Abstract
Objective:
Radiation exposure to the general public and patients undergoing diagnostic or therapeutic procedures is of great concern, especially to the medical community. Revision of Nuclear Regulatory Commission rules several years ago yield new recommendations for the administration of therapeutic doses of 131-Iodine that included the release criteria. The guidelines for ambulatory treatment included patient education and radiation safety measures to minimize exposure and contamination. Our goal in this study was to evaluate patient compliance with the radiation safety instruction protocols given to them before the therapeutic dose and monitor radiation levels in different house areas at different times after an ablation therapy of 3700MBq or more.
Method:
Patients with well differentiated thyroid cancer being evaluated for ablation therapy with 131-Iodine were invited to participate. A thorough set of instructions on radiation protection were given verbally and in writing. Patient house was assessed with a Geiger Muller detector at 24 and 72 hours or above to obtain direct radiation levels in several areas. Patient radiation levels were also monitored.
Results:
A total of 12 patients have been included, 11 females and 1 male, median age was 53 years. Tumor histology was 10 papillary, 2 papillary-follicular variant and 1 follicular carcinoma; 92% of the cases were T1, N0, M0. Home location was urban in 77% and rural in 33% of the patients; 67% of the patients had an educational level between 9-12 grade. Radioiodine doses range from 3441-5994MBq. None of the patients had a relatives or companion in the house. Mean patient exposure 24 hours after the dose at 1 meter was 12mrem/hr, 0.120mSv/h; this represented a retained dose of 2181MBq (59mCi). Only one patient (T1, Nx, M1) had an exposure rate at 1 meter of 100mrem/hr (1mSv/hr) at 24 hours. At 72 hours the exposure changed to 4mrem/hr, 0.040mSv/hr, retained dose of 725.2MBq (19.6mCi). Higher exposure rates in the house were at 24 hours in the bed and pillows (7mrem/hr), kitchen trash (13mrem/hr) and bathroom sink (8mrem/hr). The exposure rates at the toilet and shower were similar (3-4mrem/hr). There was a significant decreased in the exposure rate at 72 hours in all house areas. Patients with the higher exposure rates were those with metastatic disease, and small living facilities. Patients living in rural or urban location had no difference in the exposure rate. The educational levels were not related either to the exposure rate.
Conclusion:
Patient compliance with radiation protection instructions and Nuclear Regulatory Commission release criteria was good. Radiation exposure levels in the house areas are safe. Special instructions must be design to minimize contamination in the bathroom and kitchen Caution is recommended in the release of patients with extensive metastatic disease and doses of 5920MBq or more.
Author Contributions
Academic Editor: Manas Sahoo, Department of Nuclear Medicine Physician
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2016 Ralph Martin, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Papillary, follicular and papillary-follicular variant type thyroid carcinomas are collectively classified as Well Differentiated Thyroid Carcinomas (WDTC). These tumors are considered slow growing tumors with low mortality and morbidity. Nevertheless, their reported incidence has been rising in the United States and Puerto Rico for the past 10 years. The age adjusted incidence reported by the National Cancer Institutes for 2009 was 9.6/100,000 per year 1. From 1999 to 2003, WDTC has been the 12th most frequent cancer in PR and there has been a rise in the incidence rate among men and women from 1987 to 2010 of 9.8% and 11.4% respectively 2.
A key characteristic of WDTC is their avidity for iodine and their dependence on the thyroid’s ability to absorb and organify iodine. These factors are the basis for the use of radioactive iodine for the treatment of WDTC, a procedure that has been well established in the past 50 years 1.
Radiation exposure to the general population and patients undergoing diagnostic or therapeutic procedures is of great concern. The Nuclear Regulatory Commission (NRC) rules and guidelines for ambulatory treatment of WDTC patients with 131-Iodine (131I) encompass patient education and radiation safety measures to minimize exposure and contamination.
In the last 5-6 years, treatment guidelines for WDTC have changed. Surgery is recommended as the initial step in the management of the tumors in most cases. Radioiodine ablation aimed to destroy or ablate any residual thyroid tissue, tumor remnants, affected lymph nodes or distant metastasis is recommended for selected low risk patients and for most of the intermediate and high risk cases. Thorough pre therapy preparation of the patient is required to ensure treatment success. Preparation is aimed at increasing the avidity of the remaining functional thyroid tissue for radio-iodine by elevating thyroid stimulating hormone (TSH) levels as well as decreasing endogenous iodine.
In the initial evaluation visit, informed consent for the therapy is generally obtained and important radiation safety precautions are discussed with the patient and any involved household members. This process is in accordance with the Code of Federal Regulations (10CFR) and the NRC. Hygiene, relative isolation and other radiation safety measures are all addressed by the nuclear medicine physician to avoid cross-contamination and limit the exposure to others. These precautions are implemented for approximately seven days or more after administration of the radio-iodine.
