Abstract
Thyroid transcription factor-1 (TTF-1) is known to play key roles in thyroid organogenesis, in thyroid cell proliferation and in the expression of genes involved in thyroid differentiated function. Many human thyroid cancer cell lines keep producing TTF-1 despite the loss of differentiated gene expression, raising a question about the role of the factor in these cells. In order to investigate this point, we used a chimeric protein acting as a functional antagonist of TTF-1 transcriptional activity that was expressed conditionally in 8505C cells originating from an anaplastic thyroid carcinoma. We observed a growth arrest of 8505C thyroid cancer cells when the endogenous TTF-1 transcriptional activity was inhibited. It correlated with decreased levels of several mRNAs encoding positive effectors of cell proliferation like CDK1 and cyclinB1, and increased levels of various mRNAs encoding negative regulators of cell division like CDKN2B and DUSP6. In conclusion, the persistence of TTF-1 expression observed in the dedifferentiated human thyroid cancer cell line 8505C reflects the need of TTF-1 activity for the proliferation of these tumor cells.
Author Contributions
Academic Editor: Giovanni Mauri, IRCCS Policlinico San Donato, Unit of Radiology
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2016 Christiane Christophe-Hobertus, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Thyroid transcription factor-1 (TTF-1), also known as Nkx2.1 or T/ebp, is a homeodomain protein encoded by the Titf1 gene that is present in the thyroid, in lung epithelium, in the posterior pituitary and in a few restricted areas in the brain of the adult organism 1. In the thyroid, TTF-1 plays critical roles in organogenesis 1, 2, in thyroid cell proliferation 3 and in the transcriptional control of genes involved in thyroid hormones synthesis 4. Mice lacking a functional Titf1 gene fail to develop the thyroid gland 5. Also, inactivating mutations in the human Titf1 gene have been associated with developmental and/or functional thyroid defects 6.
Thyroid cancer is the most frequent endocrine cancer 7. Whereas it remains often in a relatively benign form, it leads to a high death rate in its most aggressive form. Various cell lines have been derived from human thyroid tumors. Most of them present a dedifferentiated phenotype evidenced by the loss of expression of thyroid-specific genes like, thyroglobulin, thyroperoxidase and thyrotropin receptor genes 8. However, nearly all of these cells still express the Titf1 gene. Very recent data indicate that this is also the case in thyroid tumors in vivo 9. The persistence of Titf1 gene expression in dedifferentiated thyroid tumor cells raises the question as to whether the presence of this transcription factor would still be required for the proliferation of these cells. In the course of a previous study of TTF-1 functions conducted in the well-differentiated rat thyroid cell line PCCl3 we gained some preliminary evidences suggesting that TTF-1 activity was required for growth in the human thyroid cancer cell lines 8505C and TPC1 (see Figure 2 and corresponding text in ref. 3). In this work, we used the 8505C cell-line, derived from an anaplastic human thyroid carcinoma 10, to confirm the function of the TTF-1 protein in the control of proliferation in this dedifferentiated thyroid cancer cell line. The 8505C cell line appeared indeed as a good model system for the study of TTF-1 function in completely dedifferentiated thyroid cancer cells still expressing TTF-1. Noteworthy, the overall gene expression profile of anaplastic carcinoma-derived cell-lines, like the 8505C cells, had been found to be more comparable to the molecular profile of the corresponding tumor tissue than in the case of follicular or papillary carcinomas-derived cell lines 8. The 8505C cells thus constituded the available experimental cell system that was closest to in vivo human thyroid tumors.
