, 68, 70, 76, 77. All but two of these 11 child studies used an AHI cutoff of 2/hr for diagnosing OSA 11, 76.
Age Group and OSA Prevalence
To assess the effect of young age on the prevalence of OSA, the studies were divided into those of children below two years of age versus those with children and adolescents who were predominantly between age 2 and 21 years. This distinction was chosen because some research suggests substantial improvement of OSA during infancy: for example, in a small retrospective study, three out of six infants who initiated CPAP had spontaneous OSA resolution after a few months of treatment 71. Four studies were found to include primarily young children under 2 years of age (N=162) 77, 75, 69, 71. The pooled estimate of OSA prevalence was 66.5% (95% CI: 35.5 to 91.9) for this young age group, although it varied substantially across studies as Goffinskil et al. reported a much higher prevalence (94.9%) than the other three studies.
The pooled estimate of OSA prevalence for youth age 2 through 21 years (16 studies; 1,234 children) was 69.9% (95% CI: 64.9 to 74.7). Finally, there were two studies that included 22 adults over age 21. The pooled estimate of OSA prevalence was 90.0% (95% CI: 73.4 to 99.5) in these studies, higher than that of the other populations, though caution should be noted given the small number of studies and participants in this analysis. Adding a third study that included individuals age 14 to 30 years 73, the pooled OSA prevalence decreased to 77.3% (95% CI: 41.5 to 100.0) for studies predominantly including adults and older adolescents.
Since the adult population uses different AHI cutoff levels (i.e., ˂5/hr indicating a lack of OSA, 5-14.9/hr = mild, 15-29.9/hr = moderate and ≥30/hr = severe), an additional analysis was conducted for this population. Since controversy about the grading system exists in adolescent OSA assessment, the three studies that included only adults and adolescents older than 14 years of age were included 5, 67, 73. Among these three studies with 59 individuals, the pooled prevalence of mild OSA was 12.9% (95% CI: 0.0 to 37.5), moderate OSA was 28.7% (95% CI: 10.6 to 50.8), and severe OSA was 35.3% (95% CI: 5.7 to 71.7); however, given the small number of individuals in these studies, exploration of the difference in OSA grading system (i.e., AHI as 1-5-10 versus 5-15-30) was not conducted.
Oxygen Desaturation Level and OSA Prevalence
Studies differ in diagnosing hypopnea (and in turn OSA) using either 3% or 4% oxygen desaturation. Accordingly, we compared studies using 3% vs. 4% cutoffs with the expectation that OSA rates would be greater among studies using the less stringent 3% cutoff. As expected the prevalence was slightly greater for the 3% oxygen desaturation cutoff, though prevalence rates were quite similar. Among the nine studies using 3% as a cutoff, the pooled prevalence of OSA was 75.9% (95% CI: 68.0 to 83.0) 11, 60, 61, 62, 70, 71, 72, 74, 76. In comparison, among the eight studies using a 4% cutoff the prevalence of OSA was 69.1% (54.5 to 82.1), suggesting that desaturation cutoff did not substantially affect the prevalence of OSA 4, 5, 6, 43, 63, 68, 73, 77.
Study Geographical Region and OSA Prevalence.
Examining whether region of study origin impacted OSA prevalence, 13 studies with 1,047 participants were conducted in the United States 3, 4, 5, 60, 63, 64, 65, 69, 71, 72, 73, 77, 78. The pooled OSA prevalence of these studies was 66.3% (95% CI: 53.0 to 78.4). Among six studies with 504 participants in Europe, the pooled OSA prevalence was 71.5% (95% CI: 61.1 to 80.9) 6, 24, 59, 61, 62, 67, 75. Among three studies with 68 participants in Asia, OSA prevalence was 62.6% (38.5 to 84.1) 66, 68, 74. While the above prevalence rates were fairly similar, rates were somewhat higher among two studies of 82 participants in Australia, with a pooled OSA prevalence of 80.1% (95% CI: 62.0 to 93.7) 70, 76. In addition, a study in Chile reported OSA prevalence of 87.5% among eight participants 11. Thus, overall, high prevalence rates of OSA among people with DS were found throughout the world.
Gender Distribution and OSA Prevalence
Males represented 56.42% of the population included in 22 studies that reported gender (809/1434). Gender, coded as the percentage of male participants in each study, was unrelated to OSA prevalence across studies (r = -.16, n.s.). In addition, examination of 11 studies that reported the prevalence of OSA separately by gender found 71.8% of males to meet criteria for OSA (95% CI: 55.8 to 85.4) compared to 65.2% of females (95% CI: 49.5 to 79.4). Likewise, among six studies examining AHI scores across gender, the mean AHI for males was 11.3 versus 10.5 for females 6, 62, 65, 67, 70, 77. Thus, although there was not a significant gender effect found across studies, it is possible that there is a slightly greater prevalence of OSA among males with DS that was not observable given the modest sample size in most of these studies.
