Abstract
The elemental analysis of the compound confirms the ratio of the compound. The sharp and well defined Bragg peaks obtained in the powder X-ray diffraction pattern confirm the crystalline nature of the compound. The single crystal unit cell parameters of the compound show that the grown crystal belongs to orthorhombic system with space group P. The optical property of the compound was ascertained through UV-visible spectral analysis. The various characteristics absorption bands in the compound were assigned through Fourier transform infra-red (FTIR) spectroscopy. Thenonlinear optical property study indicates that the compound has SHG efficiency 0.50 times greater than that of standard potassium dihydrogen phosphate (KDP).
Author Contributions
Academic Editor: Loai Aljerf, Department of Life Sciences, Faculty of Dentistry, Damascus University
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2023 S. Silambarasan, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
The nonlinear optical crystals have been given much importance, because of their potential applications such as telecommunication, optical information process, frequency conversion and optical disk data storage 1, 2. Amino acids are interesting materials for NLO applications as they contain a proton donor carboxyl acid (COO−) group and a proton acceptor amino (NH+2) group in them. Most recent works have demonstrated that organic crystals can have very large nonlinear susceptibilities compared with that of inorganic crystals, but their use is impeded by poor mechanical properties and the inability to produce large crystals 3. Organic crystals show prominent properties due to their fast and nonlinear response. Over a broad frequency range, they have not only inherent synthetic flexibility and large optical damage threshold, but also have some inherent drawbacks, such as voltality, low thermal stability and weak mechanical strength 4. The naturally occurring amino acid l–asparagine monohydrate plays a role in the metabolic control of some cell functions in nerve and brain tissues and is also used by many plants as a nitrogen reserve source 5. Recently, the growth and characterization of the single crystals of the NLO materials, viz., l-asparaginium picrate 6 and l-asparagine monohydrate 7 have been reported. Based on the above facts, we have reported the synthesis, spectral and nonlinear optical characterization of l-asparagine potassium nitrate crystal.
Synthesis of L-Asparagine Potassium Nitrate
Synthesized by L-asparagine potassium nitrate taking them in the molar ratio 1:1 according to the following reaction.
KNO3+ (C4H8N2O3)→K (C₄H₇N₂O₃) +HNO3
