Abstract
Bisphenol A (BPA) is an endocrine disruptor with a weak estrogenic effect used in industry as a component of food cans. We aimed to study the toxic effects of BPA on mRNA expression of steroidogenic genes and testicular structure in mature male rats. Animals were divided into 3 groups: vehicle control rats as first group, while second group received 10 µg/kg BW and third group received BPA 15 µg/kg BW orally every alternate day for a period of 105 successive days. Serum testosterone level, mRNA expression of genes related to steroid synthesis, histopathological examination, spermatogenesis index and number of Leydig cells were evaluated in this study. Lower serum hormone levels were observed in both BPA-treated groups as compared to the control group. The gene expression patterns of steroidogenic acute regulatory protein (StAR), cytochrome P450 17a(CYP17a) and 3β-Hydroxysteroid dehydrogenase (3β-HSD) were significantly down-regulated in BPA-treated rats compared to control group. Meanwhile, the expression of aromatase (CYP19) and lutinizing hormone receptor (LHR) was significantly up-regulated. Histopathological lesions were observed in the testes and epididymis of BPA-treated rats. Spermatogenesis index and the number of Leydig cells were significantly decreased in BPA-treated groups compared with the control group. This study highlights negative effect of BPA on steroidogenic genes and testicular structure in male rats.
Author Contributions
Academic Editor: Shabir Lone, ICAR-National Dairy Research Institute, India.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2018 Basma H Marghani,et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Bisphenol A (BPA) is a ubiquitous environmental chemical that is integrated in the manufacturing process of many industrial products such as plasticizers, as well as in the production of materials used for food and potable water, such as epoxy lining of food and beverages cans 1, 2, 3, 4. It leaches from those products causing health hazards for humans and animals 2. Its presence in biological and nonbiological samples was previously verified 5. BPA is an endocrine disruptor; it binds to estrogenic receptors mimics the action of estrogen hormone 6, also, it can bind to androgen receptors, blocking endogenous androgen action 7 resulting in impairment of male reproductive function 8, 9. BPA at a dose of 10 mg/kg bw/day orally for 14 days reduced activity of testicular mitochondrial enzymes in micewith subsequent stimulation of oxidative stress through inhibiting the activities of antioxidant enzymes 10. Alternatively, extremely low concentrations of BPA inhibited activity of steroidogenic enzymes in human and rat testicular microsomes 11. Reproductive toxicity of BPA was previously studied using different experimental animals such as mice and rats 12, 13, 14, 15, 16, evidenced by alterations in sperm parameters as well as reductions in sex hormones, testicular antioxidant enzymes and weights of reproductive organs. .
Enzymes of steroidogenesis perform a vital role in biosynthesis of different hormones. They consist of several specific cytochrome P450 enzymes (CYPs), hydroxysteroid dehydrogenases (HSDs), and steroid reductases 17. It was reported that BPA reduced the expression of the steroidogenic acute regulatory protein (StAR) and 3β -hydroxysteroid dehydrogenase (3β-HSD)18, 19. Also, protein expression of the luteinizing hormone receptor (LHCGR) in male rats was suppressed following BPA exposure, impairing the secretion of androgen hormone by testicular Leydig cells 20.
Here, using environmentally relevant doses of BPA and for long duration of exposure, we investigated the downstream pathway of chronic exposure of BPA via evaluating its effect on the expression of genes encode steroidogenesis including StAR, 3β-HSD,CYP17a, CYP19 and LHR. Moreover, its effect on serum testosterone level, the index of spermatogenesis and the number of Leydig cells. Histopathological examination of the testes and epididymis were also investigated.
Materials and Methods
Experimental Animals
Thirty mature Sprague Dawley rats weighted 250-280 g were used. Animals were purchased from the Animal House, Helwan University, Egypt and housed in separate cages in the department of Physiology, Faculty of Veterinary Medicine, Mansoura University. Rats were kept in a controlled environment, maintained under a 12 h light; dark cycle, 24⁰C (±3⁰C) and 50-70% humidity and were provided with a standard diet and water ad-libitum. Animals received human care in compliance with the guidelines of animal care of the National Institutes of Health, and all animals producers were performed in accordance with the Ethics Committee of the National Research Centre, Egypt, registration number (09/189).
Chemicals
Bisphenol-A (4,4 isopropylidenediphenol) (Sigma, Aldrich, Germany) with a molecular weight of 228.29 g/mol was dissolved in corn oil as a vehiclebefore administration. Diethyl ether was used for anesthesia.
Experimental Design
Rats were kept in polycarbonate cages. The cages as well as the water bottles used in this study were washed, rinsed, and dried several times a week in order to decrease the release of BPA from polycarbonate cages and water bottles 21. Therefore, exposure of experimental animals to phytoestrogens and BPA from these sources was minimal and equal for all groups. Animals were divided into three groups (n=10). A vehicle control group orally gavaged with corn oil, secondgroup received 10 µg/kg BW of BPA in corn oil, while third group received 15 µg/kg BW of BPA in corn oil. Administration was continued every alternate day for 105 days according to 22.