II – Surgery and OSA Outcome
Intervention and its Impact on AHI Scores among Individuals With DS
To assess the effect of AT and lingual tonsillectomy on OSA severity, 11 studies were identified for analysis 12, 43, 45, 58, 72, 80, 81, 82, 83, 84, 85. Four additional studies were excluded due to lack of sufficient information 55, 86, 87, 88, one study was excluded due to reliance on symptom improvement rather than PSG 89, and three other studies were excluded because they employed more invasive surgeries 18, 59, 78.
Seven of the 11 studies, totaling 143 individuals with DS, included the total average change in mean AHI among their cohort using AT, tonsillectomy, adenoidectomy and lingual adenoidectomy. The mean change of AHI following intervention ranged from 4.7 to 15.0, with a mean weighted pre-surgery AHI of 16.3 decreasing to 8.2 at post-surgery (weighted AHI change of 8.1), approximately a 50% decrease. Among four studies of 81 individuals who had AT, the mean change of AHI following AT ranged from 3.9 to 15.0 (weighted AHI change of 8.0) 45, 58, 72, 83. Three of these studies totaling 51 individuals included a pre-surgery weighted mean AHI of 17.8 decreasing to a post-surgery mean of 9.5. There were two studies examining the effect of tonsillectomy on 20 subjects reporting a mean change in AHI ranging from 4.8 to 6.7 (weighted average of 5.7) 45, 58. Only one of these studies documented pre- versus post-tonsillectomy levels, decreasing from a mean AHI of 9.1 to 5.2 among 10 individuals. One study examining adenoidectomy alone included four individuals with a mean change of AHI of 6.7 45. Lingual tonsillectomy effects were examined among two studies including 34 individuals and reported a mean AHI decrease of 8.2 and 10.2 (weighted mean change of AHI of 9.0) as the mean AHI decreased from 25.6 to 16.7 12, 84. Additionally, three studies included a percent normalization of AHI of less than 1/hr of 23.1% of individuals (12/52) 12, 83, 84. Two studies utilizing an AHI of 2 as a cutoff reported normalization for 17.8% of individuals (8/45) 58, 72. One study utilized an AHI of 5 and showed normalization of the AHI in 48% of individuals (12/25) 85. However, it is important to note that these prior studies used different inclusion criteria (i.e., cutoffs and oxygen desaturations) and sometimes included additional surgeries besides the four that we focus on above. Collectively, however, individuals with DS tend to exhibit a mild to moderate degree of improvement in OSA symptoms following surgery.
Discussion
This meta-analytic review indicates that approximately 70% of individuals with DS have OSA. The prevalence of OSA did not vary greatly by region, as prevalence rates were similarly high across the United States, Europe, and Asia. Examining age groups, we found a similar prevalence of OSA in children younger than 2 years of age (i.e., 66.5%) to those between 2 to 21 years of age (69.9%). A higher prevalence of OSA was found ranging from 77.3% to 90.0% among a small group of studies examining adults with DS. In addition, OSA appears to be more severe in adults (i.e., 13% had mild OSA, 29% moderate and 35% severe) compared to children (22%, 20% and 30%, respectively). The reason for possibly higher prevalence and more severe OSA among older individuals is unclear, but muscular weakness of the airway (e.g., laryngomalacia for example might be due to muscle weakness which might develop or worsen with age) and larger tongues among older individuals might explain this finding. Indeed, in a retrospective analysis of 24 children and younger adults with DS, endoscopic examination of their airway showed laryngomalacia to be the most common finding in 50% of the cases, tracheomalacia in 33.3%, and abnormal bronchial branching in 29.2%, while both tracheal bronchus and bronchomalacia were each present in 20.8% of cases 33. Only 25% of the cases had normal airway structure, with most having more than two abnormalities These upper airway abnormalities were again highlighted in an MRI study in which children with DS but without OSA had more narrow airways, with mid facial and mandibular hypoplasia, and increased fat content in the tongue, soft palate and parapharyngeal area compared to children without DS 90.
It is important to note the diversity of diagnostic criteria of OSA in children, with some studies using an AHI of 1 as the cutoff for OSA, and others using 1.5 or even 2/hr. However, we found only a modest increase in OSA prevalence with lower cutoffs, as most individuals met criteria for OSA based on AHI scores great than 2. The prevalence of OSA for an AHI cutoff of 1/hr was 78.7%, 1.5/hr was 76.8%, while 2/hr was 74.2%. In addition, while some studies have used desaturation of oxygen at different levels ranging from 2 to 5% desaturation (though most were either 3 or 4%) to diagnose hypopnea, sub-analysis of the difference between studies using 3% versus 4% did not appear to greatly affect the prevalence of OSA (75.9% vs 69.1%).