Materials and Methods
Cell Culture
DNA Constructions and Cell Transformations
The controllable production of exogenous proteins in transformed 8505C cells was achieved using the Tet-Off system (Clontech) in which the expression of the transgene is silenced in the presence of doxycycline and is induced upon removal of the agent. The DNA constructs and transfection conditions used to transform the cells have been described previously 3, 11. Human thyroid tumor cells 8505C 10 were first transformed using a pEFIN4 construct directing the expression of the doxycycline-dependent transactivator tTA. Transformed 8505C cells were selected in the presence of 400µg/mL geneticin (GIBCO) and maintained in culture medium supplemented with 200µg/mL of the agent. As the random integration of the expression construct in the genome was known to result in the obtention of cell clones displaying various levels of doxycycline-dependent transcriptional response, transient transfections with a tTA-responsive luciferase reporter construct (pTRE2hyg-Luc, Clontech) were performed on individual cell clones in order to identify the one allowing the best agent-dependent control of gene expression. Two independent transformations were then performed on this cell clone using pTRE2hyg constructs driving the conditional expression of EngrHD or EngrHDm 3 respectively. The cells were transfected in culture medium supplemented with 200 µg/mL of geneticin and 2 µg/mL of doxycycline (Sigma-Aldrich) and then selected in the presence of 200 µg/mL of both geneticin and hygromycin (HygroGold, InvivoGen) and 2 µg/mL of doxycycline. Again, as the random integration of the EngrHD or EngrHDm expression construct in the genome was known to result in the obtention of cell clones displaying various levels of doxycycline-dependent expression, transformed 8505C cells clones showing the expected conditional expression of EngrHD or EngrHDm were selected by Western-blot analysis (see text).
COS-7 cells were transformed using pEFIN4 constructs directing the constitutive expression of EngrHD or EngrHDm 3. The transformed cells were selected and maintained in the presence of 300 µg/mL of geneticin. Clones expressing EngrHD or EngrHDm at comparable levels were selected by Western-blot analysis (see text).
Microscopic Examination
Microscopic examination was performed on an Eclipse TE300 inverted microscope (Nikon) and pictures were recorded using a 3CCD colour video camera XC-003P (Sony) at a magnification of 100.
Western Blot Analysis
Western blots were performed as described before 12 using the anti-engrailed d300 antibody from Santa-Cruz at a dilution of 1/1,000.
Measure of Cell Proliferation
We used a technique developped previously (3) based on the measure of the amounts of DNA contained in dish-attached cells. About 10,000 cells (8505C) were seeded in each culture dish (3.5 cm diameter) or cells were plated at 5% of confluency (COS-7). Triplicate dishes were used at each time point in the experiments.
mRNA Analysis
Total RNA was obtained from cells at about 80-90 % confluency maintained in the presence or absence of doxycycline for one week using the RNeasy mini kit from Qiagen. Double-stranded cDNA was synthesized from 1µg of total RNA, followed by production of antisense RNA containing the modified nucleotide 5-(3-aminoallyl)-UTP using the Amino Allyl MessageAmpTM II aRNA Amplification kit (Ambion). After labelling with Cy3 or Cy5 (GE Healthcare Bio-Sciences), test/control sample pairs were hybridized onto HS1100 Human Genomic Array (Human MI ReadyArrayTM, Microarrays Inc.). Hybridizations were replicated with dye swap. Slides were scanned using a Molecular Devices 4000B laser scanner and expression levels were quantified using GenePix Pro 6.1 image analysis software (Axon Instruments). Image acquisitions were performed with automatic photomultiplier gains (PMT) adjustment. Artefact-associated spots were eliminated by both visual and software-guided flags, as well as spots with a signal/background fluorescence ratio less than 2. The fluorescence values were imported into Acuity 4.0 software package (Molecular Devices). A non-linear locally weighted scatter plot (Lowess) normalization method applied to each individual block (print-tip option) was carried out using Acuity 4.0 software package (Molecular Devices). Data obtained from mean of the normalized log2-ratio calculated for each duplicate were used for the subsequent steps. A selection was done by using a cut-off of normalized fold change >2. The data have been deposited in NCBI’s Gene Expression Omnibus and are accessible through GEO Series accession number GSE58587. For quantitative RT-PCR experiments, residual DNA was removed from the total RNA preparations using the Turbo DNA-free kit from Ambion. RNA was converted into cDNA by random priming using the Superscript IIRT system from Invitrogen. The sequences of the primers used in the qRT-PCR experiments are given in Table 1. Q-PCR mixes contained 6.25ng of cDNA-converted RNA and 200nM of each primer in the iQ™ SYBR Green Supermix (BIO-RAD). Reactions (final volume: 20 µL) were performed in a C1000™ Thermal Cycler equipped with a CFX96™ Real-Time System (BIO-RAD). Reactions were done in duplicate within each experiment and the experiments were repeated at least three times. RER1, TTC1 and CD164 genes that exhibited a well detectable and unchanged level of expression in the microarray experiments were used as reference genes. Fold change was calculated as 2-ΔΔCt.