Blood and Tissue Sampling
At the end of the experimental period, all experimental rats were anaesthetized using diethyl ether. Blood samples were collected via cardiac puncture and centrifuged at 3000 rpm for 15 minutes for serum separation and stored at -20oC until analysis. Both testes were removed; one testis was frozen in liquid nitrogen and stored at -80oC for RNA extraction, while the other testis was washed with normal physiological saline and fixed in 10% formaline for histopathological examination.
Biochemical Analysis
Serum testosterone hormone was evaluated using commercial ELISA kits (Biodiagnostic Co., Catalog number: LKTW1, Egypt) according to 23 and measured using Immulite 2000 device.
RNA Extraction and Reverse Transcription
Total RNA was extracted from 50 mg of testis using Trizol reagent according to the manufacturer,s instructions (Direct-zolTM RNA MiniPrp, catalog No. R2050). The quantity and purity were measured by using Nanodrop technique (UV-Vis spectrophotometer Q5000/USA). The cDNA of each sample was synthesized following the manufacturer's protocol (SensiFasttmTM cDNAsynthesis kit, Bioline, catalog No. Bio-65053).
Quantitative Real Time PCR
Relative quantification of mRNA levels of StAR, CYP17a, aromatase, LHR and 3β-HSD in testes of rats was performed by real-time PCR using SYBR Green PCR Master Mix (2× SensiFastTM SYBR, Biolline, catalog NO. Bio-98002). Primer sequences and the size of each amplified PCR product are shown in Table 1. The house keeping gene (B-actin) was used as an internal control. The reaction mixture was carried out in a total volume of 20 µl consisting of 10 µl 2× SensiFast SYBR, 3 µl cDNA, 5.4 µl H2O, 0.8 µl of each primer. The PCR cycling conditions were as follows: 95oC for 2 min followed by 40 cycles of 94oC for 15 sec, annealing temperatures as shown in Table 1 for 30 sec, and 72oC for 20 sec. At the end of the amplification phase, a melting curve analysis was performed to confirm the specificity of the PCR product. The relative expression of the gene in each sample was compared to that of the internal control (B-actin) and calculated according to 24.
Table 1. Oligonucleotide primer sequences, annealing temperature and PCR prduct size of the studied genes.| Gene | Oligonucleotide sequence | Annealing temp. (ºC) | Size (bp) |
| StAR | f5,- GGGCATACTCAACAACCAG-3,r5,- ACCTCCAGTCGGAACACC-3, | 58 | 111 |
| CYP17a | f5,- CTCTGGGCACTGCATCAC-3,r5,- CAAGTAACTCTGCGTGGGT-3, | 58 | 114 |
| Aromatase | f5,- GCCTGTCGTGGACTTGGT-3,r5,- GGTAAATTCATTGGGCTTGG-3, | 58 | 142 |
| LHR | f5,- CATTCAATGGGACGACTCTA-3,r5,- GCCTGCAATTTGGTGGA-3, | 55 | 130 |
| 3β-HSD | f5,- TGTGCCAGCCTTCATCTAC-3,r5,- CTTCTCGGCCATCCTTTT-3, | 56 | 145 |
| B-Actin | f5,- TCGTGCGTGACATTAAAGAG-3,r5,- ATTGCCGATAGTGATGACCT-3, | 56 | 134 |
Histopathological Examination
Twenty cross sections of testes and epididymis were taken from each group, fixed in 10% formaline and then processed routinely until being embeded in a paraffin wax. Paraffin sections of 5 µm thickness were prepared and stained with H&E according to 25. Histopathological changes were examined by light microscopy. Seminiferous tubules were evaluated for their modified spermatogenesis index in all groups using Johnson’s score in which scoring method ranged from 1 (tubular section without any cell) to 10 (tubular section with regular thickness of germinal epithelium with complete spermatogenesis stages) according to 26. The number of Leydig cells was counted in ten random fields of each testicular section per animal in H&E stained slides using (100X) objective power according to 27.
Statistical Analysis
All the data obtained from the experiment were expressed as means ± SEM. Statistical analysis of data was carried out by software SPSS program package version 17 (SPSS, 2004) 28 using the one-way analysis of variance ANOVA followed by Duncan’s Range Test (DMRT) for testing the significant differences between variables. Results were considered significant only at the level of (P < 0.05). Scores of spermatogenesis index were tested by using a Chi-Square test among the three groups.
Results
Serum Testosterone Level
We examined the effect of environmentally relevant doses of BPA on serum level of testosterone. A significant reduction (P < 0.05) was observed in serum level of testosterone (1.5 ± 0.41; 2.1 ± 0.21 ng/dl) at 10 and 15 μg/kg BPA, respectively compared with the control group (3.7 ± 0.29 ng/dl). However, no significant difference was observed between both BPA-treated groups (Figure 1).
Figure 1.Serum level of testosterone (ng/dl) in control and BPA treated rats.
