Abstract
The mammary tumor is one of the most common cancer in female dogs and, at the present days, there is a big focus on the study of the relation between this kind of tumor in animals and the cells that stay around them, like the inflammatory cells. The objective of this study was to evaluate and show where the inflammatory cells stay in simple mammary carcinomas in female dogs by immunohistochemistry. Samples of simple mammary carcinomas (tumor group; n=26) and mammary gland samples without tumor (control group; n=18) were submitted to immunohistochemical analysis for the detection of T lymphocytes, macrophages, plasma cells and the MHC-II molecule. The mast cells were evaluated by the histochemical technique (toluidine blue). Lymphocytes, macrophages and mast cells were observed distributed in the tumor stroma. MHC-II was detected in tumor cells and in the inflammatory infiltrate. Plasma cells predominated in the peritumoral stroma. Macrophages differed significantly between the two groups and predominated in the tumor group. In the comparison between histological types of mammary carcinomas, mast cells differed significantly between solid tumors of the tubular / papillary types. The cytoplasmic immunodetection of MHC-II was suggested an inefficient antigen presentation. Some of the leukocytes present in the tumor infiltrate, appear to be exerting a pro-tumor effect and allowing the progression of tubular and papillary carcinomas. But in solid carcinomas (may be poorly immunogenic), as they had the lowest proportion of leukocytes present in the tumor site. More studies are necessary to confirm these results, such as the determination of the cytokine profile and the predominant leukocyte subpopulations in the tumor microenvironment.
Author Contributions
Academic Editor: Burak Dik, Selcuk University.Veterinary Faculty.Farmacology And Toxicology, TURKEY
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2017 Anne Caroline, et al
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
The concept of immunological surveillance idealized by the German immunologist Paul Ehrlich and later defined by Lewis Thomas and Macfarlane Burnet was based on the protection of the organism against the proliferation of mutated cells, with the objective of destroying them before the tumor progression. The effectiveness of this individual immunological surveillance can be controversial, since immunocompetent individuals are capable of developing cancer 1, 2. The immune system would have the ability to react in different ways against different types of neoplasms. So, some tumor antigens may be weakly immunogenic, which attenuates the functions of the immune system, which is eventually supplanted by the tumor 3.
For their survival, tumors with rapid development create means to escape of the immune system, and this event is called escape or tumor evasion. Some of the ways of evasion was already studied, include low immunogenicity of tumor antigens, decrease or loss of class I major histocompatibility complex (MHC-I) and co-stimulatory molecule expression, reduction of tumor cell apoptosis, sub-regulation of genes associated with antigen presentation, production of immunosuppressive cytokines, tolerance of T lymphocytes to tumor antigens. All these events induce immunological tolerance 4, 5.
In medicine, studies have shown that the presence of inflammation in some types of cancer is beneficial, as in melanomas 6. However, in mammary carcinomas 7 and in oral squamous cell carcinomas 8, the presence of chronic inflammation is considered an adjuvant of tumor growth tolerance. Some patients who keeps a chronic inflammatory state have at higher risk of developing cancer 9.
Studies that evaluate the inflammatory infiltrate in animal neoplasms are still scarce, in relation to the number of works performed with several human cancer. In some studies with tumors in female dogs, have already highlighted the relationship between the immune system and the canine tumors, especially the mammary gland tumors 10. Some researchers support the hypothesis about the pro-tumor action of inflammatory infiltrate on breast tumors 11 and others point out that the inflammation associated with the tumor would be a real anti-tumor immune system response 12. For that reason, the objective of this study was to evaluate and show where and who are the subpopulations of inflammatory cells present in simple type of mammary gland carcinomas of female dogs, by immunohistochemistry, and to verify if their presence is relate to more aggressive aspects of the mammary tumor in dogs.