Table 1. Sequences of the oligonucleotides used as primers in the qRT-PCR experiments.| Name | Sequence (5’ → 3’) | Location in cDNA | Amplicon size |
| hRER1 fw | GTGTGGGAGAATCCGTCCAT | 208-227 | |
| rev | CCCCAAGGCATAGGTCACAA | 407-388 | 200 bp |
| hTTC1 fw | CCCCAGCTATATCAGGGCAAT | 605-625 | |
| rev | AGTACGAGCCGGTAGAGGAA | 891-872 | 287 bp |
| hCD164 fw | CCAACAGCCAATTCTACAGCTAAA | 533-556 | |
| rev | GGGTCTGTTTACAGAGTGTGGTA | 783-761 | 251 bp |
| hTTF-1 fw | GCCGTACCAGGACACCAT | 480-497 | |
| rev | CCGACAGGTACTTCTGTTGCT | 748-728 | 269 bp |
| hCDKN2B fw | ACTAGTGGAGAAGGTGCGACA | 435-455 | |
| rev | CACCAGCGTGTCCAGGAA | 651-634 | 217 bp |
| hCDK1 fw | GGGCTACCCGATTGGTGAAT | 92-111 | |
| rev | CATGGCTACCACTTGACCTGT | 250-230 | 159 bp |
| hcyclinB1 fw | GCCTCTACCTTTGCACTTCCT | 1150-1170 | |
| rev | ATTCTTAGCCAGGTGCTGCAT | 1411-1391 | 262 bp |
| hDUSP6 fw | GGAGATCTTGCCCTTCCTCTAC | 1104-1125 | |
| rev | CAGCCAAGCAATGTACCAAGAC | 1366-1345 | 263 bp |
Results
Expressing a Functional Antagonist of TTF-1 in the 8505C Cell-Line
Human thyroid tumor cells 8505C (10) were transformed using either a construct directing the doxycycline-dependent expression of the TTF-1 antagonist EngrHD, a chimeric protein composed of the DNA-binding TTF-1 homeodomain (HD) linked to the repressor domain of the drosophila Engrailed protein, or a construct directing the doxycycline-dependent expression of the control protein EngrHDm, a similar chimeric protein containing a mutated TTF-1 homeodomain (HDm) devoid of DNA binding activity 3, 11. Transformed 8505C cells clones showing the expected conditional expression of EngrHD or EngrHDm were selected by Western-blot analysis using an antibody directed against the Engrailed moeity of the fusion proteins (Figure 1).