Gene Expression Patterns
Steroidogenic enzymes are key factors for the biosynthesis of various steroid hormones. BPA at either 10 or 15 μg/kg significantly down-regulated the relative mRNA expression of StAR(0.67 ± 0.09, 0.53 ± 0.88, respectively) compared to control group (1.15 ± 0.16). Expression of CYP17a gene was reduced (P < 0.05) at doses of 10 (0.60 ± 0.12) and 15 μg/kg BPA (0.40 ± 0.15) compared to control group (1.17 ± 0.23). The testicular mRNA expression of 3β-HDS gene was suppressed after exposure to either 10 or 15 μg/kg BPA (0.75 ± 0.07 and 0.53 ± 0.12, respectively) compared with control group (1.44 ± 0.12) (P < 0.05).Nevertheless, BPA significantly up-regulated mRNA relative expression of CYP19 at 10 (16.14 ± 0.25) and 15 μg/kg (10.17 ± 0.29) in comparison with control group (1.68 ± 0.67). Likewise, LHR gene expression was significantly increasedin second (2.09 ± 0.52) and third groups (2.04 ± 0.19) compared to control group (0.67 ± 0.09). There was no significant difference between both BPA treated groups in all selected genes (Figure 2).
Figure 2.mRNA levels of StAR, CYP17a, aromatase, LHR and 3β-HDS in the control and BPA-treated groups. Small alphabetic letters show significance when (P < 0.05).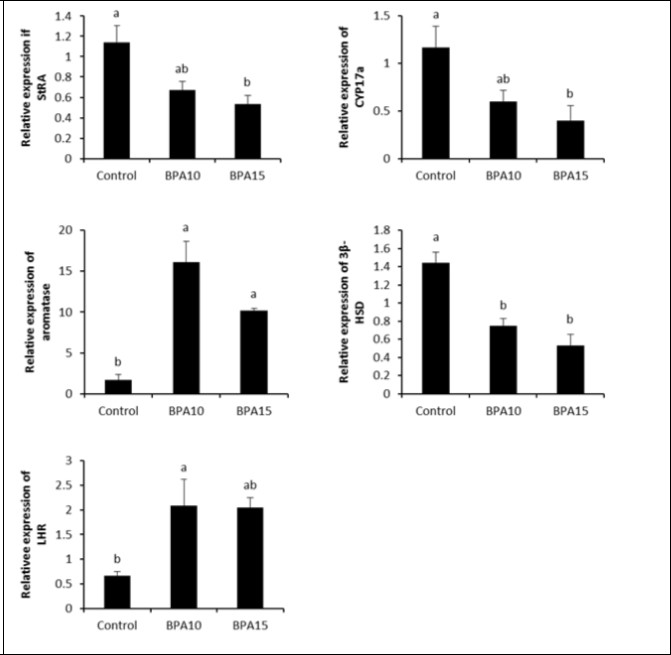
Histopathological Results
In the control group, the testes and epididymis displayed normal histological structures (Figure 3a and 3b). The testes in the second group (10 μg/kg BW BPA) showed some seminiferous tubules with few or no late spermatids and a wide, emptied lumen. Vacuolar degeneration was demonstrated in the germinal epithelium (Figure 3c) and partially emptied ducts were encountered in the epididymis (Figure 3d). Meanwhile, testes of the third group (15 μg/kg BW BPA) showed decreased sperm production as indicated by fewer spermatids, no late spermatids and a much wider lumen. Vacuolar degeneration and necrosis were demonstrated in the germinal epithelium (Figure 3e). Partially emptied ducts with marked edema widely separating epididymal ducts were observed (Figure 3f). Concerning index of spermatogenesis, the control group showed highest percentage (80%) of testicular secions scored a 10. However, the highest percentage (79%) and (76%) of testicular sections scored a 7 and 6 (Chi-Square test; value = 447.050a, df = 8, (P < 0.0001) in BPA-treated rats at 10 and 15 µg/kg BW, respectively. Moreover, necrotic interstitial Leydig cells were observed only in several sections of testes from BPA-treated groups (Figure 4a to 4c). The numbers of Leydig cells were significantly lower in BPA-treated groups compared to control (Figure 5).
Figure 3.Sections from control group; the testes (a) and epididymis (b) display normal histological structures. In second group (BPA 10 μg/kg); testes (c) with arrowheads pointing to vacuolar degeneration in germinal epithelium lining some seminiferous tubules. While, epididymis (d) shows partial emptying epidydmal ducts. In third group (BPA 15 μg/kg); Testis (e) with arrowhead pointing to vacuolar degeneration in germinal epithelium lining some seminiferous tubules and arrows pointing to necrosis in germinal epithelium. Meanwhile, epididymis (f) with a star marking severe edema and widely separating epididymal ducts (X: 100).