Material and Methods
All procedures were approved by the ethics committee on the use of animals of the same institution (CEUA 014526/12). The samples of mammary neoplasia used in this study were obtained during a period of two years, by the Veterinary and Obstetrics and Animal Reproduction Oncology Services of the Veterinary Hospital of São Paulo State University (Unesp) Faculty of Agrarian and Veterinary Sciences (FCAV), Campus of Jaboticabal, São Paulo State, Brazil.
The animals in the tumor group went through a clinical triage and they were forwarded to the surgical procedure (unilateral radical mastectomy), with inhalation anesthesia. There was no predilection for breed or age in the selection of samples from each group.Just the mammary gland tumors that were histologically classified as simple mammary carcinoma were submitted to immunohistochemistry. The mammary tissue samples were divided into two groups: control group (canine mammary without neoplasia, n = 18) and tumor group (canine mammary with simple carcinoma, n = 26). The tumor group was subdivide into three subgroups, according to the histological type of the simple carcinoma (tubular, papillary and solid).
All the samples collected were fixed in formol solution (10%), buffer with phosphates (pH 7.4), for 24 hours. After, they were processed until the inclusion in paraffin, cut in the thickness of 5μm and stain with Hematoxylin and Eosin (HE). All samples from the tumor group were classified according to the Consensus for diagnosis, prognosis and treatment of canine mammary tumors 13.
The location of the inflammatory infiltrate was determined as intratumoral or peritumoral. The presence of tumor-associated mast cells was assessed by the histochemical technique of toluidine blue (AT).
Immunohistochemically Analysis
For the immunohistochemically analysis, antibodies were used for the detection of T lymphocytes (CD3, clone F7.2.38, Dako, ref.M7254, dilution 1: 150), MHC-II (HLA-DR, clone TAL.1B5, Dako, Ref. M0746, 1: 500 dilution), plasma cells (CD138, clone N / A, Spring, ref.E4564, 1:25 dilution) and macrophages (clone MAC387, Abcam, ref. Ab22506, 1: 4000 dilution).
The immunohistochemistry protocol was used after consisted of desparaffinization of the histological sections in the drying oven (60ºC / 60 minutes). The antigenic recovery done by heat (Pascal steam cooker, DakoCytomation) and endogenous peroxidase blockade was used a solution with hydrogen peroxide solution (30 v / v, 8%) and methanol, for 30 minutes in a darkroom. To blocking non-specific reactions, a commercial product (Protein Block serum-free, Dako, ref. X0909) was used.
Primary antibody incubation was grown at 4°C for 18 hours (CD3, MAC387, HLA-DR) or and 24 hours at room temperature (CD138) in a humid chamber. Incubation with Peroxidase-bound Polymer Complex (Advance Kit, DAKO, cod. K4067) was used for CD3 and HLA-DR antibodies. The Streptavidin Peroxidase complex (Dako EnVision ™ kit, cod.K5355) was used for MAC387 and CD138. The chromogen was DAB (3,3-diaminobenzidine, Dako, cod. K3468-1, three minutes) and in the counter-staining was used the Harris Hematoxylin. Positive control of the reaction was the recommended by the manufacturers of the antibodies. The negative control was antibody diluent (Antibody diluent with background reducing components, Dako, cod. S3022) that replaced the primary antibody.
The count of immunolabellated cells and cells stained with toluidine blue was performed by a light microscope (Nikon E200) with digital photomicrograph equipment (Motic Images Plus version 2.0). For this, five fields with a 40x objective were randomly chosen, where the total number of cells marked for each antibody was considered, in an area measuring 0.19625 mm2.
Statistical Analysis
The counts of T lymphocytes, MHC-II, macrophages, plasma cells and mast cells were compared between the groups (control and tumor) by the Mann-Whitney non-parametric test. At the same way, the predominant profile of the inflammatory infiltrate was compared within the tumor group, comparison the histological types (tubular, papillary and solid), using Kruskall-Walis non-parametric tests and the Dunn test. The correlations between inflammatory infiltrate and histological type of tumor were determined using Spearman's correlation coefficient. The GraphPad Prism statistical software (version 5.00, 2007) was used for all analyzes, and differences were taken to be significant when p<0.05.