Figure 1.Doxycycline-dependent expression of EngrHD and EngrHDm in transformed 8505C cells. The production of the fusion proteins was analyzed in cell extracts from 8505C cells transformed with tTA-dependent EngrHD or EngrHDm expression constructs by Western blot using an antibody directed against the engrailed moeity of the proteins. An extract from COS-7 cells transiently expressing EngrHD 3 was used as positive control. M: size marker; +/-: presence/absence of doxycycline (“dox”) in the culture medium.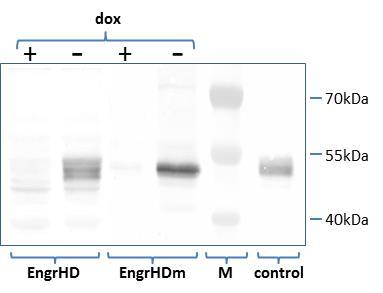
Inhibiting TTF-1 Activity Blocks 8505C Cell Proliferation
Cells not expressing (in the presence of doxycycline) or expressing (after doxycycline withdrawal) the TTF-1 antagonist EngrHD or the control protein EngrHDm were followed under microscopic examination. As shown in Figure 2A, non-expressing cells (left panels) and cells expressing EngrHDm (upper right panel) displayed similar cell densities and a similar morphology. By contrast, the cells expressing EngrHD (lower right panel) showed a clearly reduced cell density, most of them appearing as isolated cells. A more quantitative evaluation of the extent of cell proliferation was perfomed by following the increase with time in the amounts of DNA in the cells remaining anchored in the dishes (no cell death was observed in any condition). As shown in Figure 2B, cells expressing EngrHD (HD-dox curve) did not display any sign of proliferation. Parallel accumulation of DNA was observed in non-expressing cells (HD+dox and HDm+dox curves). A slighty higher accumulation rate was noted in the cells expressing EngrHDm (HDm-dox curve). This was presumably a consequence of the absence of doxycycline in the culture medium. As shown later in Figure 4, the amounts of CDKN2B and DUSP6 mRNAs were higher in doxycycline-exposed cells (expressing the control protein EngrHDm) as compared to untreated (non-expressing) cells Figure 4, HDm-dox/HDm+dox ratio). No significant change was observed in CDK1, cyclinB1 mRNAs concentrations as a control Figure 4, HDm-dox/HDm+dox ratio). The observed increase in CDKN2B and DUSP6 mRNAs in the cells kept in the continuous presence of doxycycline might likely account for the observed reduction in their growth rate. A small inhibitory effect of doxycycline on PCCl3 (normal rat thyroid) cell growth had also been observed previously 3.
Figure 2.Part A: Microscopic examination of 8505C cells expressing or not the EngrHD(m) fusion protein (uncrossed/crossed). The pictures show 8505C cells transformed with tTA-dependent EngrHD or EngrHDm expression constructs maintained for 8 days in the presence or in the absence of doxycycline (“dox/no dox”) in the culture medium. Part B: Growth curves of 8505C cells expressing or not the EngrHD(m) fusion protein. The graphs show the evolution of the amounts of DNA contained in dish-attached 8505C cells transformed with tTA-dependent EngrHD (“HD”) or EngrHDm (“HDm”) expression constructs and maintained for increasing time lengths in the presence or in the absence (+/-) of doxycycline (“dox”) in the culture medium. The product of the O.D.260 value to the value of the O.D.260/O.D.280 ratio calculated at day 3 was set to one in each case. Data are displayed as mean value ± SD.
Figure 3.Part A: Expression of EngrHD and EngrHDm in transformed COS-7 cells. The constitutive production of the fusion proteins was analyzed in cell extracts from transformed COS-7 cells by Western blot using an antibody directed against the engrailed moeity of the proteins. M: size marker. Part B: Growth curves of COS-7 cells expressing EngrHD or EngrHDm fusion protein. The graphs show the evolution of the amounts of DNA contained in dish-attached COS-7 cells expressing constitutively EngrHD or EngrHDm. The product of the O.D.260 value to the value of the O.D.260/O.D.280 ratio calculated at day 2 was set to one in each case. Data are displayed as mean value ± SD.
In order to confirm that the growth arrest we observed in 8505C cells expressing EngrHD was specific, we measured the growth rate of transformed COS-7 cells expressing EngrHD or EngrHDm constitutively at comparable levels (Figure 3A). As shown in part B of the figure, no dramatic difference in cell proliferation was observed in the COS-7 clones expressing EngrHD or the control protein EngrHDm.