OSA prevalence among people with DS did not differ by gender. Nonetheless, although there was no significant correlation between the proportion of males and OSA prevalence across studies, examination of the 11 studies that reported OSA prevalence separately by gender indicated that 72% of males had OSA compared to 65% of females, suggesting the possibility of a subtle gender difference. Likewise, among six studies including the mean AHI score across genders, the mean AHI was 11.3 for males versus 10.5 for females 6, 62, 65, 67, 70, 77. As in community samples without DS, males tend to have a higher prevalence of OSA compared to females, especially in adults, possibly due to protective effects of female hormones 91.
Given the high prevalence of OSA among individuals with DS, it is imperative to note that OSA is related to cognitive problems (for example, lack of concentration), as well as strokes, myocardial infarction, hypertension and diabetes mellitus 92. It is unknown, but possible, that OSA might contribute to the known early onset of dementia and increased death at a younger age in this population 93. Thus, diagnosing OSA using PSG should be indicated in various stages of life in this population, followed by subsequent treatment if needed.
In analyzing the effect of adenotonsillectomy as well as lingual tonsillectomy, the biggest change in AHI was found in the lingual tonsillectomy group (a mean average decrease in AHI of 9.0), compared to 8.0 in the AT group. Tonsillectomy and adenoidectomy showed the smallest decreases in AHI (5.7 and 6.7 respectively). The slightly greater improvement with adenoidectomy than tonsillectomy possibly highlights a greater role of a narrow nasal passage and the effects the adenoids exert than tonsils in this group (i.e., with the oropharynx being larger than the nasopharynx, the adenoids might cause more obstruction).
There are several limitations to the current review. First, many of the included studies likely included children treated at an earlier age with adenotonsillectomy or other OSA treatment modalities, which could lower the current prevalence of OSA among individuals with DS. Although adenotonsillectomy has been shown to be somewhat ineffective in normalizing breathing while asleep as evidenced by our analysis, the potential effect of surgery history could not be taken into account.
A second limitation involves the general lack of studies reporting the presence of obstructive hypoventilation syndrome in children with DS, possibly lowering the reported prevalence of OSA in the reviewed studies. For example, in one study of 56 children with DS, 37.5% had OSA, 19.6% had obstructive hypoventilation syndrome without OSA, and 42.9% had neither 4. Third, we were unable to examine the potential mediators of the relationship between DS and OSA as most studies present cross-sectional data. Prospective studies are needed to examine potential mediators such as body mass index (BMI). It is known that obesity contributes to the development of OSA, especially in adults, and that individuals with DS have a tendency to be obese 60. However, this was not the case in a small study of 56 children with DS in which 47% of the obese children with DS were diagnosed with SDB vs 53% of the normal weight children) 4. Additionally, DS individuals with cardiac disease might be expected to have a high prevalence of OSA; however, this was not substantiated in the latter study (50% of individuals with cardiac disease had SDB vs 50% without and 64.3% had SDB without exhibiting cardiac abnormalities 4). Other factors associated with OSA that are generally not reported in the literature but that also may serve as mediators include subglottic stenosis, macroglossia, midfacial hypoplasia and hypothyroidism.
Conclusion
There is a higher propensity of OSA among individuals diagnosed with DS that can adversely affect their health. Although screening for OSA using questionnaires might be helpful in the non-DS population, evidence for the accuracy of questionnaires in children and adults with DS is lacking, with some clinicians suggesting parental bias that it is normal for children with DS to have irregular breathing 4. Since there is a high point prevalence of OSA of close to 70% among youth and up to 90% in adults, regular and frequent PSG in all individuals with DS is advised. While the American Academy of Pediatrics in 2001 suggested testing all children with DS around 4 years of age as well as those below age 4 who are suspected of having OSA, our findings suggest a need for regular PSG screening even in the adult population with this disorder. However, further attempts are needed to standardize variables such as AHI cutoffs and the use of fixed oxygen desaturation levels (especially in PSG in children) in order to establish concrete guidelines for the diagnosis and treatment of OSA in the DS population. In addition, more epidemiological research is needed on individuals diagnosed with DS to clarify the possible role that factors such as gender, age, BMI, and other comorbid medical problems may play as risk factors for OSA. In particular, research is needed with the adult population. While we found a particularly high prevalence of OSA among adults with DS, larger samples are needed to replicate this finding. Considering quality of life, individuals with DS tend to be at increased risk for attention deficit hyperactivity disorder, the symptoms of which may improve following treatment for OSA 94, 95. Finally, it is well known that individuals with DS tend to die earlier, possibly in part due to untreated OSA. Thus, early detection and treatment is required to best manage OSA in this population.
References
- 1.Bull M J.and the Committee on Genetics, “Health Supervision for. , Children With Down Syndrome,”PEDIATRICS,Aug.2011 128(2), 393-406.