Figure 4.(a) Testis from control group shows normal germinal epithelium and interstitial Leydig cells (arrowhead). (b) Testis from second group (BPA 10 μg/kg) shows vacuolated spermatocytes (arrows) and necrotic interstitial Leydig cells (arrowheads). (c) Testis from third group (15 μg/kg) shows necrotic spermatocytes (arrow) and necrotic interstitial Leydig cells (arrowheads) (H&E, X:200).
Figure 5.Number of Leydig cells in testis from control and BPA-treated groups (10 or 15 μg/kg BW). Small alphabetic letters show significance when (P < 0.05).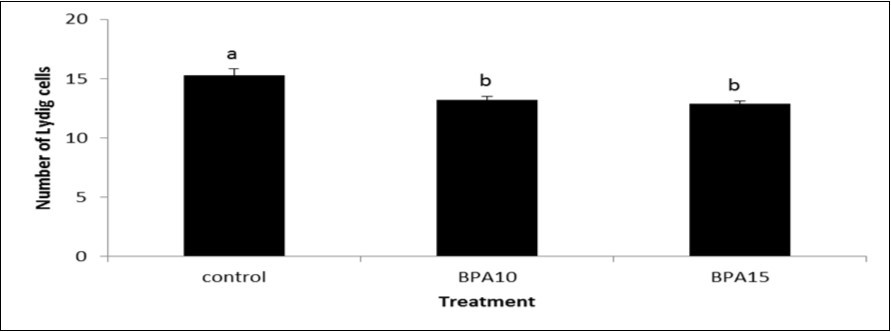
Discussion
Despite its presence in low levels in the environment, exposure to BPA is common among living beings producing several deleterious effects on various physiological mechanisms particularly reproduction 29. The reproductive toxicity of BPA was extensively studied both in vivo and in vitro in different animal species and cell lines 30, 31, 32, 33 with wide range of doses and concentrations of BPA particularly in case of in vitro studies. Even in case of in vivo studies that used very low doses of BPA, pregnant dams or their pups were used as experimental model, meaning that the exposure to the endocrine disruptor was applied in critical periods of life 34. But concerning to in vivo studies in adult animal species, most of them testified high doses of BPA (1 to 240 mg/kg) in adult male rats and mice 14, 15, 33, 35. Whereas, the current reference dose of BPA according to Environmental Protection Agency (EPA) is 50 μg/kg bw/day 36. Additionally, it is plausible that BPA is integrated in several daily-used products, thus, exposure of humans to this chemical occurs over prolonged periods of time, as evidenced by the presence of BPA in measurable levels in blood 37. In vivo studies that examined low relevant doses of BPA in adult males are relatively few. Adult male rats exposed to 2 ng to 200 mg /kg/day for 6 days showed reduction in testicular weight and daily production of sperms starting at dose of 20 μg/kg of BPA, but lower doses showed no obvious effects, it could be due to the short exposure period 38.
Moreover, chronic postnatal exposure of rats to environmentally relevant dose of BPA (2.4 μg/kg/day) for 70 days (from 21 to 90 day) resulted in elevation in serum levels of LH without variation in serum testosterone levels between BPA treated group and control group 39. While testosterone production in Lyedig cells was reduced in ex vivo experiment. Likewise, BPA at oral doses of 0.2–20 μg BPA/kg/day for 45-60 days decreased epididymal and testicular weights and induced epididymal oxidative stress in adult rats 40, 41. Also, adult male mice received BPA at very low doses (2, 25 and 100 ng/kg) for 28 days showed reduction in their fertility represented by lower pregnancy rate and increased resorption sites in female coubled with BPA-exposed males with reductions in the absolute weights of the testes 42. Similarly, BPA at a dose of 2.4 μg/kg/day for 15 day decreased serum levels of LH and testosterone as well as Leydig cell androgen biosynthetic capacity in postnatal BPA-exposed rats. While BPA at a dose of 10 μg/kg/day for 15 days showed no difference in LH or testosterone concentrations [39), suggesting that the effect of BPA influenced by the administered dose and the exposure period.
Taken together, we used in the present study, 10 and 15 μg/kg bw BPA, hence, the BPA doses, used here, were lower than the reference limit for humans, also we expand the exposure period to 15 weeks.
In consistence with previous in vitro and in vivo studies 14, 39, the results of the present study indicated that the exposure of mature male rats to BPA at either 10 or 15 µg/kg BW for 105 successive days significantly decreased the number of Leydig cells and serum testosterone level compared to the control group. Leydig cells are the major producer of testosterone under the influence of luteinizing hormone (LH), thus, lowering in the number of Leydig cells negatively affect testosterone production 43. Such reductions may be attributed to the endocrine disrupting properties of BPA 9, which possesses a weak estrogenic action 6 and anti-androgenic effects 44. Moreover, BPA acts as a mitogen in Leydig cells in rats and interfers with the proliferative activity and development of Leydig cells 20. Likewise, production of testosterone was reduced in BPA-exposed TM3 murine Leydig cells 45.
