Abstract
We report 2 cases of vitamin B12 deficiency in children due to deficient intake. These were 2 girls aged 4 years (case 1) and 6 years (case 2), respectively, hospitalized in December 2020 and March 2021 in the pediatric ward of Le Dantec hospital. Both patients presented with aregenative anemia, melanoderma and undernutrition. The bone marrow count in case 1 showed a dysmyelopoiesis with megablastosis. The blood vitamin B12 level was low in both cases. Folic acid blood levels were normal in both patients, but an associated martial deficiency was found in case 2. The dietary survey revealed a lack of intake of animal products rich in vitamin B12. Vitamin B12 replacement therapy was effective with rapid regression of all clinical signs observed in both children. The control of the vitamin B12 level after 1 month of treatment was normal in both patients. The disappearance of the symptoms under substitute treatment confirmed the deficiency of Vitamin B12 intake in both patients. Conclusion: Both of our patients had a profound Vitamin B12 deficiency in a context of deficiency in nutrition. The regression of the symptoms was spectacular under vitamin B12 replacement therapy, confirming the deficiency. We recommend in our context a contribution in micronutrients such as vitamins in children after weaning to avoid dietary errors.
Author Contributions
Academic Editor: Amal Ibrahim Hassan Ibrahim, Department of Radioisotopes, Nuclear Research Centre, Atomic Energy Authority, Egypt
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2021 Y. Keita, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Vitamin B12 or cobalamin is a water-soluble vitamin, essential for the synthesis of Desoxyribonucleic acid (DNA) and Ribonucleic acid (RNA) in the body. It is involved in erythropoiesis, as well as in the myelination of the central nervous system 1, 2, 3, 4, 5. It is not synthesized by the body and must therefore be provided by the diet. Only food of animal origin contain this vitamin in sufficient quantities 1, 2. Vitamin B12 deficiency is one of the most common vitamin deficiencies 1. It is more common in developing countries. Indeed, it affects 40% of the pediatric population 6. The deficiency can occur at any age, particularly in human being requiring high dietary intake such as infants 6. It may be related either to an abnormality of its absorption, to a defect of intracellular utilization, or to a deficiency of intake 1, 2, 3, 4, 5, 6. We report 2 cases of vitamin B12 deficiency due to intake deficiency in pediatrics.
Observations
Case 1
A 4-year-old girl, previously free of any pathology, was hospitalized on December 24, 2020 for dyspnea, anorexia, skin hyperpigmentation and failure to thrive. Examination revealed an anemic syndrome, palmo-plantar melanoderma (Figure 1a) and severe undernutrition with a weight-for-standing-height < -3 z score, a weight-for-age < -3 z score and a BMI/age of 9.18 kg/m2 (< -3 SD). The patient had a normal neurological examination. She had an anemia of 8.2g/dl normochromic normocytic aregenerative. The myelogram showed dysmyelopoiesis with megablastosis very suggestive of vitamin B12 deficiency or folate deficiency. Vitamin B12 blood test confirmed a collapsed level of 83 pg/ml (laboratory normal: 187-883 pg/ml). The level of folic acid in the blood was normal, as was serum iron. The rest of the workup (ionogram, creatinine, blood calcium) was normal. The dietary survey showed a lack of intake of meat products rich in vitamin B12 in a context of low socioeconomic level. The child received intramuscular vitamin B12 therapy at a rate of 1,000 µg per day for 7 days, followed by oral supplementation at a rate of 1,000 µg/day for 1 month. The symptoms observed on admission regressed within a month (Figure 1b). The control of vitamin B12 levels at 1 month and then at 3 months of treatment returned to normal (Table 1) thus confirming the deficiency's character. Nutritional advice was given to the parents to prevent vitamin and caloric deficits in the patient.