Results
The tumor group showed inflammatory infiltrate foci consisting predominantly of lymphocytes, macrophages, plasma cells, and mast cells.
The T lymphocyte (CD3) immunostaining was observed in the intratumoral (stroma) environment of the tubular and papillary carcinomas, specifically around the tubules (Figure 1A) and in the fibrovascular axis of the papillae. In solid carcinomas the presence of inflammation was rarely observed at the intratumoral site, occasionally in the peritumoral space.
Figure 1.Inflammatory infiltrate associated with the mammary tumor in female dogs. (A) Photomicrography of intratumoral T lymphocytes in grade I tubular carcinoma. (B) T lymphocyte median in the control and tumor groups (p = 0.221). (C) Intratumoral immunostaining of the major histocompatibility complex class 2 (MHC-II) labeling in tubular carcinoma grade III, observe the immunomarked cells in detail. (D) No significant difference was observed between the control and tumor groups (p = 0.0638). (E) Intratumoral macrophages in grade II tubular carcinoma. (F) Median macrophage markers in the control and tumor groups, with significant differences between groups (p = 0.0139). (A, C, E) Complex of peroxidase-bound polymers (bar = 50 μm). (B, D, F) Non-parametric Mann-Whitney test.
MHC-II (HLA-DR) showed cytoplasmic marking in tumor cells and mononuclear inflammatory infiltrate (Figure 1C) and showed no significant difference (p<0.05) between the tumor and control groups (Figure 1D). In the comparison between histological types, MHC-II (HLA-DR) immunostaining in tubular carcinomas was visualized in both tumor cells and leukocytes of the inflammatory infiltrate. In papillary carcinomas, marking predominated in myoepithelial cells and was scarce in inflammatory cells. Solid carcinomas showed poor marking in the tumor stroma and inflammatory cells distributed in the peritumoral space.
The macrophages (MAC387) had cytoplasmic labeling. In tubular carcinomas, macrophages were observed in small groups around the neoplastic tubules or were associated with intratumoral inflammatory infiltrate (Figure 1E). In papillary carcinomas, macrophages were scarce and observed in the interstitial of tumor papillae. In solid carcinomas, macrophages were located in the peritumoral region and rarely in the intratumoral environment. Significant differences (p<0.05) were observed between the tumor and control groups, with predominance of immunolabellated cells in the tumor group (Figure 1F).
The immunostaining of the plasma cells (CD138) occurred in the cytoplasmic membrane (Figure 2A). In tubular carcinomas, these cells were detected around the neoplastic tubules and the mammary myoepithelium. Papillary carcinomas were found near the periphery of the tumor and were absent between the tumor papillae. In the solids, scarcity of this cell type was detected, even in the peritumoral region. There was no significant difference (p<0.05) between the control and tumor groups (Figure 2B).