- 2.Dahlqvist A, Rask E, Rosenqvist C-J, Sahlin C, K A Franklin.Sleep apnea and Down’s syndrome,”. , Acta Otolaryngol. (Stockh.),Dec.2003 123(9), 1094-1097.
- 3.Basil J S, Santoro S L, Martin L J, Healy K W, Chini B A et al.. , Retrospective Study of Obesity in Children with Down Syndrome,” J. Pediatr.,Jun.2016 173, 143-148.
- 4.Shott S R, Amin R, Chini B, Heubi C, Hotze S et al.Obstructive sleep apnea: Should all children with Down syndrome be tested?,”. , Arch. Otolaryngol. Head Neck Surg.,Apr.2006 132(4), 432-436.
- 5.M S Trois.Obstructive sleep apnea in adults with Down syndrome,”. , J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med.,Aug.2009 5(4), 317-323.
- 6.J de Miguel-Díez, J R Villa-Asensi, J L Alvarez-Sala.Prevalence of sleep-disordered breathing. in children with Down syndrome: polygraphic findings in 108 children,” Sleep,Dec.2003 26(8), 1006-1009.
- 7.Constantin E, Tewfik T L, Brouillette R T.Can the OSA-18 quality-of-life questionnaire detect obstructive sleep apnea. in children?,” Pediatrics,Jan.2010 125(1), 162-168.
- 8.Borgström A, Nerfeldt P, Friberg D.Questionnaire OSA-18 has poor validity compared to polysomnography in pediatric obstructive sleep apnea,”. , Int. J. Pediatr. Otorhinolaryngol.,Nov.2013 77(11), 1864-1868.
- 9.Jheeta S, McGowan M, Hadjikoumi I.Is oximetry an effective screening tool for obstructive sleep apnoea. in children with Down syndrome?,” Arch. Dis. Child.,Feb.2013 98(2), 164.
- 10.Kaditis A, Kheirandish-Gozal L, Gozal D.Pediatric OSAS: Oximetry can provide answers when polysomnography is not available,”. , Sleep Med. Rev.,Jun.2016 27, 96-105.
- 11.P E Brockmann.Sleep-disordered breathing in children with Down syndrome: Usefulness of home polysomnography,”. , Int. J. Pediatr. Otorhinolaryngol.,Apr.2016 83, 47-50.
- 12.E J Propst.Midline posterior glossectomy and lingual tonsillectomy in obese and nonobese children with down syndrome: Biomarkers for success,”. , The Laryngoscope,Mar.2017 127(3), 757-763.
- 13.Barendregt J J, Doi S A, Lee Y Y, Norman R E, Vos T.. , Meta-analysis of prevalence,” J. Epidemiol. Community Health,Nov.2013 67(11), 974-978.
- 14.Ashworth A, C M Hill, Karmiloff-Smith A, Dimitriou D. (2015) The Importance of Sleep: Attentional Problems. in School-Aged Children With Down Syndrome and Williams Syndrome,” Behav. Sleep. Med 13(6), 455-471.
- 15.J L Bassell, Phan H, Leu R, Kronk R, Visootsak J.Sleep profiles in children with Down syndrome,”. , Am. J. Med. Genet 2015, 1830-1835.
- 16.Carter M, McCaughey E, Annaz D, C M Hill.Sleep problems in a Down syndrome population,”. , Arch. Dis. Child.,Apr.2009 94(4), 308-310.
- 17.Churchill S S, Kieckhefer G M, Bjornson K F, Herting J R.Relationship between sleep disturbance and functional outcomes in daily life habits of children with Down syndrome,”. , Sleep,Jan.2015 38(1), 61-71.
- 18.Cockerill C C, Frisch C D, Rein S E, Orvidas L J.Supraglottoplasty outcomes in children with Down syndrome,”. , Int. J. Pediatr. Otorhinolaryngol.,Aug.2016 87, 87-90.
- 19.Cotton S, Richdale A.Brief report: parental descriptions of sleep problems in children with autism. Down syndrome, and Prader-Willi syndrome,” Res. Dev. Disabil.,Apr.2006 27(2), 151-161.
- 20.S M Cotton, A L Richdale.Sleep patterns and behaviour in typically developing children and children with autism, Down syndrome, Prader-Willi syndrome and intellectual disability,” Res. Autism Spectr. Disord.,Jul.2010 4(3), 490-500.
- 21.A J Esbensen, E K Hoffman.Reliability of parent report measures of sleep in children with Down syndrome,”. , J. Intellect. Disabil. Res. JIDR,Mar.2017 61(3), 210-220.
- 22.C A Hoffmire, C I Magyar, H V Connolly, I D Fernandez, E van Wijngaarden.High prevalence of sleep disorders and associated comorbidities in a community sample of children with Down syndrome,”. , J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med.,Apr.2014 10(4), 411-419.