Figure 1.Melanoderma (1a) ; normal skin appearance after treatment (1b)
| Biological characteristics | Case 1 | ||||
| D0 | W1 | M1 | M2 | M3 | |
| Hemoglobin (g/dl) | 8.2 | 10.7 | 12.8 | 12.7 | 12.8 |
| MCV (fl) | 86 | 83 | 93 | 93.4 | 94 |
| MCHC (pg) | 33 | 34 | 31 | 30.8 | 33 |
| MCH (pg) | 28.8 | 28 | 29 | 29 | 30.1 |
| Reticulocyte rate (/mm3) | 2,300 | 40,000 | 57,000 | 65,000 | 71,000 |
| White blood cells (/mm3) | 3.080 | 3.420 | 9.010 | 11.910 | 10,000 |
| Platelets (/mm3) | 50.000 | 45,000 | 675,000 | 778,000 | 690,000 |
| Vitamin B12 (pg/ml) | 83 | - | 1.409 | - | 1200 |
Case 2
This was a 6-year-old girl, with no previous history, who had consulted for asthenia, anorexia, pallor and productive cough. She presented with anemia, Hunter's glossitis (Figure 2a), hyperpigmentation of the soles of the feet, flexion creases and palms of the hands (Figure 2c), and moderate undernutrition with a standing weight-for-height between -2 and -3 z score and a BMI of 13.6 kg/m2 between -2 and -3 SD. The neurological examination was also normal in this case 2. The anemia was profound at 3.5g/dl normocytic normochromic aregenerative with bicytopenia. The myelogram had not been performed. The vitamin B12 level was < 83 pg/ml (threshold detectable by the automaton). Blood folic acid was normal at 11.4 ng/ml (normal > 5-21 ng/mL), however, martial deficiency was present with a serum iron level of 43µg/dl (normal: 75 - 155 µg/dl) (Table 2). The frontal chest X-ray was normal. A lack of intake of animal products rich in vitamin B12 was confirmed by the dietary survey. The child was transfused twice and received intramuscular vitamin B12 therapy at a rate of 1000 µg per day for 7 days, followed by oral supplementation at a rate of 1000µg/day for 1 month. This replacement therapy was effective with rapid regression of the signs (Figure 2b, 2c). Hemoglobin and vitamin B12 levels were checked at 1 month and 3 months after treatment and the patient's blood levels returned to normal(Table 2). The parents received nutritional counseling for the child to prevent recurrence of the disease.
Figure 2.Hunter’s Glossite (2a); Disappearance of Hunter’s Glossitis (2b); Melanoderma (2c); Normal skin appearance after treatment (2d)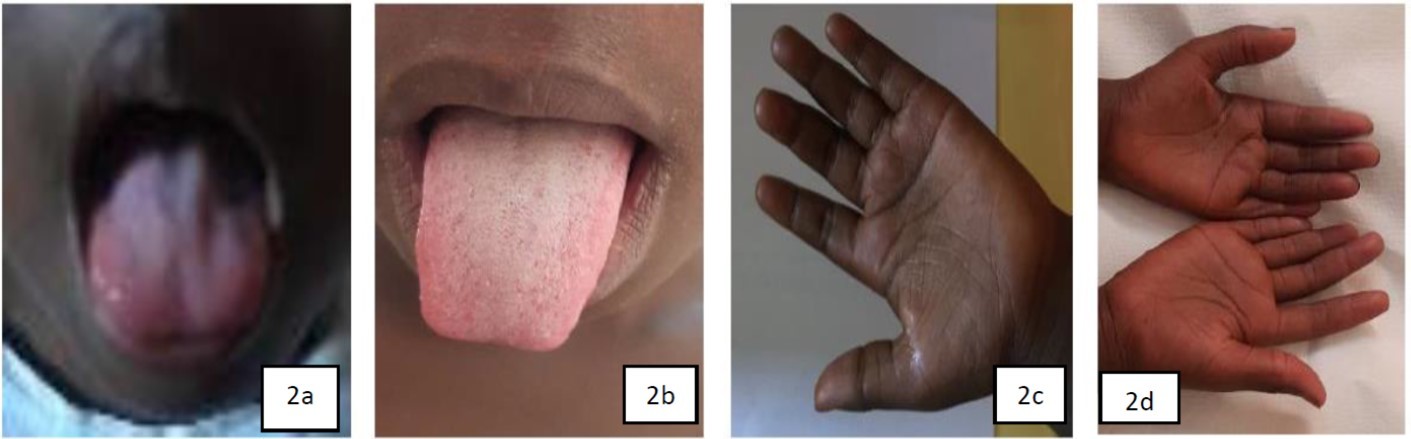
| Biological characteristics | Case 2 | ||||
| D0 | W1 | M1 | M2 | M3 | |
| Hemoglobin (g/dl) | 3.5 | 10.9 | 12.6 | 11.7 | 12 |
| MCV (fl) | 101.9 | 87.1 | 87.2 | 87 | 86 |