Figure 2.Inflammatory infiltrate associated with the mammary tumor of bitches (A) Plasm cells visualized in a papillary carcinoma grade I, observe the plasm cells in the detail. Peroxidase-bound polymer complex (bar = 50 μm). (B) Plasma cell counts in the control and tumor groups (p = 0.2597, Mann-Whitney test). (C) Intratumoral mast cell infiltrate in grade I papillary carcinoma (detail). Toluidine blue staining (bar = 100 μm). (D) Mean of mast cells in the control and tumor groups (p = 0.3846, Mann-Whitney test).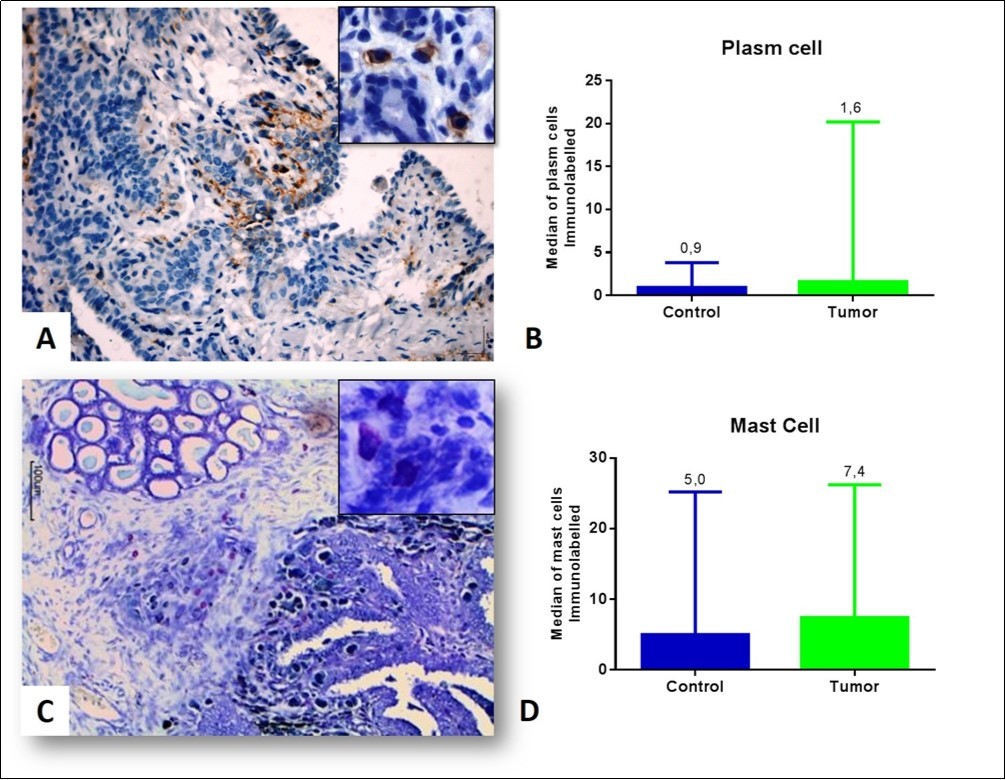
The presence of mast cells in tubular carcinomas was evident around the tubules and in the intratumoral stroma. Into the papillary carcinomas they were observed in the papillae (Figure 2C). In solid carcinomas, the density of this cell type was low within the tumor site. No significant difference (p<0.05) was found between the control and tumor groups, but the mast cells had higher density in the tumor group (Figure 2D).
In the comparison between the immunostaining inside the tumor group, no significant difference was observed for MHC-II, lymphocytes, macrophages and plasma cells for the tubular carcinomas, papillary and solid. However, and mast cells differed between the tubular and papillary histological types in relation to the solid (Figure 3).
Figure 3.Median marking of the major histocompatibility complex class 2 (MHC-II) (p = 0.4174), (B) T lymphocytes (p = 0.0504), (C) macrophages (p = 0.5297), (D) mast cells (P = 0.0286) and (E) plasma cells into the tumor group, in the three different tumor types (p = 0.3355). Kruskall-Walis test and Dunn's test.
In the correlation analysis performed inside the tumor group (Table 1), it was possible to compare the histological types with the leukocyte and MHC-II immunostaining. Tubular tumors (n=17) showed a moderate positive correlation between lymphocytes and MHC-II expression. Correlations between the histological types papillary (n=6) and solid (n= 3) were not considered, due to the small number of samples per group.