Consequences of the Loss of TTF-1 Activity on the mRNA Population
We investigated the consequences of the inhibition of TTF-1 activity on the gene expression profile of 8505C cells using microarray hybridization analysis. Only the changes in gene expression that were detected both when comparing cells expressing or not EngrHD (HD-dox or HD+dox) and when comparing cells expressing EngrHD or EngrHDm (HD-dox or HDm-dox) were taken into account. A total of 288 genes displayed upregulated expression (at least twofold) and 356 genes showed reduced expression (less than half the normal one) in these conditions. The complete data set is available in the GEO database under accession number GSE58587. For a comparison, 349 genes were found overexpressed and 173 genes displayed a reduced level of expression in a similar study performed previously in normal rat thyroid PCCl3 cells 3. Functional annotation clustering using DAVID database 13 revealed that the changes in gene expression observed in 8505C cells in the present study were most significantly related to the processes of cell division (enrichment score 30.11) and DNA replication and the chromosome (enrichment scores 19.99 and 19.2 respectively). As expected (8), the expression of the genes linked to thyroid differentiated function (thyroglobulin, thyroperoxidase,…) was barely detectable in 8505C cells and no significant effect could be detected. However, convincing effects were observed on the expression of several genes involved in cell growth. We detected decreased amounts of several mRNAs encoding positive effectors in cell proliferation (e.g.: CDK1, CDK2, cyclinA2, cyclinB1, cyclinE2) and a concomitant increase in the amounts of several mRNAs encoding negative regulators of cell division (e.g.: CDKN1A, CDKN2A, CDKN2B, DUSP4, DUSP6, DUSP10) in the cells expressing EngrHD. The changes in mRNA concentrations detected in the microarray analysis were confirmed independently in quantitative RT-PCR experiments for CDK1 and cyclinB1 mRNAs (reduced accumulation in the presence of EngrHD) and CDKN2B and DUSP6 mRNAs (increased concentrations in the presence of EngrHD) as shown in Figure 4 (HD-dox/HD+dox and HD-dox/HDm-dox ratios). Noteworthy, a direct function of TTF-1/Nkx2.1 in the control of the cell cycle has also been revealed in the mouse lung, where the cyclinB1 and the cyclinB2 genes were identified as targets of this factor in ChIP experiments and further validated in RNAi experiments 14. The control exerted by TTF-1 on the cyclinB1 gene in the 8505C cells could thus possibly constitute a direct event.
Figure 4.Microarray and quantitative RT-PCR data on CDK1, CDKN2B, cyclinB1, DUSP6 and TTF-1 gene expression in the transformed 8505C cells. The fold changes in mRNA concentrations are shown when comparing cells expressing or not the TTF-1 antogonist EngrHD (“HD-dox/HD+dox”), when comparing cells expressing or not the control protein EngrHDm (“HDm-dox/HDm+dox”), and when comparing cells expressing EngrHD or EngrHDm (“HD-dox/HDm-dox”). Microarray data are displayed as mean value of duplicates ± half range and RT-qPCR data as mean value ± SEM.
We also investigated whether reducing TTF-1 in the cells affected endogenous Titf1 gene expression. We observed a slightly reduced TTF-1 mRNA level in cells expressing EngrHD as compared to non-expressing cell or to cells expressing EngrHDm, however the difference in concentrations was less than twofold in both cases (Figure 4, HD-dox/HD+dox and HD-dox/HDm-dox ratios). If TTF-1 exerts a positive control on its own expression in 8505C cells, the extent of this control appears to be at least very limited. As expected, no difference was apparent when comparing cells expressing or not EngrHDm (Figure 4, HDm-dox/HDm+dox ratio). In our previous study in the normal rat thyroid PCCl3 cell line no effect of EngrHD expression on TTF-1 mRNA level could be detected 3.
Discussion
We have shown here that the presence of transcriptionally active TTF-1 is required for the proliferation of human thyroid tumor 8505C cells. If TTF-1 appears still required for the proliferation of dedifferentiated thyroid cancer cells, its presence is clearly not sufficient for preserving normal cell morphology and the expression of terminal differentiation in these cells. This is reminiscent of what occurs during thyroid organogenesis, where the presence of TTF-1 was shown to be required for the survival of undifferentiated early thyroid precursor cells 1, 2. But other events are required in order to activate later in time the expression of the genes involved in final thyroid differentiation.