It was proposed that StAR, CYP17a, 3β-HSD and CYP19 mRNAs were predominately expressed in gonads 46. These enzymes are responsible for the process of steroidogenesis aiming to formation of endogenous male hormones (testosterone and androstenedione) via specific cascade of reactions 43. Recent studies have been focused on the effects of BPA on steroid biosynthesis in different animal models. In mammals, BPA at various doses modulates sex hormone levels and changes the expression of steroidogenic genes including StAR, CYP17a, 3β-HSD and CYP1947, 48, 49. These data could support the present results where BPA significantly down-regulated the expression of mRNA of selected steroidogenic biomarkers with subsequent reduction in the level of testosterone attributing to the direct inhibitory action of BPA on steroidogenesis and potentially disrupts StAR phosphorylation and cholesterol transport to mitochondria 50, 51. Also, the inhibition of testicular steroidogenesis by BPA was associated with decreased steroidogenic enzyme gene expression in rat Leydig cells 39. Reduction in the activity of 3β-HSD and inhibition of CYP17a following BPA exposure were also observed in both rats and human testes microsomes 11.
Aromatase enzyme (CYP 19) is a specific form of Cytochrome P450 and is a key enzyme that catalyzes the conversion of androgen to estrogen in the steroid genesis pathway in the gonads 52. In rat testicular Leydig R2C cells, BPA induced an increase in CYP19 gene expression and its enzyme and reduced testosterone synthesis 53. On the other side, the expression of gene encode aromatase enzyme was decreased in vitro using rat Lyedig cells 39. The current data showed that BPA significantly up-regulated the expression of the CYP19 gene, it could be via the estrogenic effects of BPA 54. In the same respect, BPA stimulated CYP19 mRNAexpression and activity mediated by regulation of PKA, AKT and MAP kinase signaling pathways in mouse MA-10 and rat Leydig cells 32, 53. The induction of CYP19 expression may be contribute to decreased serum levels of testodterone.
Previous studies that examined the effect of BPA on LH levels varied in their outcomes, several studies reported inhibitory effect of BPA on seum levels of LH in rats 39, 55, others observed an increase in LH level after exposure of male rats to BPA 35, 56. But measuring of testicular expression of LHR was scarce. The expression of testicular LHR was redeuced in rats received a dosage of 200 mg/kg/day bisphenol AF (BPAF) 57. Perinatal BPA at doses of 2.5 and 25 μg/kg bw/day suppressed protein expression of the luteinizing hormone receptor (LHCGR) 20.
In the current study, the expression of LHR gene was investigated to monitor the responsiveness range of gonads to gonadotropins when exposed to BPA. A significant up-regulation of LHR in BPA-treated groups was recorded.
On the other hand, expression of LHR gene was suppressed in mice exposed to BPA 58, 59. Binding of LH to its receptor (LHR) in Leydig cells triggers a cascade of events that are catalysed by the steroidogenic enzymes to form testosterone 60. Hence, lower LHR as well as CYP19 expressions suggested a kind of struggling of steroidogenesis. Alternatively, despite BPA could reduce the production of androgen, an evidence was exist that BPA could interfere with LHR binding 61 uncoupling of LH from its receptor possibly contributes to diminished LH stimulation of steroidogenesis. The Higher levels of serum LH in BPA-treated rats were presumably resulted from a reduction in the negative feedback regulation by testosterone.on the hypothalamus and pituitary 39.
Histopathological lesions were detected in the testes and epididymis from BPA-treated rats. Seminiferous tubules showed vacuolar degeneration and necrosis inBPA-treated rats similar to those reported by Khafaga and Bayad 62. Also, seminiferous epithelial damage was observed in testes of rats exposed to BPA for 42 days represented by disruption of intercellular junctions and sloughing of germ cells into the seminiferous tubular lumen 63. Indeed, the decrease of spermatogenesis index and Leydig cell numbers in BPA-treated groups is in consistent with lower testosterone levels in BPA-treated groups compared to control group. The index of spermatogenesis is one of the most important indicators of the state of spermatogenic layer 64. The decrease in this indicator always indicates disturbances of spermatogenesis and decreased functional activity of seminal gland as well as a reduction of the functional activity of the testes. 64. Moreover, the current results are in agreement with previous studies reported the opposing effect of BPA on spermatogenesis and total sperm count 65, 66, 67.
Conclusion
BPA supplementation could affect steroidogenesis via lowering serum levels of testosterone, altering the expression patterns of steroidogenic genes, exerting histopathological lesions in the testes and epididymis and lowering index of spermatogenesis as well as the number of Leydig cells in adult male rats.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for -profit sectors.
Acknowledgements
We would like to acknowledge Barry long, Department of Animal Science, Texas A&M University, USA, Lauren Gentry, Texas A&M University Writing Center, USA, Ahmed Balboula, Cambrige University UK for their efforts in English editing of the present manuscript.
References
- 1.Fleisch A F, Sheffeid P E, C Edelstein, BL, Landrigan P J. (2010) Bissphenol A and related compounds in dental materials. , Pediatrics 126, 760-768.