Table 1. Spearman correlation between the inflammatory tumor infiltrate and the histological type of canine mammary carcinomas.| Histological Types | Antibodies | Lymphocytes | Macrophages | Mast cells | Plasm cells |
| Papilar | MHC-II | -0.543 | N | 0.314 | -0.257 |
| CD3 | - | 0.257 | N | -0.429 | |
| MAC | - | - | 0.369 | 0.371 | |
| Tubular | MHC-II | 0.4737 | N | 0.243 | N |
| CD3 | - | 0.349 | 0.171 | N | |
| MAC | - | - | N | -0.223 | |
| Sólido | MHC-II | 0.5 | 0.5 | N | N |
| CD3 | - | 1 | N | -0.866 | |
| MAC | - | - | N | -0.866 |
Discussion
In the comparison between the tumor and control groups, only the macrophages had a statistically significant difference between the two groups. Macrophages are cells that adapt to changes in microenvironments and perform diverse functions14, 15. For this reason, in human literature, these leukocytes are called tumor-associated macrophages (TAMs) and exert essential functions for the tumor progression 14, 16, 17. The number of macrophages did not differ statistically between the histological types of canine mammary tumors, however the tubular carcinoma had the highest median (6.6), followed by papillary (2.0) and solid (0.4). In the routine analyzes, histological sections stained with hematoxylin and eosin, it was possible to verify that solid tumors were the histological type that had absence or shortage of intratumoral inflammation. These findings coincide with the immunostaining of the present study. These tumors are considered the most aggressive histological types, perhaps the scarcity of leukocytes infiltrated into the tumor may suggest less expression of MHC-II or other molecules that attract inflammatory cells to the tumor site.
In the present study, macrophages were detected by the antibody MAC387, which binds to the protein calprotectin, that is expressed on neutrophils, monocytes and actives young macrophages. Future studies are needed to assess whether these macrophages detected in the tumor group would have a higher proportion of the M2 phenotype, depending on the histological type of mammary carcinoma; methods that specifically identify macrophage are necessary to clarify and support, because just the presence of cells positive for the MAC387 antibody can’t sustain that young macrophages are attracted to the tumor site.
In the comparison between tumor histological types (tubular, papillary and solid tumors), there was a statistically significant difference only for mast cell, which were observed in a greater proportion in the papillary carcinoma group. Tubular and solid histological types are carcinomas more aggressive than papillary carcinomas 13. Perhaps the expression of MHC-II or the release of activating factors of the immune system is reduced in these aggressive histological types, favoring an inefficient response of leukocytes associated with the tumor.
Mast cells are considered possible coadjuvants of tumor development, as they are attracted to the neoplastic growth site in response to various chemoattractants derived from tumors 21. The sensitized mast cells by the tumor release mediators that can change in the maturation of dendritic cells, keeping them immature and leading to immune tolerance of T lymphocytes 22, 23. It is believed that this T lymphocyte depletion results in uncontrolled degranulation, since T lymphocytes help in the regulation of this mast cell activity 24, 25. Thus, the increased amount of histamine released at the tumor site may benefit the development of neoplasia 26. In the present study, mast cells differed statistically between histological types of mammary carcinoma, which were more numerous in the papillary type. Although mast cells have been significantly observed in invasive mammary carcinomas of women 27, and their true role in tumor growth has not been elucidated. There is controversy about its presence and good prognosis in breast tumors in women 28 and in gastric carcinomas in humans 28, 29. In the veterinary, the presence of mast cells has been related to poor prognosis, since they stimulate tumor angiogenesis 30.
MHC-II expression was observed in the cytoplasm of inflammatory and neoplastic cells. The cytoplasmic immunodetectionof MHC-II was suggested an inefficient antigen presentation to the naive T lymphocytes. MHC class I is expressed in all cell types, whereas MHC class II is limited to specific cell types such as macrophages, B lymphocytes and DCs, which may present tumor antigens to T lymphocytes 20. The detection of MHC-II in the neoplastic mammary epithelium suggests a mechanism of immune evasion of the tumor, since in the mammary gland without tumor this expression does not occur. Some studies suggest that this aspect would be related to the action of hormones or cytokines present in the tumor 31. This may be the result of a delicate balance between intrinsic factors of the tumor and factors of the host that tries to regulate the immune response 32.
According to previous studies, a higher proportion of immature dendritic cells was detected in the same tumor types of the present study. The higher proportion of immature dendritic cells confirmed the inefficient antigenic presentation ability of these cells, since there is no expression of MHC molecules in the cytoplasmic membrane 19. The results of this study reinforce the findings of the previous study, since the MHC-II marking was observed in the cytoplasm and not in the inflammatory cell membrane.