Table 2. Comparison of changes in the expression level of selected genes resulting from the inhibition of TTF-1 transcriptional activity by EngrHD expression in human thyroid tumor 8505C and normal rat thyroid PCCl3 cell lines as measured in microarray hybridization experiments.| 8505C | PCCl3 | |
| CDKN1A | + | 0 |
| CDKN2A | + | 0 |
| CDKN2B | + | ++ |
| CDKN2C | (-) | 0 |
| CDKN3 | (-) | 0 |
| cyclinA2 | - | (-) |
| cyclinB1 | - | nd |
| cyclinB2 | 0 | (-) |
| cyclinE2 | - | 0 |
| CDK1 | - | 0 |
| CDK2 | (-) | 0 |
| DUSP4 | + | 0 |
| DUSP6 | + | ++ |
| DUSP10 | + | (+) |
| GADD45 α | 0 | ++ |
| GADD45 β | 0 | + |
| GADD45 γ | 0 | + |
In the normal thyroid cell line PCCl3, the inhibition of TTF-1 transcriptional activity entailed the loss of expression of the genes involved in thyroid differentiated function and induced an epithelial-to-mesenchymal transition process in addition to growth inhibition 3. As the 8505C cells diplay an altered cell morphology and have already lost the expression of the differentiation genes, we could only observe a growth arrest in this cancerous cell line. At the molecular level, comparing our present data with those gained in this previous work revealed the existence of several differences in the list of genes depending on TTF-1 activity in the two distinct cell types. As displayed in Table 2, the inhibition of TTF-1 activity impacted CDK1 and CDK2 genes expression in the 8505C cells only and the GADD45 genes (α,β,γ) in the PCCl3 cells exclusively. A number of CDKN genes (1A,2A,2B,2C,3) were underexpressed in the absence of TTF-1 in 8505C cells whereas only the expression of CDKN2B was affected in PCCl3 cells. Among the DUSP genes, DUSP6 was clearly the most downregulated in PCCl3 cells whereas DUSP4, DUSP6 and DUSP10 were equally impacted in 8505C cells. The effect on the expression of the cyclinA2 and cyclinE2 genes was also most pronounced in the 8505C cells than in the PCCl3 cells. The observed differences could either reflect the existence of species-specific differences between human and rat or they could result from the cancerous transformation of the 8505C cells as compared to the PCCl3 cells. The loss of the control of GADD45 genes expression by TTF-1 in the 8505C cells could possibly fit with this last case. The GADD45α protein is well known to be required at the G2/M checkpoint for entry into the M phase 15. And the GADD45β gene has been found to act as a pituitary tumor suppressor gene in man 16. The loss in the control of expression of these genes could thus possibly constitute a mark of the cancerous transformation in 8505C cells. However, the possibility that GADD45 gene expression is depending differently on TTF-1 activity in human and rat thyroid still remains to be examined. If TTF-1 transcriptional activity is clearly required for cell proliferation in both human 8505C and rat PCCl3 cells, the precise molecular mechanisms involved in this phenomenon are not necessarily identical in both cases.
Confronting our observations gained in the thyroid to those made in the lung reveals the existence of striking similarities regarding the role of TTF-1 in both of these tissues. TTF-1 is known to be required for lung epithelium morphogenesis and differentiated function as well as it is needed for thyroid organogenesis and specialized function 1. In lung tumors, both the absence and the overexpression of TTF-1 are linked to a poor prognosis 17, 18. In a study performed in vivo in the mouse, its absence was shown to result in an increased metastatic potential of the tumor cells 19, and independently, TTF-1 was identified as a potent inhibitor of TGFβ-mediated EMT in lung adenocarcinoma cells, able to restore the epithelial phenotype when expressed in these cells 20. Thus, the epithelial phenotype appears to be controlled by TTF-1 in both thyroid 3 and lung epithelia, in addition to the control exerted by the same factor on the distinct tissue-specific differentiated functions of both of these cell types. In several lung tumors, the Titf1 gene has been shown to be amplified and the resulting overexpression of TTF-1 was associated with increased lung tumorigenesis and poor prognosis 17, 18. The overexpression of TTF-1 in thyroid tumors has never been reported as yet to our knowledge, but a genome-wide association study in the Icelandic population revealed the existence of an association of common variant of the Titf1 gene with increased risk of thyroid cancer 21. The reduction of endogenous TTF-1 expression in lung cancer cells overexpressing the factor resulted in a decreased cell proliferation 22, 23. These reports nicely agree with our present observation in the thyroid cancer cell line 8505C.