- 2.Goodson A, Robin H, Summerfield W, Cooper I.Migration of bisphenol A from can coatings effects of damage, storage conditions and heating (2004) Food Addit. , Contam 21, 1015-1026.
- 3.Michalowicz J. (2014) Bisphenol A-Sources, toxicity and biotransformation. , Environ. Toxicol. Pharmacol 300, 1301-1310.
- 5.Mikołajewska K, Stragierowicz J, Gromadzińska J. (2015) Bisphenol A - Application, sources of exposure and potential risks in infants, children and pregnant women. , Int. J. Occup. Med. Environ. Health 28, 209-241.
- 6.Xi W, Lee C K, Yeung W S, Giesy J P, Wong M H. (2011) Effect of perinatal and postnatal bisphenol A exposure to the regulatory circuits at the hypothalamus-Pituitary-gonadal axis of CD-1 mice. , Reprod. Toxicol 31(4), 409-417.
- 7.Wetherill Y B, Akingbemi B T, Kanno J, McLachlan J A, Nadal A. (2007) . , Reprod. Toxicol 24, 178-198.
- 8.Lang I A, Galloway T S, Scarlett A, Henley W E, Depledge M. (2008) Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. , JAMA 300, 1301-1310.
- 9.Saal Vom, FS, Hughes C.An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment (2005) Environ. Health Perspect. 113, 926-933.
- 10.Anjum S, Rahman S, Kaur M, Ahmad F, Rashid H.Melatonin ameliorates bisphenol A induced biochemical toxicity in testicular mitochondria of mouse (2011) Food Chem. , Toxicol 49, 2849-2854.
- 11.Ye L, Zhao B, Hu G, Chu Y, Ge R S. (2011) Inhibition of human and rat testicular steroidogenic enzyme activities by bisphenol A. , Toxicol. Lett 207, 137-142.
- 12.Gupta C. (2000) Reproductive malformation of the male offspring following maternal exposure to estrogenic chemicals. , Proc. Soc. Exp. Biol. Med 224, 61-68.
- 13.Dobrzyńska M M, Radzikowska J. (2013) Genotoxicity and reproductive toxicity of bisphenol A and X-ray/bisphenol A combination in male mice Drug Chem. , Toxicol 36, 19-26.
- 14.Nakamura D, Yanagiba Y, Duan Z, Ito Y, Okamura A. (2010) Bisphenol A may cause testosterone reduction by adversely affecting both testis and pituitary systems similar to estradiol. , Toxicol. Lett 194, 16-25.
- 15.El-Shafey M. (2012) Lipoic acid mitigates bisphenol A-induced testicular mitochondria toxicity in rats. , Toxicol. Ind. Health 29, 875-887.
- 16.Kourouma A, Peng D, Chao Q, Lopez Y T, Changjiang L. (2014) Bisphenol A induced reactive oxygen species (ROS) in the liver and affect epididymal semen quality in adults Sprague-Dawley rats. , J. Toxicol. Environ. Health Sci 6(4), 103-112.
- 18.Hatef A, Alavi SMH, Abdulfatah A, Fontaine P, Rodina M. (2012) Adverse effects of bisphenol A on reproductive physiology in male goldfish at environmentally – relevant cncentrations. , Ecotoxicol. Environ. Saf 76, 56-62.
- 19.D´Cruz S C, Jubendradass R, Jayakanthan M, Rani S J, Mathur P P.Bisphenol A impairs insulin signaling and glucose hemeostasis and decreases steroidogenesis in rat testis: an in vivo and in silico study (2012) Food Chem. , Toxicol 50, 1124-1133.
- 20.Nanjappa M K, Simon L, Akingbemi B T. (2012) The industrial chemical bisphenol A (BPA) interfers with proliferative activity and development of steroidogenic capacity in rats Leydig cells. , Biol. Reprod 86, 135.
- 21.Howdeshell K L, P H, Judy B M, Taylor J A, Orazio C E.Bisphenol A is released from used polycarbonate animal cages into water at room temperature (2003) Environ. Health Perspect. 111, 1180-1187.
- 22.FRS Vom Saal, Cooke P S, Palanuza P, Palanza P, Thayer K A. (1998) A physiological based approach to the study of Bisphenol- A and other estrogenic chemicals on the size of reproductive organs, daily sperm production and behavior. , Toxicol. Ind. Health14(1-2) 239-260.
- 24.Pfaffi M W.A new mathematical model for relative quantification in real time RT-PCR (2001) Nucleic Acids Res. 119, 209-217.
- 25.Bancroft J D, Gamble M. (2007) . Theory and Practice of Histological Techniques. 5thEd.Churchill Livingstone.London.UK .
- 26.Johnson S G. (1970) Testicular biopsy score count-a method for 12 registration of spermatogenesis in human testes: normal value and results of 355 hypogonadal males. , Hormones 1(1), 2-25.