MHC-II expression is thought to be a good prognosis for breast tumors, as well as tumors of the larynx 33 and colo-rectal tumors in humans 20. In the literature it is highlighted that the reduced expression of MHC-II can be influenced by the increase of IL-10 (immunosuppressive cytokine), which contributes to the absence of stimulation of T lymphocytes 2. In this study, the abnormal expression of MHC-II molecules could be related to inefficient maturation of DCs present in the tumor microenvironment 19. In the literature it is highlighted that the increase of IL-10 (TH2 response) may cause the decrease of MHC II expression in the initial phases of the immune response, contributing to the inhibition of T cell activation 2. In previous studies high immunostaining of IL-10 positive cells has been found in the tumor environment 18.
Plasma cells can be present into the inflammatory response to mammary neoplasia 14; however, there is little evidence in the literature of the true role of plasma cells inside the tumor microenvironment. Some authors note that B lymphocytes and plasma cells appear to be unrelated to the prognosis in human’s lung carcinomas and have been shown to exert a pro-tumor effect 34. In undifferentiated canine mammary carcinomas, the infiltration of plasma cells is described at the periphery of the cells, indicating a possible association of plasma cells with tumor development 35. In women, the presence of these cells infiltrating the ductal and lobular carcinomas of the breast was associated with a worse prognosis 36. In the present study, these cells were not statistically relevant between groups and histological types. In routine analyzes of breast tumors, this cell type is always present in the inflammatory infiltrate associated with the tumor. Other research groups observed a higher proportion of B lymphocytes in canine mammary tumors with regional lymph node metastasis 12.
The true role of tumor-associated inflammatory infiltrate is unclear. The response of the patient to any type of cancer, whether human or animal, may be variable and influenced by the tumor cells themselves, since the behavior of the immune response is also individual. However, understanding the behavior of the tumor and its interaction with the stroma and immune system is essential for determining the prognosis and for more efficient antitumor therapies.
Conclusion
In conclusion, T lymphocytes, macrophages, and mast cells were located into the tumor; the molecules of MHC-II and the plasm cells were founded into microenvironment. Under the conditions of this study, it was possible to realize that types of more aggressive tumor, like solid carcinomas, have a poor infiltrate inflammatory. Some kind of inflammatory populations, like mast cells and macrophages can be associated with the tumor microenvironment, could possibly failed to exert antitumor effector functions and thus favored tumor growth. Future studies that evaluate the cytokine profile and subpopulations of leukocytes predominant in the tumor microenvironment may confirm this hypothesis.
Acknowledgements: The authors wish to acknowledge the Serviço de Oncologia Veterinária e Serviço de Obstetrícia Veterinária of Unesp Jaboticabal.
Funding: Financial assistance was provided by FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo; Procedural number 2012/09385-0). Mayara C. Rosolem was supported by a grant from FAPESP (Procedural number 2011/03510-4).
References
- 1.Kumar V, Abbas A K, Fausto N. (2005) . Robbins & Cotran - Patologia - Bases Patológicas das Doenças. Elsevier, Rio de Janeiro,1592 .
- 2.Abbas A K, Lichtman A H. (2007) Reconhecimento antigênico no sistema imune adaptativo. In: Ibid. (Eds), Imunologia Básica: Funções e Distúrbios do Sistema Imunológico. Elsevier Brasil, Rio de Janeiro 69-90.
- 3.Kah-Wai L, Jacek T, Jacek R. (2006) Dendritic cells heterogeneity and its role in cancer immunity. , J. Cancer Res. Ther 2, 35-40.
- 4.Igney F H, Krammer P H. (2002) Immune escape of tumors: apoptosis resistance and tumor counterattack. , J Leukoc Biol 71(6), 907-20.
- 5.Gabrilovich D. (2004) Mechanisms and functional significance of tumour induced dendritic-cell defects. , Nat. Rev 4, 941-952.