The preparatory process is also addressed in this initial evaluation visit. Prior to the administration of the 131I dose, TSH should be elevated (above 30 IU) to allow a greater uptake of radioiodine. A low iodine diet is also recommended to deplete the remaining tissue and enhance radio-iodine absorption. Radioactive iodine (131-I) is then administered orally.
Prior to 1997, the NRC required that all 131I therapies exceeding 1,110MBq (30mCi) were administered in the hospital, with patient isolation rules to avoid public exposure and /or contamination. Revision of these rules by the NRC produced new recommendations for the administration of therapeutic doses of 131I. Recommendations included that the release criteria require a total effective dose equivalent of no more than 0.05mSv (5mrem) per hour at 1 meter from the patient, a total effective dose of no more than 5mSv (500mrem) in a single year to any person in close contact with the patient. A stricter dose limit of 1mSV (100mrem) per year applies to children, pregnant women, and anyone not involved in the care of the patient 3.
During the past decades, various investigators have gradually helped shape regulations by setting out to measure household radiation exposure in WDTC therapies, including patients, family members and pets. 3, 6, 9.
Our goal was to evaluate our patient compliance with the radiation safety instruction protocols by measuring radiation levels at different times in the most used house areas, including the bathroom, bedroom and kitchen, with ablation doses of 3700MBq (100mCi) or above.
Methods
Adult and pediatric patients with WDTC, referred to our center for post-operative ablation therapy with 131I were invited to participate. Instructions for radiation safety precautions were provided in both verbal and written form by the authorized user on the initial visit and were reviewed again with the patient on the day of the dose administration. Instructions included information related to the house facilities, number of persons living in the house and the presence of children and/or pregnant females. A Geiger Muller (GM) detector was used to monitor the radiation levels in the living area. Surveys were performed at 24 and 72 hours after the radioactive 131I dose was administered. Patient radiation levels were also monitored after the dose administration and at the time of the home surveys. We defined contamination as any house area with radiation levels above background. Descriptive statistics were used for the analysis of the study population data.
All patients and/or legal guardian signed an Informed Consent; pediatric patients also completed the Assent Form. The study and the protocol were approved IRB and Radiation Safety Committee.
Results
A total of 12 patients were included, 11 women and 1 man with a median age was 53 years (11-65). Tumor histology in 97% of the cases was Papillary and Papillary-Follicular variant. Most of the patients were Stage 1, T1, N0. One patient had distant metastasis; two had T3-4 tumors (see Table 1).
Home location was urban in 75% of the cases; in two patients the living facilities were very small (studio apartment). None of the patients had a relatives or companion in the house. The majority of the patients had a high school diploma, 33% had a university degree.
Table 1. Patient demographics| Patient No. | Age | Home* | Education | Ocupation | Histology | T-N-M | RISK | Tx Dose (mCi) |
| 1 | 41 | U | 11th grade | Housewife | Papillary | T1-N0-M0 | LOW | 147.1 |
| 2 | 57 | U | 12th grade | N/A | Pap-Foll | T1-N0-M0 | LOW | 96.4 |
| 3 | 50 | U | 9th grade | Housewife | Papillary | T1-N1-M0 | INT | 97.4 |
| 4 | 62 | R | 9th grade | Housewife | Papillary | T1-N0-M0 | LOW | 100.8 |
| 5 | 65 | U | Papillary | T3-N0-M0 | LOW | 94.9 | ||
| 6 | 39 | U | BA | Housewife | Pap-Foll | T1-N0-M0 | LOW | 95.8 |
| 7 | 68 | U | BA | Teacher | Follicular | T4-N0-M1 | HIGH | 162.3 |
| 8 | 11 | U | Special Ed | Student | Papillary | T1-N1-M0 | INT | 147.1 |
| 9 | 53 | R | 12th grade | Housewife | Papillary | T1-N0-M0 | LOW | 92.7 |
| 10 | 64 | R | 12th grade | Retired | Papillary | T1-N0-M0 | LOW | 100.4 |
| 11 | 57 | U | BA | Eldercare | Papillary | T1-N0-M0 | LOW | 96 |
| 12 | 20 | U | BA | Student | Papillary | T1-N0-M0 | LOW | 94.4 |
Radioiodine doses range from 3441-5994 MBq (See Table 1). Mean 24-hour patient measurements at 1 meter were 12mrem/hr (0.120mSv/h); this represented a retained dose of 2181 MBq (59mCi). Only one patient (T1, NX, M1) had an exposure rate of 100mrem/hr (1mSv/hr) at 1 meter. By 72 hours the mean levels decreased to 4mrem/hr (0.040mSv/hr), equivalent to a retained dose of 725.2MBq (19.6mCi) (see Graph 1).