The calculated amount of L - asparagine was first dissolved in 60 ml of deionized water. Then potassium nitrate was added to the solution slowly by continuous stirring and a homogeneous mixture was obtained. Then the solution was left undisturbed. After about (Figure 1)
Characterization of L-Asparagine Potassium Nitrate Crystals
Powder X-ray Diffraction Analysis
Powder X-ray diffraction studies have been carried out for L-asparagine potassium nitrate using Rigaku X-ray diffractometer with Cu Kα (λ= 1.5405Å) radiation over the range 10–90° at room temperature. The Powder X-ray diffraction pattern of L-asparagine potassium nitrate crystal obtained is shown in The well-defined sharp peaks in the XRD pattern confirm the high crystallinity of the grown L-asparagine potassium nitrate. The observed Powder X-ray diffraction pattern was indexed using JCPDS (PDF # 301529) 11. The L-asparagine potassium nitrate crystals have orthorhombic structure with lattice parameters a = 12.895 Å, b = 6.6023 Å, c = 4.645 Å, α=90°, β=90°, γ=90o and V = 363.2 Å3. (Figure 2)
Fourier Transform Infrared (FTIR) spectral analysis
Fourier Transform Infrared (FTIR) Spectroscopy is used to identify the functional groups and modes of vibration of the synthesized L-asparagine potassium nitrate. In order to analyze qualitatively the presence of functional groups in L-asparagine potassium nitrate, the FTIR spectrum was recorded using a Perkin Elmer spectrometer II in the range 400 - 4000 cm-1. The FT-IR spectrum of L-asparagine potassium nitrate is shown in Assignments for vibration frequencies observed in FTIR spectrum of L-asparagine potassium nitrate are shown in These are in good agreement with the reported values 8. The band in the region 1560 cm-1 is due to the symmetric stretching mode of vibrations of CO2 molecules and the bands at 2929 cm-1 and 3395 cm-1 are due to O-H stretching vibrations of Water molecules absorbed from air. (Figure 3)
Figure 3. Fourier Transform Infrared (FTIR) spectral analysis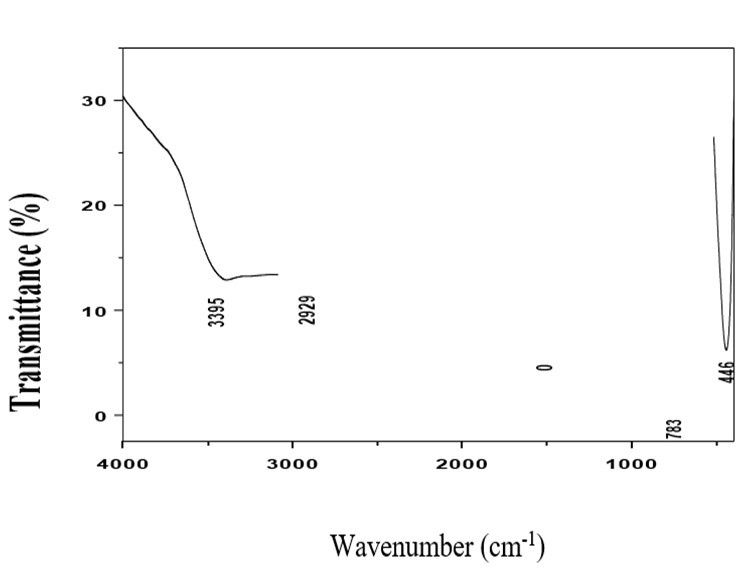
Photoluminescence Studies
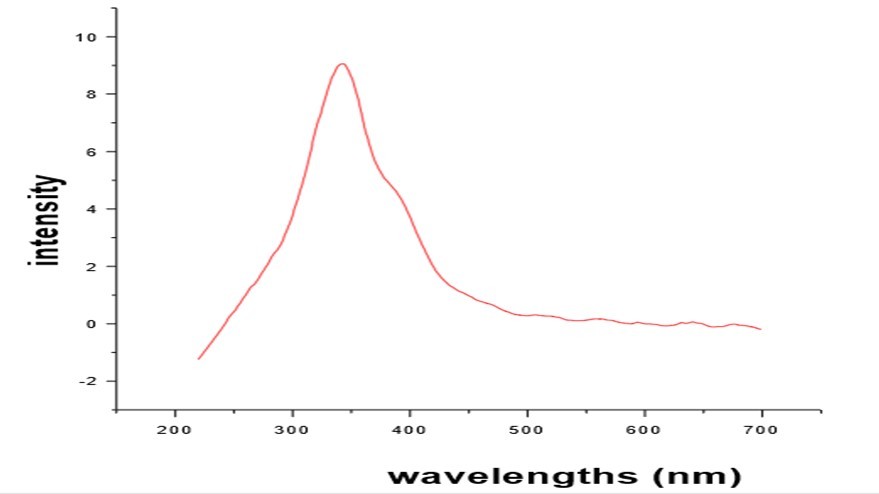
The optical behavior of the L-asparagine potassium nitrate was analyzed by PL measurements. The photoluminescence spectrum was recorded for L-asparagine potassium nitrate using Perkin Elmer LS45 Luminescence spectrometer in the range of 200 and 700 nm. The excitation wavelength used was 247nm. Spectrum shows a broad emission peak centered at 343nm. It corresponds to Ultraviolet (Figure 4)
Energy Dispersive X-ray Analysis
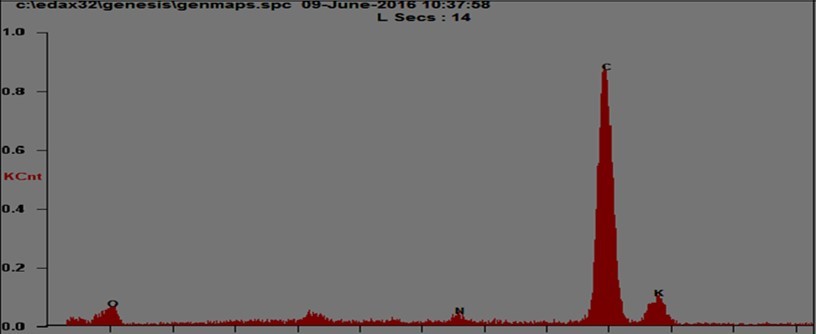
