Abstract
Carbofuran is a broad spectrum pesticide used in agricultural fields and domestic places throughout the world. It is one of the deadly toxic carbamate pesticide that kills the pest by inhibiting the crucial enzyme of nervous system known as acetyl cholinesterase. In the present study, we report how carbofuran increases the different spectrum of cholesterols, including free cholesterol and esterified cholesterol in the fish hepatocytes. It is observed that induced-cholesterol can inhibit the enzymatic activity such as Ca++-ATPase, which is a critical protein for maintaining the calcium homoeostasis in the cellular microenvironment. Carbofuran integrates into human body through foods and drinks. As trace of carbofuran is identified in our daily food and drinks, we examined the homology of Ca++-ATPase between the fish and human, so our data can illuminate the effects of carbofuran on this crucial enzyme. While studying the homology with the help of bioinformatics, we recognized that there is around 70% homology in the protein sequence of Ca++-ATPase between fish Heteropneustesfossilisand human (Homo sapiens), which appears as sufficient to simulate our fish-model data in human. This study demonstrates that carbofuran affects our day-to-day life by inhibiting Ca++-ATPase through modulation of lipid synthesis, a critical regulatory system that controls overall homeostasis in our body.
Author Contributions
Academic Editor: Shitao Li, Department of Microbiology & Immunobiology Harvard Medical School, Boston.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2014 Manik C.Ghosh, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Carbofuran (trade name is furadon) is one the most commonly used pesticide in agricultural fields and domestic places 1. The mechanism of its toxic action is mediated through irreversible binding of a crucial enzyme of nervous system known as acetyl cholinesterase, which breaks the acetylcholine in acetic acid and choline in physiological system2. The extended half life of carbofuran on the soil ecosystem makes it more venerable to disperse from one ecosystem to other 1. Surprisingly, traces of carbofuran were detected in the foods and drinks, which is consumed day-to-day in our life 3, 4, 5. The biochemical structure of carbofuran is similar to steroid hormone with an organic benzene nucleus which makes it a more lipophilic compound 6. New study revealed that carbofuran can disrupt the steroid hormone homoeostasis by binding its receptor and inducing a mock signaling 1. Abnormal sperm count was observed upon intake of carbofuran in rat model 7. Chronic exposure to this pesticide seemed to exert a carcinogenic effect on the baby in mother’s womb 5, 7, 8. A mood disorder was observed to the patients, which might be due to its ability of inhibiting to acetyl cholinesterase, a critical enzyme in our nervous system
9. The acute toxicity of carbofuran is fatal to human and animals if exposed in high dose. Thousands of birds and wild animals die due to ingestion of granular form of carbofuran in agricultural fields 10.
The detoxification of carbofuran occurs in the liver. Our laboratory reported that cytochrome P4501a might be involved in degradation of this pesticide in hepatocytes 11, 12, 13. The mechanism of degradation is also associated with the induction of several critical pathways including, PI3 kinase, PKC, and HSP70 12. There are lots of report indicated that, upon metabolism, carbofuran produces 3-keto carbofuran and 3-hydroxycarbofuran 14. The metabolic bi-products of carbofuran are relatively water soluble and therefore excrete from our body, however; there is no knowledge about the cellular compartmentalization of these products in the hepatocytes while it undergoes detoxification. In this manuscript, we have identified its different metabolites in subcellular fractions upon detoxification, such as, nucleus, cytosol, mitochondria, and membrane by high pressure liquid chromatography (HPLC). Membrane Ca++-ATPase is one of the critical enzymes that work in the endoplasmic reticulum (ER) and maintain the homeostasis of calcium inside the cell 14, 15. Disruption of Ca++-ATPase leads to dysregulation of many signaling pathways which may result in apoptosis of cells 16. Here we observed that carbofuran inhibits the Ca++-ATPase activity dose dependently in the cultured catfish hepatocytes. While investigating the homology of Ca++-ATPase between fish Heteropneustesfossilis and human Homo sapiens, we observed that there is around 70% homology between these two species, indicating that our data can be simulated with human system. Few earlier reports indicated that pesticides have the ability to disrupt the Ca++-ATPase activity, however, there was no clear mechanism 17, 18, 19. While searching the underlying molecular mechanism, we observed that carbofuran induced the spectrums of cholesterol in the hepatocytes membrane. The elevated cholesterol could be responsible for the attenuation of membrane Ca++-ATPase activity. Our observations revealed a new mechanism of carbofuran toxicity, which is one of most useful pesticides being utilized nowadays to save crops and domestic products. The data also signify that carbofuran when enters our body through food and water could inhibit the Ca++-ATPase via modulation of lipid synthesis or its metabolism, and affects the overall Ca++ homeostasis in liver.
Materials and Methods
Materials:
Carbofuran (98.5% pure), 3-keto carbofuran, and 3-hydroxycarbofuran were obtained as a generous gift from Rallis India Inc. (Karnataka, India).Dichloromethane water and de-ionized water HPLC grade were purchased from Spectrochem, India. Silica gel (60-120 mesh sige), alumina were purchase from SRL, India.
Culture of Catfish Hepatocytes
Maintenance of catfish (Heteropneustesfossilis) in institute aquarium, treatment, sacrifice, and collection of liver were undertaken in compliance with the regulatory rules of Bose Institute Animal Ethics Committee (No#95/99/CPCSEA). Isolation of hepatocytes from catfish liver and their culture were done following the methods routinely used in our laboratory 11.