Graph 1.Mean Patient Radiation Levels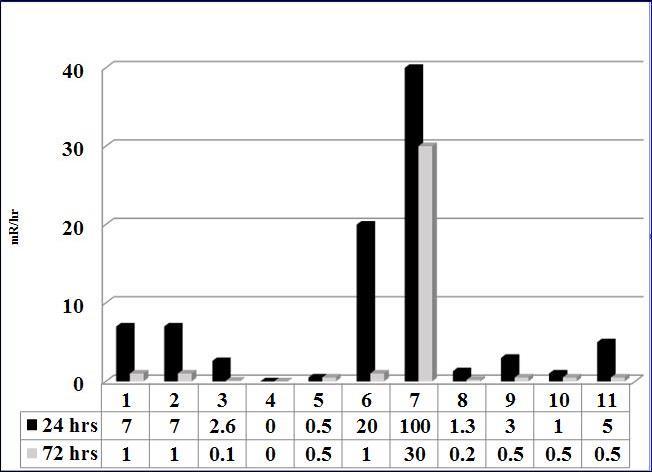
The highest exposure rates in the living areas were at 24 hours in the pillows (22mrem/hr), the kitchen trash bin (19mrem/hr), and the bathroom sink (10mrem/hr). Toilet and shower measurements were similar (3-4mrem/hr). As expected, there was a significant decreased in the levels at 72 hours in all the living areas (see Graph 2).
Graph 2.Radiation Levels in Living Areas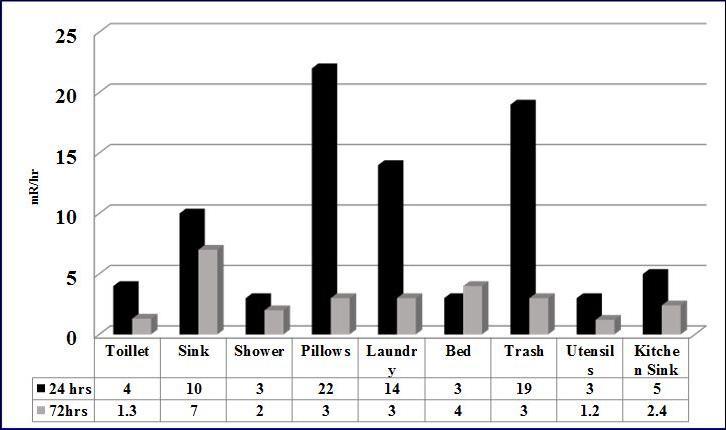
Patients with the higher measurements were those with distant metastasis and small living facilities. We could not identify any trends based on education level or in the rural or urban settings.
Discussion
Even though NRC guidelines for the release of patients were revised several years ago, it is still an important concern to the scientific community and to the general public the radiation exposure from patients receiving ambulatory radioiodine therapies. The changes in the regulations allowed patients receiving more than 1110MBq of 131I to be released from the medical facility if the authorized user presented a plan to his/her licensing authority that the patient is able to conform to reasonable isolation instructions so that no member of the public is likely to be exposed beyond 500mSv.
The evolution of these guidelines has been influenced by continued research in patient and public exposure.Pant, G.S. et al used TLD of CaSO4:Dy discs to measure exposure of 295 relatives of thyroid cancer patients, subdivided according to the administered dose in the range of 0.925-7.4 GBq (25-200mCi). TLD Discs were given to family members in the form of a locket and were used for 15 days from the time 131I was administered. Of all the relatives measured, 66% received < 1mSv of exposure and of the remaining33% that received >1mSv, 10 shared a bed for the first 3 days and traveled on the same car the day of treatment administration 6. Gigsby, P.W. et al measured the exposure of family members and pets and the radiation levels in several rooms for 10 days after receiving 131I doses ranging from 2.8 -5.6GBq (75-150mCi), TLD dosimeters were also used. Patients were instructed to sleep alone, drink fluids, and avoid close personal contact with others for 2 days. Relatives were instructed to resume normal daily activities and dosimeters were used 24hrs/day for 10 days. The dosages to family members ranged from 0.02 to 1.11mSv; exposure dosages to pets ranged between 0.02-1.11mSv. The measured radiation at home was greatest in the patient’s bedroom (0.01-2.89 mSv). This study states, as a limitation, the compliance of relatives and patients in the proper use of the dosimeter. They understand that the radiation doses measured on the living areas and in the relatives are based on 100% compliance7.