The energy dispersive X-ray analysis (EDAX) is an important tool for identifying the elements present in a material and hence to determine the chemical composition of these elements in the material. The L-asparagine potassium nitrate was subjected to EDAX using FEI QUANTA FEG 250 scanning electron microscope. EDAX spectrum of L-asparagine potassium nitrate is shown in EDAX quantification data of L-asparagine potassium nitrate is presented in It is mentioned. (Figure 5) (Table 1)
Table 1.| Element | Wt% | At |
| OK | 31.52 | 71.59 |
| CK | 13.02 | 9.36 |
| NK | 5.69 | 4.22 |
| K | 49.77 | 14.83 |
| Matrix | Correction | ZAF |
Thermal studies (TG/DTA)
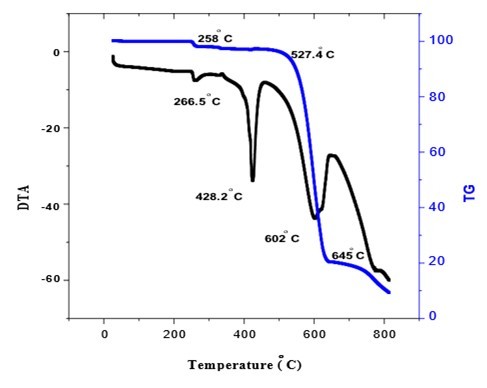
TG and DTA spectra of L-asparagine potassium nitrate were recorded using NETSZCH STA 409C thermal analyzer. The sample was heated from 30 to 800 °C at a scanning rate of 20K/min in nitrogen atmosphere. shows the TG-DTA thermo grams of L-asparagine potassium nitrate This indicates that the material is thermally stable up to 258°C. The material undergoes decomposition in three phase transitions. The first phase transition occurs at 258°C and corresponds to a weight loss of 2%. The second phase transition occurs at 428°C and has no weight loss. This indicates melting of material. The major weight loss of 77.7% starts at 527.4°C and it continues up to 645°C. This weight loss indicates that the material undergoes decomposition during this transition and it may be due to the evaporation of volatile material in the compound. Beyond 645°C, no weight loss is observed. In the DTA thermo gram an endothermic peaks is observed at 428.2 °C and 602.5°C. (Figure 6)
Conclusions
L-asparagine potassium nitrate crystals are successfully synthesized by chemical method.
Good quality L-asparagine potassium nitrate crystals are grown by slow evaporation method at room temperature using water as solvent.
Powder XRD studies reveal that L-asparagine potassium nitrate crystal has orthorhombic structure.
The Fourier transform Infra-Red analysis confirms the functional groups present in the L-asparagine potassium nitrate.
The UV-Vis studies show that both the crystals are optically transparent in the entire UV-Vis region. The UV cut off wavelength for L-asparagine potassium nitrate is 247nm. The optical band gap energies of L-asparagine potassium nitrate .
The Kurtz Perry powder SHG studies indicate that the SHG efficiency of L-asparagine potassium nitrate is 4.06 times that of KDP
Photoluminescence excitation studies show that when excited by light of wavelength 235nm and 247nm respectively, L-asparagine potassium nitrate.
EDAX confirm the presence of elements contained in L-asparagine potassium nitrate.
TGA/DTA studies reveal that the L-asparagine potassium nitrate is thermally stable up to 258 °C.