- 23.A F Lukowski, H M Milojevich.Sleep problems and temperament in young children with Down syndrome and typically developing controls,”. , J. Intellect. Disabil. Res. JIDR, Mar.2017 61(3), 221-232.
- 24.Maris M, Verhulst S, Wojciechowski M, Heyning P Van de, Boudewyns A.Sleep problems and obstructive sleep apnea in children with down syndrome, an overwiew,”. , Int. J. Pediatr. Otorhinolaryngol.,Mar.2016 82, 12-15.
- 25.Rosen D, Lombardo A, Skotko B, E J Davidson.Parental perceptions of sleep disturbances and sleep-disordered breathing. in children with Down syndrome,” Clin. Pediatr. (Phila.),Feb.2011 50(2), 121-125.
- 26.Sanders E, C M Hill, H J Evans, Tuffrey C. (2015) The Development of a Screening Questionnaire for Obstructive Sleep Apnea. in Children with Down Syndrome,” Front. Psychiatry 6, 147.
- 27.I N Jacobs, R F Gray, N W Todd.Upper airway obstruction in children with Down syndrome,”. , Arch. Otolaryngol. Head Neck Surg.,Sep.(1996) 122(9), 945-950.
- 28.M T Truong, V G Woo, P J Koltai.Sleep endoscopy as a diagnostic tool in pediatric obstructive sleep apnea,”. , Int. J. Pediatr. Otorhinolaryngol.,May2012 76(5), 722-727.
- 29.Fung E, Witmans M, Ghosh M, Cave D, El-Hakim H.Upper airway findings in children with Down syndrome on sleep nasopharyngoscopy: case-control study,”. , J. Otolaryngol. - Head Neck Surg. J. Oto-Rhino-Laryngol. Chir. Cervico-Faciale,Apr.2012 41, 138-144.
- 30.A M Coverstone.Overnight pulse oximetry for evaluation of sleep apnea among children with trisomy 21,”. , J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med.,Dec.2014 10(12), 1309-1315.
- 31.R J Stores, Stores G.The significance of aspects of screening for obstructive sleep apnoea in children with Down syndrome,”. , J. Intellect. Disabil. Res. JIDR,Apr.2014 58(4), 381-392.
- 32.Andreou G, Galanopoulou C, Gourgoulianis K, Karapetsas A, Molyvdas P.Cognitive status in Down syndrome individuals with sleep disordered breathing deficits. , (SDB),” Brain Cogn.,Oct.2002 50(1), 145-149.
- 33.Bertrand P, Navarro H, Caussade S, Holmgren N, Sánchez I.Airway anomalies in children with Down syndrome: endoscopic findings,” Pediatr. , Pulmonol.,Aug.2003 36(2), 137-141.
- 34.Brouilette R.A diagnostic approach to suspected obstructive sleep apnea in children,”. , J. Pediatr.,Jul.1984 105(1), 10-14.
- 35.Chen C-C J J, Spanò G, J O Edgin.The impact of sleep disruption on executive function. in Down syndrome,” Res. Dev. Disabil.,Jun.2013 34(6), 2033-2039.
- 36.Clausen J, E A Sersen, Lidsky A.Sleep patterns in mental retardation:. , Down’s syndrome,” Electroencephalogr. Clin. Neurophysiol.,Aug.1977 43(2), 183-191.
- 37.Esbensen A J.Sleep problems and associated comorbidities among adults with Down syndrome,”. , J. Intellect. Disabil. Res. JIDR,Jan.2016 60(1), 68-79.
- 38.Ferri R, Curzi-Dascalova L, S Del Gracco, Elia M, Musumeci S A et al.Heart rate variability and apnea during sleep in Down’s syndrome,”. , J. Sleep Res.,Dec.(1998) 7(4), 282-287.
- 39.Fukuma E, Umezawa Y, Kobayashi K, Motoike M. (1974) Polygraphic study on the nocturnal sleep of children with Down’s syndrome and endogenous mental retardation,” Folia Psychiatr. , Neurol. Jpn 28(4), 333-345.
- 40.Hamaguchi H, Hashimoto T, Mori K, Tayama M. (1989) . Sleep in the Down syndrome,” Brain Dev 11(6), 399-406.
- 41.K M Jensen.Greater risk of hospitalization. in children with Down syndrome and OSA at higher elevation,” Chest,May2015 147(5), 1344-1351.
- 42.Konstantinopoulou S.Relationship between obstructive sleep apnea cardiac complications and sleepiness. in children with Down syndrome,” Sleep Med.,Jan.2016 17, 18-24.
- 43.C L Marcus, T G Keens, D B Bautista, Pechmann W S von, S L Ward.Obstructive sleep apnea. in children with Down syndrome,” Pediatrics,Jul.1991 88(1), 132-139.
- 44.Miano S.Sleep phenotypes of intellectual disability: a polysomnographic evaluation in subjects with Down syndrome and Fragile-X syndrome,”. , Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol.,Jun.2008 119(6), 1242-1247.