Conclusion
In the present study, the presence of transcriptionally active TTF-1 has been shown to be required for the proliferation of human thyroid tumor 8505C cells. As many cell lines derived from human thyroid tumors display common characters 8, and notably the continuous production of TTF-1 despite the loss of normal cell morphology and differentiated function, the conclusion we reached here using the 8505C cell line may possibly also apply to many of these other human thyroid cancer cell lines. It may also possibly apply to many tumors in vivo, as most of thyroid malignant tissues were recently shown to keep producing TTF-1 mRNA 9. Noteworthy, TTF-1 expression was also recently shown to be required for the tumorigenicity of thyroid cells harbouring the RET/PTC rearrangement, one of the most common genetic alteration found in papillary thyroid carcinomas 24. We have reported here an initial observation in a model cell line in vitro that needs now to be confirmed in tumor tissues in vivo. The present on-going development of efficient techniques for genome editing or for the precise manipulation of genomic expression 25 opens the way to performing a selective desactivation of the Titf1 gene in model tumors in vivo. If confirmed in tumors in vivo the requirement of TTF-1 activity for thyroid cancer cell proliferation would make of this transcription factor a potential therapeutic target. Although transcription factors were essentially not considered as useful targets for a therapeutic approach until now, a small-molecule inhibitor of transcription factor LSF activity has recently been shown to be able to specifically inhibit hepatocellular carcinoma tumor growth in a mouse model 26. A problem in the case of TTF-1 is that its activity is also required for proper lung function which would preclude the systemic administration of an eventual specific chemical inhibitor. The expected future development of efficient somatic genome editing methods 25 could however make the local inactivation of the Titf1 gene in the thyroid an alternative therapeutic approach worth to be explored if appropriate. Of course, this local inhibition of TTF-1 activity would also suppress the production of thyroid hormones in the normal tissue adjacent to the tumor, but this loss could be easily compensated by the oral administration of synthetic hormone.
Acknowledgements
This work was supported by grants from the Belgian Fonds David et Alice Van Buuren and the Belgian Fonds de la Recherche Scientifique Médicale (FRSM). D.C. is a research director of the Belgian Fonds National de la Recherche Scientifique (FNRS).
References
- 1.M De Felice, R Di Lauro. (2004) Thyroid development and its disorders: genetics and molecular mechanisms. , Endocr. Rev 25, 722-746.
- 2.Fernandez L P, Lopez-Marquez A, Santisteban P. (2015) Thyroid transcription factors in development, differentiation and disease. , Nat. Rev. Endocrinol 11, 29-42.
- 3.Christophe-Hobertus C, Lefort A, Libert F, Christophe D. (2012) Functional inactivation of thyroid transcription factor-1 in PCCl3 thyroid cells. , Mol. Cell. Endocrinol 358, 36-45.
- 4.Damante G, Tell G, R Di Lauro. (2001) A unique combination of transcription factors controls differentiation of thyroid cells. , Prog. Nucleic Acid Res 66, 307-356.
- 5.Kimura S, Hara Y, Pineau T, Fernandez-Salguero P, Fox C H. (1996) The T/ebp null mouse: thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. , Gene Dev 10, 60-69.
- 6.Montanelli L, Tonacchera M. (2010) Genetics and phenomics of hypothyroidism and thyroid dys- and agenesis due to PAX8 and TTF-1 mutations. , Mol. Cell. Endocrinol 322, 64-71.
- 7.Vu-Phan D, Koening R J. (2014) Genetics and epigenetics of sporadic thyroid cancer. , Mol. Cell. Endocrinol 386, 55-66.
- 8.van Staveren WCG, Solis D W, Delys L, Duprez L, Andry G. (2007) Human thyroid tumor cell lines derived from different tumor types present a common dedifferentiated phenotype. , Cancer Res 67, 8113-8120.
- 9.Batista F A, Ward L S, Marcello M A, Martins M B, Peres K C.(Epub ahead of print, September14,2015)Gene expression of thyroid-specific transcription factors may help diagnose thyroid lesions but are not determinants of tumor progression. doi: 10.1007/s40618-015-0386-4.J. Endocrinol. Invest
- 10.Ito T, Seyama T, Hayashi T, Dohi K, Mizuno T. (1994) Establishment of 2 human thyroid-carcinoma cell-lines (8305C, 8505C) bearing P53 gene-mutations. , Int. J. Oncol 4, 583-586.