Treatment of the Hepatocytes with Carbofuran (CF)
Carbofuran was added at a concentration of 1 uM to the culture media and incubated for 24h. Treated hepatocytes were harvested and pooled for further experiments. Pellet was obtained from harvested hepatocytes upon centrifugation at 500 x g for 5 minutes followed by measuring the wet-weight of hepatocytes. Pellet was sonicated by a sonicator (Proscientific, oxford, CT ) in 20 mM of Tris buffer with 6 strokes for 10 sec each. Nucleus, mitochondria, cytosol, and membrane were separated by differential centrifugation. Nucleus was obtained at 500 x g, where as mitochondria was obtained at 10,000 x g from post nuclear supernatant. Membrane was obtained by ultracentrifugation at 100,000 x g for 1 h. The supernatant was collected as a cytosol. Those four fractions were freezed down at -80ºC for carbofuran and its metabolites extraction.
Preparation of Samples for HPLC
Extraction of carbofuran and its metabolites was performed following the method of Chatterjee et al. 20, 21. The fractions containing carbofuran and its metabolites were cleaned by passing through a column packed with silica gel (mesh size 60-120) and alumina separated by a layer of sodium sulfate. The column was pre-eluted with dichloromethane followed by addition of extract. The final elution was done with dichloromethane that eluted the carbofuran and its metabolites from the column and filtered through a filter paper (pore size 0.22 uM) (Millipore, Billirica, MA,). Elutes were concentrated by a gentle stream of nitrogen before loading in HPLC column.
Quantification of Carbofuran, 3-Ketocarbofuran and 3-Hydroxycarbofuran by HPLC
HPLC is a powerful tool for measuring the concentration of macromolecules at pictogram or below level. Water HPLC with binary pump and reverse phase C-18 column (Microbondapak, Waters Inc., MS, USA) was used in this experiment. The post-column detection was done by UV detector at 265 nm. A mobile phase of water : methanol (55:45) was used. The flow rate was maintained at 1 ml/min in isocratic condition at ambient temperature. The identification of carbofuran, 3-hydroxycarbofuran, and 3-Ketocarbofuran were done by comparing the retention time with standard. HPLC was run according to the standard protocol by performing all the washing and pre-optimization with internal solvent control. The quantification and computation were made using Milleneum-2010 chromatographic software of Waters Inc.
Estimation of Membrane Cholesterol by Analytical and Thin Layer Chromatography
2.5 g of wet hepatocytes was used for collection of total lipid. The membrane was obtained from post-mitochondrial supernatant by ultracentrifugation as mentioned before. The total lipid was extracted from this fraction according to the method of Bligh and Dyer 22.
Estimation of Cholesterol by Thin Layer Chromatography Plate (TLC)
Silica-G (E. Merck, Germany) plates (18 x 12 cm2) with 0.5 mm thickness were used for total cholesterol analysis. Equal amount of total lipid was charged to the TLC plate at 3 cm above the lower edge. Petroleum ether, diethyl ether, and acetic acid at the ratio of (90:10:1) was used as a solvent system. A standard of cholesterol (Sigma chemicals, MO, USA) were used in parallel with the unknown samples. The plate was developed by solvent and chromatogram was obtained. Detection and quantification of cholesterol on the plate were performed using the staining reagents, a mixture of Ferric chloride, acetic acid, and sulfuric acid solution. The staining reagents were sprayed uniformly on the plate followed by heating at 100-110ºC for 20 minutes. Cholesterol were detected as a violet spot on the TLC plate. The stained spots were quantified by a densitometer against the standard using the software (Bio-Rad 700-GS, Imaging Densitomter, Japan).
Analytical Estimation of Cholesterol from Total Lipid
Assay of Membrane Ca++-Atpase from the Hepatocytes
Insertion of Cholesterol in the Hepatocytes Membrane Followed by Measurement of Ca++-Atpase Activity
Insertion of cholesterol in the hepatocytes membrane was performed according to the method of Warren et al. 27. In brief, different amount of cholesterol (4.3, 8.6, and 13 mM) was mixed with Na-cholate in Tris-sucrose buffer (pH 7.4) in separate tubes. The ratio of cholesterol and cholate were always maintained 1:2 and the final volume was 3 ml. The mixture was added to the hepatocytes and sonicated with 6 pulses for 10 second each. The extra cholesterol that did not include in the hepatocytes membrane was excluded by ultracentrifugation in sucrose gradient at 100,000 x g for 1 hr. Then cholesterol-inserted membrane was thoroughly dialised against 20 mM of Tris-buffer which depleted the amount of cholate from the membrane. It was necessary to leach out the cholate because it inhibits the membrane bound enzymatic activity irreversibly 27. Ca++-ATPase activity from cholesterol inserted membrane was measured as mentioned before.
Identifying the Homology of Ca++-ATPase Enzyme
The homology of protein sequence was examined using the pairwise sequence alignment tool from European Bioinformatics Institute (Clustal W and Clustal X, Version-2 ) following the method as mentioned before 28.
Statistical Analysis of the Data
Each experiment was repeated at least three times using multiple samples. Statistical analysis of the data was done by Student’s t-test between two groups and Duncan’s Multiple Range Test among various groups. Significance level was considered at p<0.05.
Results
Subcellular Membrane Fraction Demonstrated Maximum Amount of Deposition of Carbofuran and its Derivatives Upon Degradation.
The data of HPLC demonstrated that the retention time of carbofuran, 3-hydroxycarbofuran, and 3-ketocarbofyran were 3.83, 3.1, and 2.57 minutes respectively. These three consecutive peaks can be treated as unit in the detection of peaks of the unknown samples (Figure 1). The data of HPLC showed that carbofuran along with their metabolites became compartmentalized in various subcellular fractions, such as, membrane, cytosol, mitochondria, and nucleus within 24 hours of exposure in the culture media. The presence of carbofuran and its metabolites were found to be more in membrane compared to cytosol, mitochondria, and nucleus (Figure 2). Carbofuran remained almost double in membrane compared to cytosol and mitochondria (Table 1). The nature of distribution of 3-hydroxycarbofuran and 3-ketocarbofuran was also similar to carbofuran demonstrating a significant higher amount in membrane compared to mitochondria and nucleus (Table 1). 3-hydroxycarbofuran was found to be least in the nucleus among all the metabolites subsided in the cell.