- 45.Mims M, P J Thottam, Kitsko D, Shaffer A, Choi S.. Characterization of Sleep Architecture in Down Syndrome Patients Pre and Post Airway Surgery,” Cureus,Jan.2017 9(1), 983.
- 46.L C Nisbet, N, T F Hoban, L M O’Brien.Effect of body position and sleep state on obstructive sleep apnea severity in children with Down syndrome,”. , J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med.,Jan.2014 10(1), 81-88.
- 47.G M Nixon, S N Biggs, Jitpiriyaroj S, Horne R S C.The Relationship Between Sleep-Disordered Breathing Severity and Daytime Adaptive Functioning. in Children with Down Syndrome,” CNS Neurosci. Ther.,Nov.2016 22(11), 936-937.
- 48.D M O’, Horne R S C, M J Davey, S A Hope, A M Walker et al.The heart rate response to spontaneous arousal from sleep is reduced in children with Down syndrome referred for evaluation of sleep-disordered breathing,”. , Am. J. Physiol. Heart Circ. Physiol.,Jun.2010 298(6), 1986-1990.
- 49.Ono J, Chishaki A, Ohkusa T, Sawatari H, Nishizaka M et al.Obstructive sleep apnea-related symptoms. in Japanese people with Down syndrome,” Nurs. Health Sci.,Dec.2015 17(4), 420-425.
- 50.Senthilvel E, Krishna J.Body position and obstructive sleep apnea in children with Down syndrome,”. , J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med.,Apr.2011 7(2), 158-162.
- 51.D P Southall, V A Stebbens, Mirza R, M H Lang, C B et al.Upper airway obstruction with hypoxaemia and sleep disruption in Down syndrome,”. , Dev. Med. Child 29(6), 734-742.
- 52.V A Stebbens, Dennis J, M P Samuels, C B, D P Southall.Sleep related upper airway obstruction in a cohort with Down’s syndrome,”. , Arch. Dis. Child.,Nov.1991 66(11), 1333-1338.
- 53.Sudarsan S S, Paramasivan V K, Arumugam S V, Murali S, Kameswaran M.Comparison of treatment modalities in syndromic children with obstructive sleep apnea--a randomized cohort study,”. , Int. J. Pediatr. Otorhinolaryngol., Sep.2014 78(9), 1526-1533.
- 54.Shott S R, Donnelly L F.Cine magnetic resonance imaging: evaluation of persistent airway obstruction after tonsil and adenoidectomy. in children with Down syndrome,” The Laryngoscope,Oct.2004 114(10), 1724-1729.
- 55.Donnelly L F, CR LaRose Shott, Chini B A, Amin R S.Causes of persistent obstructive sleep apnea despite previous tonsillectomy and adenoidectomy in children with down syndrome as depicted on static and dynamic cine. , MRI,” AJR Am. J. Roentgenol.,Jul.2004 183(1), 175-181.
- 56.Shott S R.Down syndrome: analysis of airway size and a guide for appropriate intubation,”. , The Laryngoscope,Apr.2000 110(4), 585-592.
- 57.N A Goldstein, D R Armfield, L A Kingsley, L M Borland, G C Allen et al.Postoperative complications after tonsillectomy and adenoidectomy in children with Down syndrome,”. , Arch. Otolaryngol. Head Neck Surg 124(2), 171-176.
- 58.Maris M, Verhulst S, Wojciechowski M, Heyning P Van de, Boudewyns A.Outcome of adenotonsillectomy in children with Down syndrome and obstructive sleep apnoea,”. , Arch. Dis. Child 102(4), 331-336.
- 59.Maris M, Verhulst S, Wojciechowski M, Heyning P Van de, Boudewyns A.. Prevalence of Obstructive Sleep Apnea in Children with Down Syndrome,” Sleep,Mar.2016 39(3), 699-704.
- 60.C B Shires, S L Anold, R A Schoumacher, G W Dehoff, S K Donepudi et al.Body mass index as an indicator of obstructive sleep apnea in pediatric Down syndrome,”. , Int. J. Pediatr. Otorhinolaryngol.,Jul.2010 74(7), 768-772.
- 61.C M Hill.Prevalence and predictors of obstructive sleep apnoea. in young children with Down syndrome,” Sleep Med.,Dec.2016vol.27–28 99-106.
- 62.E M.Austeng et al., “Obstructive sleep apnea in younger school children with Down syndrome,”. , Int. J. Pediatr. Otorhinolaryngol.,Jul.2014 78(7), 1026-1029.
- 63.L J Brooks, M N Olsen, A M Bacevice, Beebe A, Konstantinopoulou S et al.Relationship between sleep, sleep apnea, and neuropsychological function. in children with Down syndrome,” Sleep Breath. Schlaf Atm.,Mar.2015 19(1), 197-204.