| MCHC (pg) | 35.5 | 28.8 | 33.4 | 33 | 33.4 |
| MCH (pg) | 28 | 28.8 | 29.2 | 29 | 28.8 |
| Reticulocyte rate (/mm3) | 25,000 | 59,000 | 61,900 | 158.782 | 198.000 |
| White blood cells (/mm3) | 7.060 | 2.760 | 3.340 | 5.660 | 7300 |
| Platelets (/mm3) | 46,000 | 42,000 | 125,000 | 125,000 | 469000 |
| Vitamin B12 (pg/ml) | < 83 | - | 1,000 | - | 810 |
| Serum iron (µg/dl) | 43 | - | 100 | - | 79 |
Discussion
Vitamin B12, or cobalamin, is derived from foods of animal origin [5,6]. The main sources of vitamin B12 and B6 in the student’s diet were chicken white meat (51.8-53.7 %), beef (45-63 %), cream (62.2 -72.1 %), sardines in oil (47.9-52.2 %), tuna (55.2 -60.4 %), cheese edamer (80.1%) and cheese feta (67.4%-73%) 5. In the United States, the estimated average daily intake of vitamin B12 is about 5 µg/d for men and 3.5 µg/d for women. The recommended intake for vitamin B12 is 2.4 µg/d 7. Its deficiency is one of the most common vitamin deficiencies. Deficits can occur at any age 1, 8. The mean age of the patients was 8±4.8 months 9. In our two patients, the signs of deficiency were found after infant age. Indeed, during the first 1,000 days, the vitamin B12 needs of both children are covered by breast milk and dietary intake by diversification 10. Ablactation could be the cause of this deficiency in the context of an overall intake deficit in our 2 patients. The tendency towards late revelation in these two cases should prompt prevention of micronutrient deficiencies in children after weaning.
Vitamin B12 is a cofactor such as pyridoxine (vitamin B6) and folic acid (vitamin B9) in the conversion of homocysteine to methionine. It plays several other roles including structural and functional integrity of myelin, hematopoiesis and DNA and RNA synthesis 6, 7. Its deficiency is mainly revealed by haematological and neurological signs 11, 12. Epilepsy is a rare condition in children and adults with VB12 deficiency 9. However, other organs may be affected 13, 14. In our two patients, the disease was manifested by hematological and cutaneous signs. Anemia was more profound when associated with martial deficiency in case 2 requiring transfusion. The disappearance of melanoderma that led to the diagnosis in our two patients was correlated with the biological cure of the disease.
Several etiologies of vitamin B12 deficits are founded in children. These are mainly lack of intake, malabsorption, lack of intracellular use, and metabolic disorders 15, 16, 17, 18.