Figure 1.High pressure liquid chromatography (HPLC) of standard carbofuran, 3-keto carbofuran, and 3-hydroxy carbofuran: A standard of carbofuran, its metabolites 3-keto carbofuran, and 3-hydroxy carbofuran were run in HPLC using methanol-water isocratic solvent system as described in ‘Materials and methods’. The flow rate was maintained 1ml/min. The retention time of standard carbofuran, 3-keto carbofuran, 3-hydroxy carbofuran were used for detection of the unknown samples in later experiments. Retention time and area of the graph in terms of their amount from HPLC column were shown underneath the picture as a table.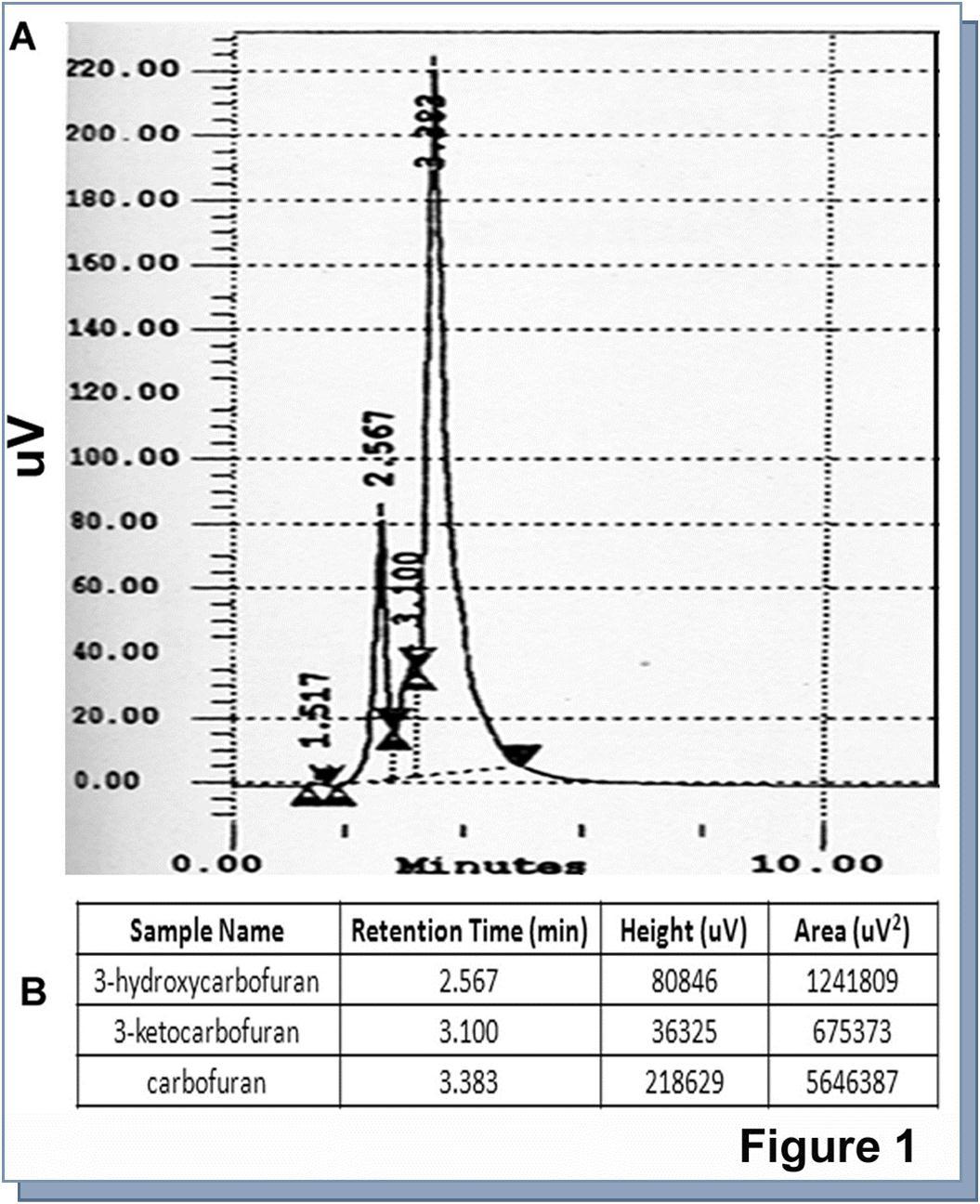
Figure 2.Quantification of carbofuran, 3-ketocarbofuran, and 3-hydroxy carbofuran from various subcellular compartments of cultured catfish hepatocytes by HPLC. Cultured hepatocytes were treated with carbofuran for 24 hrs. Nucleus, mitochondria, cytosol, and membrane were isolated from sonicated cells by differential centrifugation. CF and its metabolites were extracted from these cellular fractions as mentioned in ‘Materials and methods’. Retention time and area of the graph in terms of their amount from HPLC column were shown underneath the picture for each sample as a table. Amount of CF and its metabolites were detected from its standard curve presented in Figure 1. The combined results were presented in Table-1. A, B, C, and D represented cytosol, membrane, nucleus and mitochondria respectively.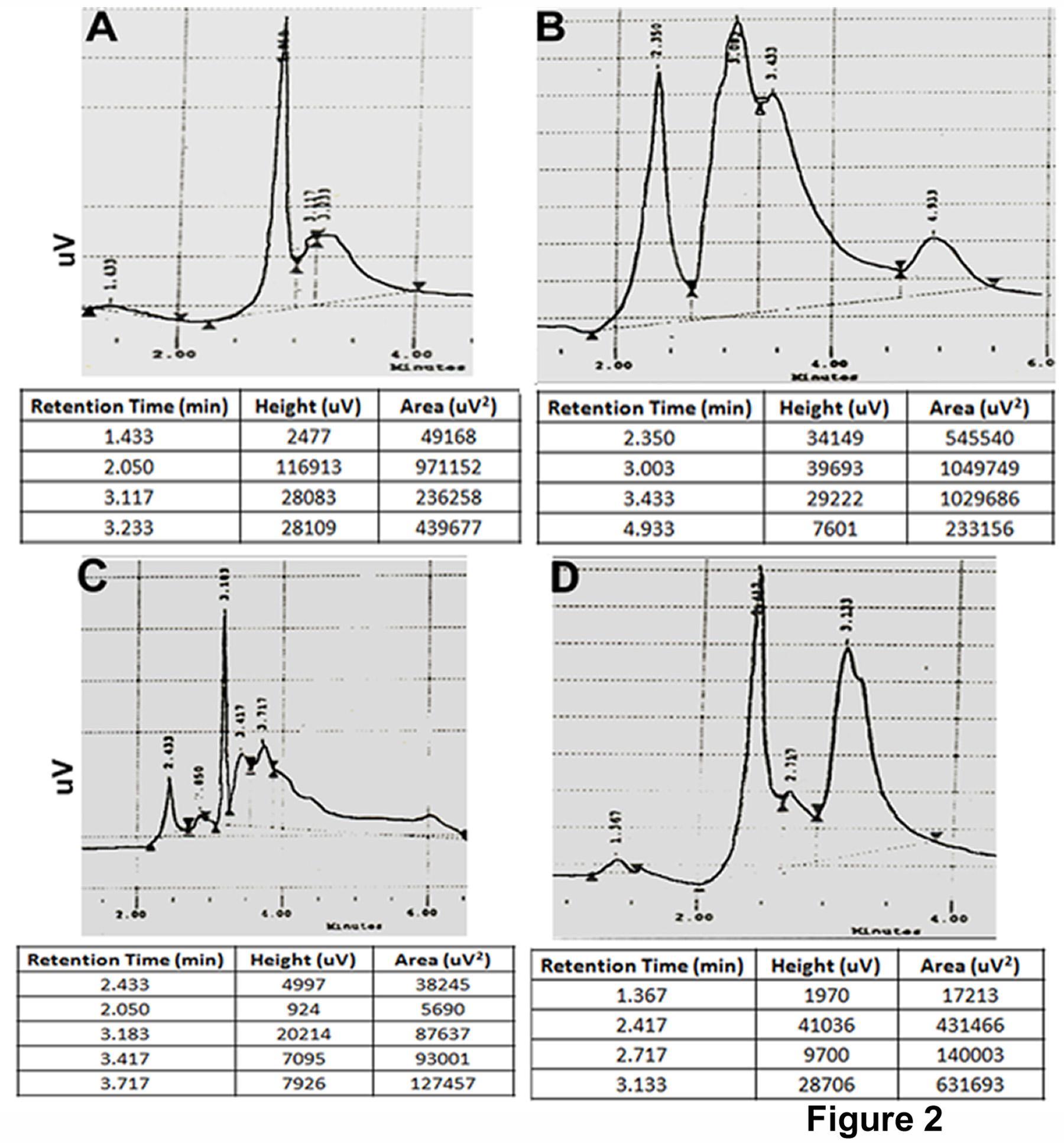
| Subcellular part | Carbofuran | 3- hydroxycarbofuran | 3- ketocarbofuran |
| Membrane | 1.57 ± 0.071b, A | 0.522 ± 0.034NS, C | 0.341 ± 0.011a |
| Cytosol | 0.851 ± 0.036NS, A | 0.485 ± 0.023b,A | 0.079 ± 0.005c |
| Mitochondria | 0.843 ± 0.024a,A | 0.215 ± 0.008a,A | 0.046 ± 0.003c |