- 64.Breslin J, Spanò G, Bootzin R, Anand P, Nadel L et al.Obstructive sleep apnea syndrome and cognition. in Down syndrome,” Dev. Med. Child Neurol.,Jul.2014 56(7), 657-664.
- 65.M E Dyken, D C Lin-, Poulton S, M B Zimmerman, Sedars E.Prospective polysomnographic analysis of obstructive sleep apnea in down syndrome,”. , Arch. Pediatr. Adolesc. Med.,Jul.2003 157(7), 655-660.
- 66.D K Ng.Obstructive sleep apnoea. in children with Down syndrome,” Singapore Med. J.,Sep.2006 47(9), 774-779.
- 67.Resta O.Sleep related breathing disorders in adults with Down syndrome,”. , Syndr. Res. Pract. J. Sarah Duffen Cent.,Aug.2003 8(3), 115-119.
- 68.Levanon A, Tarasiuk A, Tal A.Sleep characteristics in children with Down syndrome,”. , J. Pediatr.,Jun.1999 134(6), 755-760.
- 69.R B Mitchell, Call E, Kelly J.Diagnosis and therapy for airway obstruction in children with Down syndrome,”. , Arch. Otolaryngol. Head Neck Surg.,Jun.2003 129(6), 642-645.
- 70.D A Fitzgerald, Paul A, Richmond C.Severity of obstructive apnoea in children with Down syndrome who snore,”. , Arch. Dis. Child.,May.2007 92(5), 423-425.
- 71.Rosen D.Some infants with Down syndrome spontaneously outgrow their obstructive sleep apnea,”. , Clin. Pediatr. (Phila.),Nov.2010 49(11), 1068-1071.
- 72.Shete M M, Stocks RMS, Sebelik M E, Schoumacher R A.Effects of adeno-tonsillectomy on polysomnography patterns in Down syndrome children with obstructive sleep apnea: a comparative study with children without Down syndrome,”. , Int. J. Pediatr. Otorhinolaryngol.,Mar.2010 74(3), 241-244.
- 73.G T Capone, J M Aidikoff, Taylor K, Rykiel N.Adolescents and young adults with Down syndrome presenting to a medical clinic with depression: co-morbid obstructive sleep apnea,”. , Am. J. Med. Genet 2013, 2188-2196.
- 74.Banjar H H. (2013) Sleep study abnormalities in patients with Down syndrome.,”. , Curr. Pediatr 17(1).
- 75.Linz A, M S Urschitz, Bacher M, P E Brockmann, Buchenau W et al.Treatment of obstructive sleep apnea in infants with trisomy 21 using oral appliances,”. , Cleft Palate-Craniofacial J. Off. Publ. Am. Cleft Palate-Craniofacial Assoc.,Nov.2013 50(6), 648-654.
- 76.S C Lin, M J Davey, Horne R S C, G M Nixon.Screening for obstructive sleep apnea in children with Down syndrome,”. , J. Pediatr.,Jul.2014 165(1), 117-122.
- 77.Goffinski A.Obstructive sleep apnea in young infants with Down syndrome evaluated in a Down syndrome specialty clinic,”. , Am. J. Med. Genet 2015, 324-330.
- 78.A J Esbensen, D W Beebe, K C Byars, E K Hoffman.. Use of Sleep Evaluations and Treatments in Children with Down Syndrome,” J. Dev. Behav. Pediatr. JDBP,Oct.2016 37, 629-636.
- 79.Health Quality Ontario, “Polysomnography in patients with obstructive sleep apnea: an evidence-based analysis,”. , Ont. Health Technol. Assess. Ser.,2006 6(13), 1-38.
- 80.G J Wiet, Bower C, Seibert R, Griebel M.Surgical correction of obstructive sleep apnea in the complicated pediatric patient documented by polysomnography,”. , Int. J. Pediatr. Otorhinolaryngol.,Aug.1997 41(2), 133-143.
- 81.J A Merrell, S R.OSAS in Down syndrome: T&A versus T&A plus lateral pharyngoplasty,”. , Int. J. Pediatr. Otorhinolaryngol.,Aug.2007 71(8), 1197-1203.
- 82.P J Thottam, Choi S, J P Simons, D J Kitsko.Effect of Adenotonsillectomy on Central and Obstructive Sleep Apnea in Children with Down Syndrome,” Otolaryngol.--Head Neck Surg. , Off. J. Am. Acad. Otolaryngol.-Head Neck Surg.,Oct.2015 153(4), 644-648.
- 83.P J Thottam, Trivedi S, Siegel B, Williams K, Mehta D.Comparative outcomes of severe obstructive sleep apnea in pediatric patients with Trisomy 21,”. , Int. J. Pediatr. Otorhinolaryngol.,Jul.2015 79(7), 1013-1016.