Malabsorption is the most common cause of cobalamin deficiency. Diseases Leading to Insufficient Cobalamin Absorption are Imerslund-Gräsbeck syndrome, atrophic gastritis or partial gastrectomy, inadequate functional gastric mucosa, atrophy of the gastric parietal cells, Lacking or inhibition of pancreatic proteases, competition in the intestines with vitamin B12 by parasites or Small Intestinal bacterial overgrowth (SIBO), transcobalamin II deficiency. Also long-term anti-acid therapy, insufficient pepsin or gastric secretion and inadequate proteolytic digestion, are others causes of cobalamin deficiency due to malabsorption 16, 17, 18.
Biermer’s disease is a relatively common autoimmune gastritis affecting mostly women. All age groups can be affected and it is often associated with other autoimmune diseases including type 1 diabetes, thyroiditis or vitiligo. It is characterized with fundic atrophy by involvement of parietal cells leading to achlorhydia and reactive hypergastrinemia. Antibodies are directed against gastric parietal cells (sensitivity 90%, specificity 50%) and against intrinsic factor (sensitivity 50%, specificity 90%). Iron deficiency is frequently associated due to achlorhydria 8, 9, 10. In our two patients, gastric biopsy and anti-parietal cell antibodies assay were not indicated in this context of undernutrition. Indeed, in view of the clinical signs, the results of the medullogram and the dietary investigation, undernutrition was the most probable hypothesis.
Imerslund-Gräsbeck disease is a congenital and elective malabsorption of cobalamins, with autosomal recessive transmission, by mutation of 2 different genes (cubulin and amnionless) coding for 2 subunits of the physiological receptor of the intrinsic factor-vitamin B12 complex in the ileal mucosa. These 2 proteins are also present in the renal proximal tubule, which explains the presence of non-selective proteinuria in the majority of cases 19, 20. During this disease, the intrinsic factor activity increased 21. We also ruled out this disease in our two patients because of the favorable clinical response to replacement therapy.
Defects in intracellular utilization due to congenital transcobalamin II deficiency are also a cause of vitamin B12 deficiency in children 22, 23. Intrinsic factor activity assay and genetic study were not performed in view of the favorable clinical response after therapeutic testing.
Intake deficiency was the cause of vitamin B12 deficiency in our two patients. The clinical signs, which are most often, are at the origin of the diagnosis as in our 2 cases (failure to thrive, melanoderma and anemia) 13. In contrast to adults, this deficiency in children has rapid and sometimes irreversible repercussions, particularly on the neurological system 10. In our two reported cases, we did not found neurological signs. Psychomotor development was normal in our patients. However, neurological damage is more severe in infants with a predominant cerebral atrophy, while in our two patients it is mainly bone marrow and skin damage.
Intake deficiency is generally reported in children breastfed by strictly vegetarian mothers or mothers with undiagnosed or untreated Biermer's disease . Anemia appears very early and the diagnosis is carried out with dietary investigation and maternal exploration (NFS, vitamin B12 assay, intrinsic anti-factor antibody assay). Health screening is therefore essential to specify the maternal diet, family and personal history in order to identify dysimmune condition 10. A case of Biermer’s disease in a woman explaining vitamin B12 deficiency in exclusively breastfed children was reported by Cariou et al 19. The deficiency occured after a few months of exclusive breastfeeding. A study of 40 exclusively breastfed children with vitamin B12 deficiency showed an average age at diagnosis at 4.4 2.5 months 19, 24. In our two reported cases, vitamin B12 deficiency was related to lack of intake after weaning, hence the interest of primary prevention in our often unfavourable socio-economic context.
Conclusion
These two sporadic cases of vitamin B12 deficiency must draw attention to the reality of micronutrient deficiencies in developing countries. Both cases reported a deep deficiency of Vitamin B12 in a context of undernutrition corrected by a replacement therapy thus confirming the lack of intake. Food diversification, food enrichment and access to food help to prevent micronutrient deficiencies such as vitamin B12 in children.
References
- 1.Sahel H.Hyperpigmentation revealing severe vitamin. B12 deficiencyJ. de Pediatrie et de Pueric.2019 ; 32(2), 99-104.