- 11.Christophe-Hobertus C, Christophe D. (2007) Human Thyroid Oxidases genes promoter activity in thyrocytes does not appear to be functionally dependent on Thyroid Transcription Factor-1 or Pax8. , Mol. Cell. Endocrinol 264, 157-163.
- 12.Christophe-Hobertus C, Christophe D. (1999) Critical residues of the homeodomain involved in contacting DNA bases also specify the nuclear accumulation of thyroid transcription factor-1. , Eur. J. Biochem 265, 491-497.
- 13.Huang D W, Sherman B T, Lempicki R A. (2009) Systematic and integrative analysis of large gene lists using DAVID Bioinformatics Resources. , Nature Protoc 4, 44-57.
- 14.Tagne J-B, Gupta S, Gower A C, Shen S S, Varma S. (2012) Genome-wide analyses of Nkx-2.1 binding to transcriptional target genes uncover novel regulatory patterns conserved in lung development and tumors. , PLoS ONE 7, 29907.
- 15.Siafakas A R, Richardson D R. (2009) Growth arrest and DNA damage-45 alpha (GADD45α). , Int. J. Biochem. Cell Biol 41, 986-989.
- 16.Michaelis K A, Knox A J, Xu M, Kiseljak-Vassiliades K, Edwards M G. (2011) Identification of growth arrest and DNA-damage-inducible gene β (GADD45β) as a novel tumor suppressor in pituitary gonadotrope tumors. , Endocrinology 152, 3603-3613.
- 17.Yamaguchi T, Hosono Y, Yanagisawa K, Takahashi T. (2013) NKX2-1/TTF-1: an enigmatic oncogene that functions as a double-edged sword for cancer cell survival and progression. , Cancer Cell 23, 718-723.
- 18.Mu D. (2013) The complexity of Thyroid Transcription Factor 1 with both pro- and anti-oncogenic activities. , J. Biol. Chem 288, 24992-25000.
- 19.Winslow M M, Dayton T L, RGW Verhaak, Kim-Kiselak C, SnyderEL. (2011) Suppression of lung adenocarcinoma progression by Nkx2-1. , Nature 473, 101-104.
- 20.Saito R-A, Watabe T, Horiguchi K, Kohyama T, Saitoh M. (2009) Thyroid Transcription Factor-1 inhibits Transforming Growth Factor-β-mediated epithelial-to-mesenchymal transition in lung adenocarcinoma cells. , Cancer Res 69, 2783-2791.
- 21.Gudmundsson J, Sulem P, Gudbjartsson D F, Jonasson J G, Sigurdsson A. (2009) Common variants on 9q22.33 and 14q13.3 predispose to thyroid cancer in European populations. , Nat. Genet 41, 460-464.
- 22.Kwei K A, Kim Y H, Girard L, Kao J, Pacyna-Gengelbach M. (2008) Genomic profiling identifies TITF1 as a lineage-specific oncogene amplified in lung cancer. , Oncogene 27, 3635-3640.
- 23.Tanaka H, Yanagisawa K, Shinjo K, Taguchi A, Maeno K. (2007) Lineage-specific dependency of lung adenocarcinomas on the lung development regulator TTF-1. Cancer Res. 67, 6007-6011.
- 24.Endo T, Kobayashi T. (2013) Concurrent overexpression of RET/PTC1 and TTF-1 confers tumorigenicity to thyrocytes. , Endocr.-Relat. Cancer 20, 767-776.
Cited by (1)
- 1.Teixeira Mariana Pires, Haddad Natalia Ferreira, Passos Eliza Freitas, Andrade Marcelle Novaes, Campos Maria Luisa Arantes, et al, 2022, Ouabain Effects on Human Anaplastic Thyroid Carcinoma 8505C Cells, Cancers, 14(24), 6168, 10.3390/cancers14246168