- 84.Prosser J D, O Rodriguez Shott, Simakajornboon N, Meinzen-Derr J, Ishman S L.Polysomnographic outcomes following lingual tonsillectomy for persistent obstructive sleep apnea in down syndrome,”. , The Laryngoscope,Feb.2017 127(2), 520-524.
- 85.Maris M, Verhulst S, Saldien V, Heyning P Van de, Wojciechowski M et al.Drug-induced sedation endoscopy in surgically naive children with Down syndrome and obstructive sleep apnea,”. , Sleep Med.,Aug.2016 24, 63-70.
- 86.Khalid-Raja M, Tzifa K.Current demand of paediatric otolaryngology input for children with Down’s syndrome in a tertiary referral centre,”. , J. Laryngol. Otol.,Nov.2016 130(11), 995-1000.
- 87.A C Yumusakhuylu, Binnetoglu A, Demir B, Baglam T, Sari M.Is it safe to perform adenotonsillectomy in children with Down syndrome?,”. , Eur. Arch. Oto-Rhino-Laryngol. Off. J. Eur. Fed. Oto-Rhino-Laryngol. Soc. EUFOS Affil. Ger. Soc. Oto-Rhino-Laryngol. - Head Neck Surg.,Sep.2016 273(9), 2819-2823.
- 88.D L Price, L J Orvidas, A L Weaver, S A Farmer.Efficacy of adenoidectomy in the treatment of nasal and middle ear symptoms in children with Down syndrome,”. , Int. J. Pediatr. Otorhinolaryngol.,Jan.2004 68(1), 7-13.
- 89.C M Bower, Richmond D.Tonsillectomy and adenoidectomy in patients with Down syndrome,”. , Int. J. Pediatr. Otorhinolaryngol.,Oct.1995 33(2), 141-148.
- 90.E C Uong.Magnetic resonance imaging of the upper airway in children with Down syndrome,”. , Am. J. Respir. Crit. Care Med.,Mar.2001.vol.163,no.3Pt1 731-736.
- 91.C K Pickett, J G Regensteiner, W D, D, J V Weil et al. (1989) Progestin and estrogen reduce sleep-disordered breathing in postmenopausal women,”. , J. Appl. Physiol. BethesdaMd1985,Apr.1989 66(4), 1656-1661.
- 92.S R Coughlin, Mawdsley L, J A Mugarza, Calverley P M A, Wilding J P H.Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome,”. , Eur. Heart J.,May2004 25(9), 735-741.
- 93.R S Bucks, Olaithe M, Eastwood P.Neurocognitive function in obstructive sleep apnoea: a meta-review,” Respirol. , Carlton Vic,Jan.2013 18(1), 61-70.
Cited by (7)
- 1.Diskin Catherine, McVeigh Terri P., Cox Des W., 2020, Sleep disordered breathing in children with Down syndrome in the Republic of Ireland, American Journal of Medical Genetics Part A, 182(12), 2847, 10.1002/ajmg.a.61855
- 2.Karlik Joelle B., Raol Nikhila, Gilbertson Laura, 2020, Hypoglossal Nerve Stimulator Placement for Pediatric Trisomy 21 Patients with Refractory Obstructive Sleep Apnea: A Case Series, Children, 7(8), 81, 10.3390/children7080081
- 3.Lee Paul B., Chung Michael T., Johnson Jared, Lucas Jordyn, Priest Caitlin R., et al, 2021, Tongue-Based Procedures in Treating Refractory Obstructive Sleep Apnea in Down Syndrome Patients: A Systematic Review and Meta-Analysis, FACE, 2(1), 65, 10.1177/2732501621992447
- 4.Wagner J. H., McPherson Pamela, Pistorius Rebecca, Shukla Anuj, Parvataneni Swathi, 2020, , , (), 367, 10.1007/978-3-030-46835-4_23
- 5.Bartolucci Maria Lavinia, Berteotti Chiara, Alvente Sara, Bastianini Stefano, Guidi Sandra, et al, 2021, Obstructive sleep apneas naturally occur in mice during REM sleep and are highly prevalent in a mouse model of Down syndrome, Neurobiology of Disease, 159(), 105508, 10.1016/j.nbd.2021.105508
- 6.Uchi Takafumi, Konno Shingo, Kihara Hideo, Sugimoto Hideki, 2024, A Multifaceted Approach to Seizure Management in a Patient With Down Syndrome, Obstructive Sleep Apnea, and Hypothyroidism: A Case Report, Cureus, (), 10.7759/cureus.55465
- 7.Callegari Marilia Rezende, dos Santos Kelly Brito, de Oliveira Barbara Valente, Amorim Ana Rita Avelino, Cymrot Raquel, et al, 2023, Sleep assessment in adults with Down syndrome: correlation between functionality and polysomnographic findings, Arquivos de Neuro-Psiquiatria, 81(06), 544, 10.1055/s-0043-1768670