| Nucleus | 0.123 ± 0.008A | 0.002 ± 0.0001 | 0.029 ± 0.001 |
Carbofuran Abrogated the Membrane Ca++-Atpase Activity.
Membrane Ca++-ATPase activity from carbofuran treated hepatocytes demonstrated a dose dependent activity. A gradual abrogation in Ca++-ATPase activity occurred in the membrane when exposed to higher amount of carbofuran indicating an involvement of cellular mechanism regulating this crucial enzymatic activity. The activity showed an optimum level at untreated cell followed by gradual inhibition and reduced to minimum at 10 uM of carbofuran (Figure 3). This result induced our interest to search the possible mechanism underlying the inhibition of this enzymatic activity.
Figure 3.Quantification of membrane Ca++-ATPase activity from carbofuran treated primary culture of catfish hepatocytes. Ca++-ATPase activity was measured from cultured hepatocytes according to ‘Materials and methods’. Each value represented the mean ± SE of six culture plates. a, indicated the level of significance p<0.001 compared to control. A (p<0.001), B (p<0.01) indicated the level of significance compared to particular dose with next high dose. NS, indicated the value was not significant compared to control.
Carbofuran Induced Cholesterol in Hepatocytes.
The estimation of total cholesterol with the help of thin layer chromatography (TLC) demonstrated that there was a significant increase of cholesterol in carbofuran treated hepatocytes compared to control (Figure 4A and B). Analytical estimation of different species of cholesterol, such as, total, free, and esterified reinstated the data of TLC showing all the species of cholesterol increased significantly in carbofuran treated cell compared to the corresponding control (Figure 4C).