Other groups used GM hand detectors to measure radiation exposure. Mulazimoglu, M. et al. measured household member exposure in patients treated for both thyroid cancer and hyperthyroidism. Exposure was measured at the level of abdomen at 1 meter at the time of dose administration and subsequently at 3 days. Measurements of 99.7% of patients were under 30μSv/hr (3mrem) 8. Panzegrau B. et al, also measured outpatient exposure to radiation in 48 patients after 5 days of treatment, at 1 and 3 feet after doses of 100, 150 and 200 mCi of 131I. Background measured in this study was 0.02mR/h. Results of exposure were all under 0.5mR/h for doses of 100 and 150 mCi; with a dose of 200 mCi at 1ft the exposure was 2.5mR/h and 0.79 at 3 feet3.
Either using TLD or GM methods to measure of radiation exposure resulting from the radioiodine ablation doses, all studies seem to agree that a well-informed patient will minimize the risk of exposure to radiation given by 131I. If a set of rules for hygiene, and proper isolation precautions are followed, the exposure of family members should not exceed the established 5mSv per year.
Even though these studies provided some indication that household radiation exposure is within current NRC safety levels, the study populations have been limited in numbers and not classified.
Our study group evaluated patients with varied educational levels, rural vs. urban house location and a range of therapeutic doses between 100-160 mCi. No difference was found in exposure rates between rural vs, urban house location or throughout the different educational levels. As found in other studies, the mean exposure rate of patients was higher at 24 hours and decreased significantly at 48-72 hours to levels below 1mSv, even in patients with M1. The living areas with greatest contamination were, as expected, in the bedroom, kitchen trash binand bathroom and the exposure rates decreased significantly at 48-72 hours.
Our initial goal was to include measurements for other household members in our study, however, after the initial orientation, patients with other household members made arrangements for them to stay elsewhere during the post treatment isolation period.
Although our study included a small sample size, the results confirm that our patients are in compliance with radiation protection instructions, providing further reassurance to our staff and patients that radiation exposure is kept to a minimum when following the ATA and NRC recommendations. In accordance with these recommendations we will continue to provide a detailed, complete set of oral and written instructions before the therapeutic dose. Nevertheless, we believe several adjustments may be required in education strategies and protocols for patients with more extensive disease in order to further reduce exposure. These adjustments may include protective covers for the bedding in those with excessive sweating or salivation at night, as well as trash management strategies and further emphasis on bathroom precautions. We also believe that special care may be required with small living quarters, including measures such as temporarily relocating other household members.
Conclusions:
Patient compliance with radiation protection instructions and NRC release criteria was good, even though our study had a small sample size. Radiation exposure levels in the house areas are safe for relatives and/or companions. Special instructions must be emphasized to minimize contamination in the bed, bathroom and kitchen. Based on the experience at our center, caution is recommended in the release of patients with extensive metastatic disease and doses of 5920MBq or more. A larger multicenter study should be considered in the near future to confirm our findings.
References
- 2.Puerto Rico.Central Cancer Registry, Puerto Rico Cancer Incidence File, Division of Epidemiology. 2006-2010.
- 4.Surveillance Epidemiology and End Results,Fact Sheet:Thyroid. www.seer.cancer.gov/statfacts/ html/thyro.html
- 8.Mulazimoglu M, Edis N, Tamam M O, Uyanik E, Ozpacaci T. (2010) . Radiat Prot Dosimetry 141(3), 233-238.
Cited by (3)
- 1.Salman K.H., Wagih Sh., Munshi T., Almalki M., Zatari S., et al, 2018, Measurement of radiation exposure to household contacts of patients with Graves’ disease treated with low dose radioactive iodine (131I) on outpatient basis, The Egyptian Journal of Radiology and Nuclear Medicine, 49(4), 1125, 10.1016/j.ejrnm.2018.07.011
- 2.Kadhim Ali Abdulhasan, Sheikhzadeh Peyman, Farzanefar Saeed, Yavari Shima, Ay Mohammad Reza, 2020, RADIATION DOSE ASSESSMENT TO FAMILY MEMBERS TAKING CARE OF NON-CANCEROUS THYROID PATIENTS TREATED WITH I-131 THERAPY IN NUCLEAR MEDICINE DEPARTMENT, Radiation Protection Dosimetry, 190(2), 208, 10.1093/rpd/ncaa092
- 3.Salman Khaled, Wagieh Shereen, Bakhsh Aquib, Al-Monshy Tarek, Talaat Omnia, et al, 2020, Measurement of cumulative radiation exposure to children and adolescents in contact with outpatients treated with low dose radioactive iodine (131I), Journal of the Egyptian National Cancer Institute, 32(1), 10.1186/s43046-019-0013-0