- 2.Demir N, Koc A, Ustyol L.Clinical and neurological findings of severe vitamin B12 deficiency in infancy and importance of early diagnosis and treatment. , J. Paediatr. Child Health,2013 : 49(10), 820-4.
- 3.Pawlak R, Parrott S J, Raj S, Cullum-Dugan D, Lucus D.How prevalent is vitamin B12 deficiency among vegetarians?. Nutr Rev.2013;71 (2) : 110-7.
- 5.Nafija S, Arzija P, Vedran D, Muris P, Namik T. (2019) The Vitamine Source, Usual Food Intake at Students. Mater Sociomed. 31(1), 53-56.
- 8.LeGuenno G, Quilliot D. (2014) Management of cobalamin deficiency. , Nutr. Clin. et Metab.: 28(2), 130-34.
- 9.Kirik S, Çatak Z. (2021) . Vitamin B12 Deficiency Observed in Children With First Afebrile Seizures. Cureus 13(3), 13745.
- 10.Mathey C, Di Marco JN, Poujol A, Cournelle M A, Brevaut V et al. (2007) Failure to thrive and psychomotor regression revealing vitamin B12 deficiency in 3 infants. Arch Pediatr. 5(14), 467-71.
- 11.Yilmaz S, Serdaroglu G, Tekgul H, Gokben S. (2016) Different neurologic aspects of nutritional B12 deficiency in infancy. , J Child Neurol 31, 565-68.
- 12.Black M M. (2008) Effects of vitamin B12 and folate deficiency on brain development in children. Food Nutr Bull. 29, 126-31.
- 13.P De Lonlay, Fenneteau O, Touati G, Mignot C, T Billette de Villemeur et al. (2002) Haematological manifestations of inborn errors of metabolism. Arch Pediatr. 8(1), 822-35.
- 14.Avci Z, Turul T, Aysun S, Unal I. (2003) Involuntary movements and magnetic resonance imaging findings in infantile cobalamine (vitamin B12) deficiency. Pediatrics. 112(3), 684-6.
- 15.Briani C, Dalla Torre C, Citton V, Manara R, Pompanin S.. Cobalamin Deficiency : Clinical Picture and Radiological Findings. Nutrients 2013 ; 5 : 4521-39.
- 16.Antony A C. (2003) Vegetarianism and vitamin B-12 (cobalamin) deficiency. , Am. J. Clin. Nutr 78, 3-6.
- 17.M J Nielsen, M R Rasmussen, C B Andersen, Nexo E, S K Moestrup. (2012) Vitamin B12 transport from food to the body’s cells—A sophisticated, multistep pathway. , Nat. Rev. Gastroenterol. Hepatol 9, 345-54.
- 18.Herrmann W, Schorr H, Obeid R, Geisel J. (2003) Vitamin B-12 status, particularly holotranscobalamin II and methylmalonic acid concentrations, and hyperhomocysteinemia in vegetarians. , Am. J. Clin. Nutr 78, 131-36.
- 19.Cariou M E, Joncquez A L, Prades N, Schmitt F. (2013) Vitamin B12 deficiency in a five-month-old infant: A case report. , IMMUNO-ANAL BIOL. SPE 28(2-3) 133-6.
- 20.Choquet P, Levrat V, Pondarre C, Vianney C, Guffon N. (2009) Imerslund-Gräsbeck Syndrome. Arch Pediatr. 16(12), 1559-61.
- 21.Guéant J L, Coelho D, Nicolas J P. (2014) Vitamin B12 and related genetic disorders. , Bull. Acad. Natle Méd 198(9), 1141-56.
- 22.Dumont B, Gay C, Rapin S, J F Benoist, Acquaviva-Bourdain C. (2016) Anomalie d’utilisation intracellulaire de la vitamine B12 par défaut de ABCD4. , Cobalamine J. Arch Pediatr 23(12), 1295-304.