Figure 4.Estimation of cholesterol from carbofuran treated hepatocytes by thin layer chromatography (TLC) and effect of cholesterol on membrane Ca++-ATPase. A. Cholesterol was extracted from the membrane of carbofuran treated hepatocytes. Thin layer chromatography was performed to quantify the extracted cholesterol and chromatogram was developed. B. Spots were collected from the TLC plate and amount of cholesterol was estimated. * indicated the value was significant compared to control (p<0.001). C. Total, free, and esterified cholesterol were estimated from treated hepatocytes. * indicated the value was significant compared to corresponding control (p<0.05). D. Cholesterol was inserted in the catfish hepatocytes with the help of Na-cholate followed by measurement of Ca++-ATPase activity. * indicated the value was significant compared to control (p<0.05).** indicated the value was significant compared to control (p<0.01). NS, indicated the value was not significant.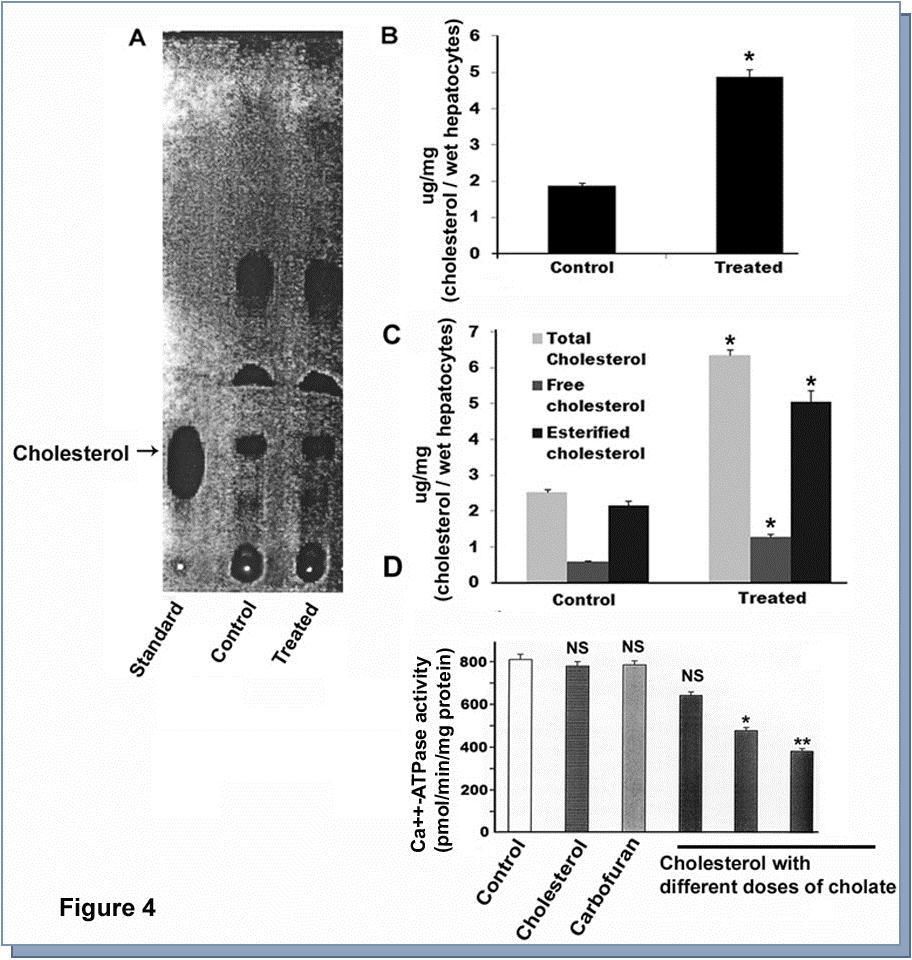
Carbofuran Abrogated the Membrane Ca++-Atpase Activity Through Activation of Cholesterol.
Findings of previous workers along with our current data indicated that cholesterol might be involved in modulation of membrane Ca++-ATPase activity. In order to examine that, we biochemically inserted cholesterol into the cell membrane followed by assessment of Ca++-ATPase activity. The only carbofuran or cholate treated cholesterol did not show any alteration of Ca++-ATPase activity; however, there was significant abrogation in Ca++-ATPase activity in cholesterol inserted membrane (Figure 4D). Out of three cholesterol inserted membrane preparation, 4.3 mM failed to show any significant inhibition but higher dose with 8.6 and 13 mM exhibited a significant attenuation compared to untreated membrane preparation (Figure 4D). Using pairwise sequence alignment tool developed by European Bioinformatics Institute (Clustal W and Clustal X, Version 2), we examined the homology of Ca++-ATPase between Heteropneustesfossilis and Homo sapiens. The data demonstrated that there was a homology of 69.7% between these two species (Figure 5).