- 27.Otoom S, Bataineh H, Hassan Z, Daoud A. (2004) Effect of long term use of Topiramate on fertility and growth parameters in adult male rats. , Neuro. endocrinol. Lett 25(5), 351-365.
- 29.Sweeney M F, Hasan N, Soto A M, Sonnenschein C. (2015) Environmental endocrine disruptors: Effects on the human male reproductive system. , Rev. Endocr. Metab. Disord 16, 341-357.
- 30.Deng M X, Wu D S, Chen X G, Zhang L S, Xu P Y. (2004) Experimental studies on male reproductive toxicity of bisphenol A in vitro and vivo. , Chin. J. Prev. Med 38, 383-387.
- 31.Zhang X, Chang H, Wiseman S, He Y, Higley E.Bisphenol A disrupts steroidogenesis in human H295R cells (2011) Toxicol Sci. , J 121, 320-327.
- 32.Lan H C, Wu K Y, Lin I W, Yang Z J, Chang A A. (2017) Bisphenol A disrupts steroidogenesis and induces a sex hormone imbalance through c-Jun phosphorylation in Leydig cells. , Chemosphere 185, 237-246.
- 33.Samova S, Patel C N, Doctor H, Pandyab H A, Verma R J. (2018) The effect of bisphenol A on testicular steroidogenesis and its amelioration by quercetin: an in vivo and in silico approach. , Toxicol. Res 7, 22-31.
- 34.Kawai K, Nozaki T, Nishikata H, Aou S, Takii M. (2003) Aggressive behavior and serum testosterone concentration during the maturation process of male mice: the effects of fetal exposure to bisphenol A Environ. Health Perspect. 111, 175-178.
- 35.Tohei A, Suda S, Taya K, Hashimoto T, Kogo H. (2001) Bisphenol A inhibits testicular functions and increases luteinizing hormone secretion in adult male rats. , Exp. Biol. Med 226(3), 216-221.
- 36.U S EPA. (2002) A Review of the reference dose and reference concentration process. Environmental Protection Agency;. , Washington DC U.S.:
- 37.Takeuchi T, Tsutsumi O. (2002) Serum bisphenol a concentrations showed gender differences, possibly linked to androgen levels. , Biochem. Biophys. Res.Commun 291, 76-78.
- 38.Sakaue M, Ohsako S, Ishimura R, Kurosawa S, Kurohmaru M. (2001) Bisphenol-A Affects Spermatogenesis in the Adult Rat Even at a Low Dose. , J. Occup. Health 43, 185-190.
- 39.Akingbemi B T, Sottas C M, Koulova A I, G R Klinefelter, M P Hardy. (2004) Inhibition of testicular steroidogenesis by the xenoestrogeen bisphenol a is associated with reduced pituitary luteinizing hormone secretion and decreased steroidogenic enzyme gene expression in rat Leydig cells. , Endocrinology 145, 592-603.
- 40.Chitra K C, Latchoumycandane C, Mathur P P.Induction of oxidative stress by bisphenol A in the epididymal sperm of rats (2003a). , Toxicol 185, 119-127.
- 41.Mathur P P.Effect of bisphenol A and co-administration of bisphenol A and vitamin C on epididymis of adult rats: a histological and biochemical study (2003b). , Asian J. Androl 5, 203-208.
- 42.Al-Hiyasa t AS, Darmani H, Elbetieha A M. (2002) Effects of bisphenol A on adult male mouse fertility. , Eur. J. Oral Sci 110, 163-167.
- 43.Tomáš J, Bistáková J, Greifová H, Tvrdá E, Lukáč N. (2017) Male Reproduction: One of the Primary Targets of Bisphenol, Bisphenol A Exposure and Health Risks, Dr. Pinar Erkekoǧlu (Ed.)doi:. 10.5772/intechopen.68629. InTech
- 44.Teng C, Goodwin B, Shockley K, Xia M, Huang R. (2013) Bisphenol A affects androgen receptor function via multiple mechanisms. , Chem. Biol. Interact 556-564.
- 45.Gonçalves G D, Semprebon S C, Biazi B I, Mantovani M S, Fernandes GSA. (2018) . , Reprod. Toxicol 76, 26-34.
- 46.Wang J, Liu X, Wang H, Wu T, Hu X. (2010) Expression of two cytochrome P450 aromatase genes is regulated by endocrine disrupting chemicals in rare minnow Gobiocypris rarus Juveniles. , Comp. Biochem. Physiol. C Toxicol. Pharmacol 152, 313-320.
- 47.Grasselli F, Baratta L, Baioni L, Bussolati S, Ramoni R. (2010) Bisphenol A disrupts granulosa cell function. , Domest. Anim. Endocrinol 39, 34-39.
- 48.Peretz J, Gupta R, Singh J, Hernández-Ochoa I, J A Flaws. (2011) Bisphenol A impairs follicle growth inhibits steroidogenesis and downregulates rate limiting enzymes in the estradiol biosynthesis pathway. , Toxicol. Sci 2011, 351-365.