- 6.Veronese L A, MEA Marques. (2004) Critérios anatomopatológicos para melanoma maligno cutâneo: análise qualitativa de sua eficácia e revisão de literatura. , J Bras Patol Med Lab 40(2), 99-112.
- 7.PC Abreu e Lima, MCC Abreu e Lima, ATCU Camacho, Da Paz AR. (2010) Avaliação dos fatores preditivos de invasão neoplásica do complexo areolomamilar em pacientes com câncer de mama. , J Bras Patol Med Lab 46(3), 245-251.
- 8.FLD Vieira, Vieira B J, MAM Guimaraes, Aarestrup F M. (2008) Cellular profile of the peritumoral inflammatory infiltrate in squamous cells carcinoma of oral mucosa: Correlation with the expression of Ki67 and histologic grading. , BMC Oral Health 8(25), 1-8.
- 9.Karin M, Greten F R. (2005) NF-kappaB: linking inflammation and immunity to cancer development and progression. , Nat Rev Immunol 5(10), 749-59.
- 10.Saeki K, EndO Y, Uchida K, Nishimura R, Sasaki N et al. (2012) Significance of tumor-infiltrating immune cells in gland tumor: 140 cases spontaneous canine Mammary. , J. Vet. Med. Sci 74(2), 227-230.
- 11.Aspord C, Pedroza-Gonzalez A, Gallegos M, Tindle S, E C Burton et al. (2007) Breast cancer instructs dendritic cells to prime interleukin 13–secreting CD4+ T cells that facilitate tumor development. , J Exp Med 204(5), 1037-1047.
- 12.Estrela-Lima A, Araujo M S S, J M Costa-Neto, Teixeira-Carvalho A, S M Barrouin-Melo et al. (2010) Immunolphenotypic features of tumor infiltrating lymphocytes from mammary carcinomas in female dogs associated with prognostic factors and survival rates. , BMC Cancer 10, 256-1.
- 13.Cassali G D, Lavalle G E, Ferreira E, Estrela-Lima A, De Nardi AB. (2014) Consensus for the Diagnosis, Prognosis and Treatment of Canine Mammary Tumors-2013. , Braz. J. Vet. Pathol 7, 38-69.
- 14.Whiteside T L. (2008) The tumor microenvironment and its role in promoting tumor growth. , Oncogene 27(45), 5904-5912.
- 15.Laoui D, Movahedi K, Overmeire E V, Den Bossche JV, Schouppe E. (2011) Tumor-associated macrophages in breast cancer: distinct subsets, distinct functions. , Int. J. Dev. Biol.55(7-9): 861-867.
- 16.Klimp A H, EG De Vries, ScherphoF G L, Daemen T. (2002) A potential role of macrophage activation in the treatment of cancer. Crit Rev Oncol Hematol. , Boca Raton 44(2), 143-161.
- 17.Lin E Y, Pollard J W. (2004) Macrophages: modulators of breast cancer progression. In: Novartis Foundation Symposium. 256, 158-68.
- 18.Garrido E, TLL Castanheira, Rosolem M C, Matsui A, Vasconcelos R O. (2015) A interleucina-10 e seu papel nos carcinomas mamários caninos. , ARS Veterinária 31(1), 037-041.
- 19.Rosolem M C, Vasconcelos R O, Garrido E, TLL Castanheira, PRR Moreira et al. (2015) Immunodetection of myeloid and plasmacytoid dendritic cells in mammary carcinomas of female dogs. , Pesq. Vet. Bras 35(11), 906-912.
- 20.Warabi M, Kitagawa M, Hirokawa K. (2000) Loss of MHC class II expression is associated with a decrease of tumor-infiltrating T cells and an increase of metastatic potential of colorectal cancer: immunohistological and histopathological analyses as compared with normal colonic mucosa and adenomas. , Pathol Res Pract 196(12), 807-815.
- 21.Theoharides T C, Conti P. (2004) Mast cells: the JEKYLL and HYDE of tumor growth. , Trends Immunol 25(5), 235-241.