Figure 5.Determination of homology of Ca++-ATPase enzyme between Heteropneustes fossilis and Homo sapiens. With the help of pairwise sequence alignment tool from European Bioinformatics Institute, ‘Clustal W and Clustal X (Version2)’ the homology of Ca++-ATPase enzyme was examined. It appeared that there was 69.7% identity of Ca++-ATPase enzyme between these two species.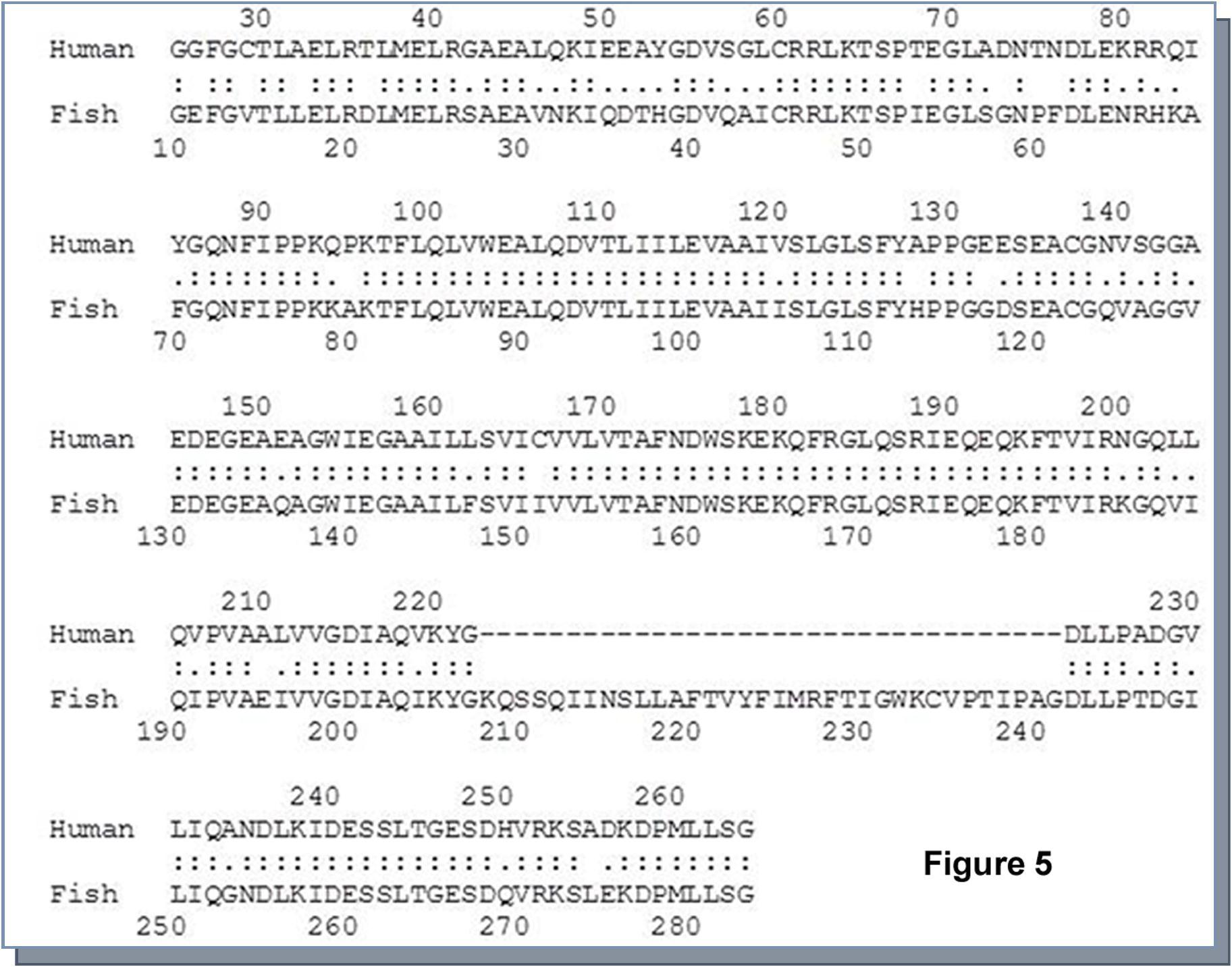
Discussion
Absorption is the fundamental criteria related to toxicokinetics of any toxic substances. It is the process of transfer of the chemical in our body from the site of administration. Lipid soluble chemicals can readily dissolve in the membrane and therefore diffuse across the cell membrane. In contrast ionized compounds do not readily surpass the lipid-lipid bilayer of the membrane 29, 30. Carbofuran being available from the agricultural field through water interacts with the cell surface of the living body. The solubility of carbofuran in water is 0.07% 1. This level can cause sufficient toxicity to the aquatic organism particularly to the primary producer 31. Irrespective of the solubility water acts as vehicle for transporting carbofuran from agricultural field to aquatic ecosystem.
The HPLC data indicated that maximum amount of CF or its metabolites accumulated in the hepatocytes membrane rather than cytosol, mitochondria, and nucleus. The amount of carbofuran that goes to the cells from culture medium was 3.5% in respect to total amount administered. Out of that, about 46% of that carbofuran accumulated in the membrane and rest of the amount spreads over to other parts of the cell. This could be due to relative lipophilicity of the pesticide. The lipophilic property of CF prevents its quick distribution in all cellular compartments. The hydroxy and keto products of carbofuran are more hydrophilic, and therefore surpass the membrane rapidly and distribute all over the tissue. The metabolites of CF are equally toxic as reported previously 1. The 3-hydroxycarbofuran stays longer in environment with the extended half life with severe anti-cholinestarase activity. It gets trapped into enterohepatic cycle even when the parent compound exists in the body. Irrespective of the disputed toxicity of parent compound or its metabolites, our investigation are extremely pointed towards the toxic effect of carbofuran in subcellular level along with the role of cytochrome P450 in detoxification 11, 13.
The deposition of carbofuran in the membrane lipid bilayer caused the elevation of membrane cholesterol level, which was assured by decrease in the Ca++-ATPase activity. CF-induced elevation of cholesterol in the liver of fish was reported by many workers 20, 32. The fish treated with carbamate pesticide showed the higher lipase activity, which might result the mobilization of fatty acids and glucerol from tissue fat depot. Cholesterol is the necessary compounds for the synthesis of many other compounds. Gupta et al. and other workers reported that intra-peritoneal administration of carbofuran resulted an increase in the total lipid, cholesterol, triglycerides, and phospholipids, including its fractions (lecithin, lysolecithin, phosphatidylethanolamine, andlysophosphatidylethanolamine) in liver, kidney, serum with corresponding decrease in lipase activity in liver 1, 33, 34, 35. It can therefore be assumed that increased amount of total and free cholesterol in liver of the experimental catfish upon CF treatment as obtained in our study might be linked to accumulation of lipids due to decreased lipase activity rather than new synthesis.