- 49.Zhou W, Liu J, Liao L, Han S, Liu J.Effect of bisphenol A on steroid hormone production in rat ovarian theca-interstitial and granulosa cells (2008) Mol. Cell Endocrinol. 283, 12-18.
- 50.Allen J A, Shankara T, Janus P, Buck S, Diemer T. (2006) Energized, Polarized, and actively respiring mitochondria are required for acute Leydig cell steroidogenesis Endocrinology. 147, 3924-3935.
- 51.Chouhan S, Yadav S K, Prakash J, Westfall S, Ghosh A. (2015) Increase in the expression of inducible nitric oxide synthase on exposure to bisphenol A: A possible cause for decline in steroidogenesis in male mice. , Environ. Toxicol. Pharm 50, 1124-1133.
- 52.Carreau S, Wolczynski S. (2010) Galeraud-Denis I Aromatase, oestrogens and human male reproduction. , Phil. Trans. R. Soc. B 365, 1571-1579.
- 53.Kim J Y, Han E H, Kim H G, Oh K N, Kim S K. (2010) BPA-induced aromatase activation is mediated by cyclooxygenase-2 up-regulation in rat testicular Leydig cells. , Toxicol. Lett 193, 200-208.
- 54.Canton D F, Sanderson J T, Letcher R J, Bergman A, Berg M van den. (2005) Inhibition and induction of aromatase (CYP19) activity by brominated flame retardants in H295R human adrenocortical carcinoma cells. , Toxicol. Sci 88, 447-455.
- 55.Wisniewski P, Romano R M, Kizys MML, Oliveira K C, Kasamatsu T. (2015) Adult exposure to bisphenol A (BPA) in Wistar rats reduces sperm quality with disruption of the hypothalamic-pituitary-testicular axis. , Toxicol 329, 1-9.
- 56.Gámez J M, Romina Penalba R, Cardoso N, Osvaldo Ponzo O, Carbone S. (2014) Low dose of bisphenol A impairs the reproductive axis of prepuberal male rats. , J. Physiol. Biochem 70, 239-246.
- 57.Feng Y, Yin J, Jiao Z, Shi J, Li M. (2012) Bisphenol AF may cause testosterone reduction by directly affecting testis function in adult male rats. , Toxicol. Lett 211, 201-209.
- 58.Eo J, Lim H. (2008) Effects of Bisphenol A. on Gene Expression and Apoptosis of Leydig Cells in the Mouse Testis , Kor. J. Fertil. Steril 35, 181-191.
- 59.Savchuk I, Söder O, Svechnikov K. (2013) Mouse leydig cells with different androgen production potential are resistant to estrogenic stimuli but responsive to bisphenol a which attenuates testosterone metabolism. PLoS ONE 8 71722.
- 60.Abdou H S, Bergeron F, Tremblaya J J. (2014) A cell-autonomous molecular cascade initiated by AMP-activated protein kinase represses steroidogenesis. , Mol. Cell. Biol 34, 4257-4271.
- 61.Nikula H, Talonpoika T, Kaleva M, Toppari J. (1999) Inhibition of hCG-stimulated steroidogenesis in cultured mouse Leydig tumor cells by bisphenol A and octylphenols. , Toxicol. Appl. Pharmacol 157, 166-173.
- 62.Khafaga A F, Bayad A E. (2016) Impact of Ginkgo biloba Extract on Reproductive Toxicity Induced by Single or Repeated Injection of Cisplatin in Adult Male Rats. , Int. J. Pharmacol 12, 340-350.
- 63.Gurmeet K S, Rosnh I, Normadiah M K, Das S, Mustafa A M. (2014) Detrimental effects of bisphenol A on development and functions of the male reproductive system in experimental rats. , EXCLI J 13, 151-160.
- 64.Potemina T E. (2008) Impairment of Spermatogenesis in Male Rats during Stress. , Bull. Exp. Biol. Med 145, 700-702.
- 65.S De Flora, R T Micale, M S La, Izzotti A, D’Agostini F. (2011) Upregulation of clusterin in prostate and DNA damage in spermatozoa from bisphenol A-treated rats and formation of DNA adducts in cultured human prostatic cells. , Toxicol. Sci 122, 45-51.
Cited by (2)
- 1.Marghani Basma H., Ezz Mohamed Aboul, Ateya Ahmed I., Fehaid Alaa, Saleh Rasha M., et al, 2023, Comparative effects of finasteride and laser-irradiated silver nanoparticles on testicular function and histology in testosterone induced benign prostatic hyperplasia in rats, Life Sciences, 324(), 121747, 10.1016/j.lfs.2023.121747
- 2.Malek Mastura Abd, Dasiman Razif, Khan Nor-Ashikin Mohamed Noor, Mohamed-Akhlak Sofee, Mahmud Mohd-Hafizi, 2022, The protective effects of Procyanidin C-1 on bisphenol a-induced testicular dysfunction in aged mice, Food Science and Human Wellness, 11(4), 965, 10.1016/j.fshw.2022.03.020