- 22.Bacci S, Pimpinelli N, Romagnoli P. (2010) Contacts between mast cells and dendritic cells in the human skin. , Ital J Anat Embryol,115(1-2): 25-30.
- 23.Dudeck A, Suender C A, Kostka S L, E Von STebut, Maurer M. (2011) Mast cells promote Th1 and Th17 responses by modulating dendritic cell maturation and function. , Eur J Immunol 41(7), 1883-1893.
- 24.Gri G, Piconese S, Frossi B, Manfroi V, Merluzzi S. (2008) CD4+ CD25+ regulatory T cells suppress mast cell degranulation and allergic responses through OX40-OX40L interaction. , Immunity 29(5), 771-781.
- 25.Mekori Y A, Hershko A Y. (2012) T Cell-mediated modulation of mast cell function: heterotypic adhesion-induced stimulatory or inhibitory effects. , Front Immunol 3(6), 1-6.
- 26.Dabiri S, Huntsman D, Makretsov N, Cheang M, Gilks B et al. (2004) The presence of stromal mast cells identifies a subset of invasive breast cancers with a favorable prognosis. , Mod Pathol 17(6), 690-695.
- 27.Rajput A B, Turbin D A, MCU Cheang, Voduc D K, Leung S et al. (2008) Stromal mast cells in invasive breast cancer are a marker of favourable prognosis: a study of 4,444 cases. , Breast Cancer Res Treat 107(2), 249-257.
- 28.Jiang Y A, Zhang Y Y, Luo H S, Xing S F. (2002) cell density and the context of clinicopathological parameters and expression of p185, estrogen receptor, and proliferating cell nuclear antigen in gastric carcinoma. , World J Gastroenterol 8(6), 1005-1008.
- 29.Heidarpour M, Rajabi M A, Rajabi P, Farkhani H S. (2008) Mast cells in invasive ductal breast carcinoma. , J Res Med Sci 13(5), 255-259.
- 30.Lavalle G E, Bertagnolli A C, Tavares WLF, Ferreira MAND, Cassali G D. (2010) Mast cells and angiogenesis in canine mammary tumor. , Arq Bras Med Vet Zootec 62(6), 1348-1351.
- 31.Tabibzadeh S S, Sivarajah A, Carpenter D, Ohisson-Wilhelm B M, Satyaswaroop P G. (1990) Modulation of HLA-DR expression in epithelial by interleukin 1 and estradiol 17 beta. , J Clin Endocrinol Metab 71(3), 740-747.
- 32.Thibodeau J, Bourgeois-Daigneault M C, Lapointe R. (2012) Targeting the MHC Class II antigen presentation pathway in cancer immunotherapy. , OncoImmunology 1(6), 908-916.
- 33.Concha A, Esteban F, Cabrera T, Ruiz-Cabello F, Garrido F. (1991) Tumor aggressiveness and MHC class I and II antigens in laryngeal and breast cancer. , Semin Cancer Biol 2(1), 47-54.
- 34.Al-Shibli K, Al-Saad S, Andersen S, Donnem T, Bremnes R M et al. (2010) The prognostic value of intraepithelial and stromal CD3-, CD117- and CD138-positive cells in non-small cell lung carcinoma. , APMIS 118(5), 371-82.
Cited by (2)
- 1.Liu Yunhan, Huo Bin, Chen Zhongping, Wang Kun, Huang Lingjie, et al, 2023, Effects of Organic Chromium Yeast on Performance, Meat Quality, and Serum Parameters of Grow-Finish Pigs, Biological Trace Element Research, 201(3), 1188, 10.1007/s12011-022-03237-z
- 2.Raffo-Romero Antonella, Aboulouard Soulaimane, Bouchaert Emmanuel, Rybicka Agata, Tierny Dominique, et al, 2023, Establishment and characterization of canine mammary tumoroids for translational research, BMC Biology, 21(1), 10.1186/s12915-023-01516-2