Based upon the spatial organization membrane Ca++-ATPase is topologically inserted in the lipid-lipid bilayer containing substantial proportion in the lipid 36. The structure of the membrane is designed in such a way that it can exclude the direct interaction of cholesterol with protein. This exclusion is caused by the single shell of phospholipid bilayer that binds relatively tightly to the protein, which is termed annulus 37. Biochemical and spin level evidence proposed a structural model in which about 30 phospholipid molecules interact directly with the hydrophobic surface of the Ca++-ATPase 38. In general condition, cholesterol does not significantly affect the interaction of phospholipid with Ca++-ATPase, but in presence of higher concentration of cholesterol, it interacts with the phospholipid annulus and replaces the phospholipid from the annulus resulting a sharp inhibition of activity 39. Cholesterol may be inhibitory for many enzymatic activities because it does not meet the requirement of protein for maintenance of the specific structure in the annulus 40. On the other hand the presence of very rigid structure of the cholesterol with the hydrophobic surface of the protein would also responsible for inhibition of the Ca++-ATPase activity 39. The inactivated Ca++-ATPase can be reactivated by insertion of dioelyl lecithin 41. In our experiment Ca++-ATPase activity decreased in the membrane of cultured hepatocytes in parallel with CF treatment, although the decrease was not sharply corelated with the dose of CF. The inhibition was not due to accumulation of CF at 0 h in the cultured hepatocytes or by Na-cholate. The enzymatic activity showed a proportional inhibition with the amount of inserted cholesterol, where as no inhibition was found in case of only CF or cholate.
During course of evolution the protein sequence of Ca++-ATPase stays almost conserved; however, it was diversified in different organs of human body as isoforms 42. The 70% homology in the protein sequence between fish and human indicated that the derogatory effect of carbofuran, which was shown in our current investigation, could be extended in human liver as carbofuran was reported to be ingested through foods and drinks in our day to day life. However, this interpolation of the Ca++-ATPase data was drawn based on global protein sequences of this enzyme of fish and human; it would have been strengthened if we could perform the comparative homology of either specific cell type versus cell type, or organ versus organ between these two organisms. This is one of the limitation of our study.
Conclusions
Our data showed that carbofuran treatment increased total, free, and esterified cholesterol, which in turn attenuated Ca++-ATPase activity in the liver. Enhancement of cholesterol by pesticide exposure is not only limited to the ability of carbofuran rather it is almost a global phenomenon, because apart from carbamate pesticide, organochlorine and organophosphate were also linked to similar activation of different cholesterol species in the liver 43, 44. Therefore, this study sheds light on the underlying biochemical mechanism of dysregulation of Ca++-ATPase in our body via involvement of agricultural pesticides, and unravels an intricate relation of how ecology is involved in human health at proteomics and genomics level.
Acknowledgements
We acknowledge Department of Science and Technology (DST) of India for funding this work.
References
- 2.Pohanka M. (2011) Cholinesterases, a target of pharmacology and toxicology. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 155, 219-229.
- 3.Chowdhury M A, Banik S, Uddin B, Moniruzzaman M, Karim N. (2012) Organophosphorus and carbamate pesticide residues detected in water samples collected from paddy and vegetable fields of the Savar and Dhamrai Upazilas in Bangladesh. , Int J Environ Res Public Health 9, 3318-3329.
- 4.Soler C, Hamilton B, Furey A, James K J, Manes J. (2007) Liquid chromatography quadrupole time-of-flight mass spectrometry analysis of carbosulfan, carbofuran, 3-hydroxycarbofuran, and other metabolites in food. , Anal Chem 79, 1492-1501.
- 5.Dulaurent S, Gaulier J M, Zouaoui K, Moesch C, Francois B. (2011) Surprising hair analysis results following acute carbofuran intoxication. Forensic Sci Int. 212, 10-14.
- 6.Goad R T, Goad J T, Atieh B H, Gupta R C. (2004) Carbofuran-induced endocrine disruption in adult male rats. , Toxicol Mech Methods 14, 233-239.
- 7.Gallegos-Avila G, Ancer-Rodriguez J, Niderhauser-Garcia A, Ortega-Martinez M, Jaramillo-Rangel G. (2010) Multinucleation of spermatozoa and spermatids in infertile men chronically exposed to carbofuran. Reprod Toxicol. 29, 458-460.
- 8.Klys M, Kosun J, Pach J, Kamenczak A. (1989) Carbofuran poisoning of pregnant woman and fetus per ingestion. , J Forensic Sci 34, 1413-1416.
- 9.Hernandez-Moreno D, Perez-Lopez M, Soler F, Gravato C, Guilhermino L. (2011) Effects of carbofuran on the sea bass (Dicentrarchus labrax L.): study of biomarkers and behaviour alterations. , Ecotoxicol Environ Saf 74, 1905-1912.
- 10.Hill E F, Camardese M B. (1984) Toxicity of anticholinesterase insecticides to birds: technical grade versus granular formulations. , Ecotoxicol Environ Saf 8, 551-563.
- 11.Ghosh M C, Ghosh R, Ray A K. (2000) Induction of CYP1A by carbofuran in primary culture of fish hepatocytes. , J Biochem Mol Toxicol 14, 204-209.
- 12.Ghosh M C, Ray A K.Regulation of cytochrome P4501A by protein kinase C: the role of heat shock protein70. , J Cell Commun Signal
- 13.Ghosh M C, Ray A K. (2013) Membrane phospholipid augments cytochrome P4501a enzymatic activity by modulating structural conformation during detoxification of xenobiotics. , PLoS One 8, 57919.
- 14.Salama A K. (1998) Metabolism of carbofuran by Aspergillus niger and Fusarium graminearum. , J Environ Sci Health B 33, 253-266.
- 15.Damiani E, Spamer C, Heilmann C, Salvatori S, Margreth A. (1988) Endoplasmic reticulum of rat liver contains two proteins closely related to skeletal sarcoplasmic reticulum Ca-ATPase and calsequestrin. , J Biol Chem 263, 340-343.
- 16.Paszty K, Antalffy G, Hegedus L, Padanyi R, Penheiter A R. (2007) Cleavage of the plasma membrane Ca+ATPase during apoptosis. , Ann N Y Acad Sci 1099, 440-450.
- 17.R Souza da Silva, Cognato Gde P, Vuaden F C, Rezende M F, Thiesen F V. (2003) Different sensitivity of Ca(2+)-ATPase and cholinesterase to pure and commercial pesticides in nervous ganglia of Phyllocaulis soleiformis (Mollusca). , Comp Biochem Physiol C Toxicol Pharmacol 135, 215-220.
- 18.Ueda T, Hirai K, Ogawa K. (1985) Effects of paraquat on the mitochondrial structure and Ca-ATPase activity in rat hepatocytes. , J Electron Microsc (Tokyo) 34, 85-91.
- 19.Petushkova E B, Semina T K. (1976) [Influence of dinitrophenol, octanol and toluene upon pH-dependence of ca-ATPase activity of heavy meromyosin]. , Biokhimiia 41, 2062-2067.
- 20.Chatterjee S, Ghosh R. (1995) Toxicity of carbofuran technical 75DB to the fertilization of eggs of catfish, Heteropneustes fossilis (Bloch). , Bull Environ Contam Toxicol 55, 111-115.
- 21.Chatterjee S, Kumar Dasmahapatra A, Ghosh R. (2001) Disruption of pituitary-ovarian axis by carbofuran in catfish. , Heteropneustes fossilis (Bloch). Comp Biochem Physiol C Toxicol Pharmacol 129, 265-273.
- 22.Bligh E G, Dyer W J. (1959) A rapid method of total lipid extraction and purification. , Can J Biochem Physiol 37, 911-917.
- 23.Courchaine A J, Miller W H, Stein D B. (1959) Rapid semi-micro procedure for estimating free and total cholesterol. , Clin Chem 5, 609-614.
- 24.Zlatkis A, Zak B, Boyle A J. (1953) A new method for the direct determination of serum cholesterol. , J Lab Clin Med 41, 486-492.
- 25.Michaelis E K, Michaelis M L, Chang H H, Kitos T E. (1983) High affinity Ca2+-stimulated Mg2+-dependent ATPase in rat brain synaptosomes, synaptic membranes, and microsomes. , J Biol Chem 258, 6101-6108.
- 26.Nishie I, Anzai K, Yamamoto T, Kirino Y. (1990) Measurement of steady-state Ca2+ pump current caused by purified Ca2(+)-ATPase of sarcoplasmic reticulum incorporated into a planar bilayer lipid membrane. , J Biol Chem 265, 2488-2491.
- 27.Warren G B, Houslay M D, Metcalfe J C, Birdsall N J. (1975) Cholesterol is excluded from the phospholipid annulus surrounding an active calcium transport protein. , Nature 255, 684-687.
- 28.Larkin M A, Blackshields G, Brown N P, Chenna R, McGettigan P A. (2007) Clustal W and Clustal X version 2.0. , Bioinformatics 23, 2947-2948.
- 29.Roberts A, Renwick A G. (1989) The pharmacokinetics and tissue concentrations of cyclohexylamine in rats and mice. , Toxicol Appl Pharmacol 98, 230-242.
- 30.Roberts A, Renwick A G, Ford G, Creasy D M, Gaunt I. (1989) The metabolism and testicular toxicity of cyclohexylamine in rats and mice during chronic dietary administration. , Toxicol Appl Pharmacol 98, 216-229.
- 31.Jaffery F N, Misra V, Viswanathan P N. (1992) Rational model for comparing vulnerability to environmental health risks at different locations. , Qual Assur 1, 181-191.
- 32.Ram R N, Singh S K. (1988) Carbofuran-induced histopathological and biochemical changes in liver of the teleost fish, Channa punctatus (Bloch). Ecotoxicol Environ Saf. 16, 194-201.
- 33.Gupta M, Mukherjee S, Gupta S D, Dolui A K, Dey S N. (1986) Changes of lipid spectrum in different tissues of Furadan-treated mice. , Toxicology 38, 69-79.
- 34.Kamboj A, Kiran R, Sandhir R. (2006) N-acetylcysteine ameliorates carbofuran-induced alterations in lipid composition and activity of membrane bound enzymes. , Mol Cell Biochem 286, 107-114.
- 35.Rai D K, Rai P K, Gupta A, Watal G, Sharma B. (2009) Cartap and carbofuran induced alterations in serum lipid profile of Wistar rats. , Indian J Clin Biochem 24, 198-201.
- 36.Cornea R L, Thomas D D. (1994) Effects of membrane thickness on the molecular dynamics and enzymatic activity of reconstituted Ca-ATPase. , Biochemistry 33, 2912-2920.
- 37.Mascioni A, Karim C, Barany G, Thomas D D, Veglia G. (2002) Structure and orientation of sarcolipin in lipid environments. , Biochemistry 41, 475-482.
- 38.Simmonds A C, East J M, Jones O T, Rooney E K, McWhirter J. (1982) Annular and non-annular binding sites on the. (Ca2+ + Mg2+)-ATPase. Biochim Biophys Acta 693, 398-406.
- 39.Cheng K H, Lepock J R, Hui S W, Yeagle P L. (1986) The role of cholesterol in the activity of reconstituted Ca-ATPase vesicles containing unsaturated phosphatidylethanolamine. , J Biol Chem 261, 5081-5087.
- 40.Hui S W, Sen A. (1989) Effects of lipid packing on polymorphic phase behavior and membrane properties. , Proc Natl Acad Sci U S A 86, 5825-5829.
- 41.Gonzalez J M, Jost L J, Rouse D, Suki W N. (1996) Plasma membrane and sarcoplasmic reticulum Ca-ATPase and smooth muscle. , Miner Electrolyte Metab 22, 345-348.
- 42.Pestov N B, Dmitriev R I, Kostina M B, Korneenko T V, Shakhparonov M I. (2012) Structural evolution and tissue-specific expression of tetrapod-specific second isoform of secretory pathway Ca2+-ATPase. Biochem Biophys Res Commun. 417, 1298-1303.
