Abstract
The apical meristem of the growing point of plants contains proplastids, precursors of chloroplasts. The main attention of investigators was paid to the transformation of proplastids into chloroplasts. The formation of proplastids of the apical meristem of wheat seedlings was investigated in the present work and described for the first time in the scientific literature. Ultrastructural images of apical meristem areas showed that the formation of the proplastide body includes several stages: localization of plastid DNA in the cytoplasmic matrix in close contact with cytoplasmic ribosomes, the formation of membrane vesicles containing plastid DNA, the step-by-step filling of these vesicles with dense contents, and the formation of mature proplastids.
Author Contributions
Academic Editor: Gopal Pandi, School of Biotechnology Madurai Kamaraj University Madurai
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2018 Galina A. Semenova
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
The apical meristem of the growing point of plants contains proplastids, precursors of chloroplasts. Proplastids are colorless spherical organelles 1–2 μm in diameter surrounded by a membrane. These organelles are composed of a dense matrix, ribosomes, and plastid DNA. They have no inner membranes, i.e. thylakoids. The studies on the ultrastructure of the apical meristem of a plant shoot are few. In the review of Pyke 1 on the biogenesis of plastids, only a few papers are devoted to proplastids, and even these studies have been performed mainly on the apical meristem of roots.
The results of studies on the apical meristem and leaf primordia of a variety of plants have shown that proplastids seen on micrographs already contain thylakoids, although they are few in number 2, 3, 4, 5. The main concern of investigators was with the transformation of proplastids into chloroplasts, and the object of investigations was often young leaves of three- to seven-day-old cereal shoots at the base of which the intercalary meristem is located. In this meristem, proplastids already have a flattened form, containing single thylakoids and prolamellar bodies, and are actually young chloroplasts 6, 7, 8.
The apical meristem of the growing point of wheat seedlings is hidden at the base of a plant, is buried into soil, and is tightly covered with the bases of two to three leaves. Therefore, this meristem can contain proplastids that still have no internal membranes.
The ultrastructure of the apical meristem of wheat seedlings has not been studied earlier. In this work, the images of the formation of proplastids of the apical meristem of wheat seedlings were obtained and described for the first time in the scientific literature.
Materials and Methods
The object of the investigation was the 12- to 14-day-old seedlings of the wheat Triticumaestivum L. Seedlings were grown in vials with soil under natural illumination at room temperature (20–23ºС) to an age of 10–12 days. Samples from five independent experiments were examined. All samples showed a similar ultrastructural organization.
The materials were fixed and prepared for examination by standard methods. Pieces of shoots adjacent to the caryopsis 2 to 3 mm in height were fixed in a 3% glutaraldehyde solution on phosphate buffer (pH 7.4) for 2 h followed by the postfixation in a 1% OsO4 (Reakhim, Russia) solution. After dehydration in alcohols of increasing concentrations 50-100% and 100% acetone, samples were embedded in Epon-812 (Fluka, Germany).
Ultrathin sections were contrasted with a saturated aqueous uranyl acetate (Sewa, Czech Republic) solution and 0.25% lead citrate (British Drug Houses, England) by the conventional methods and examined in a JEM-100B electron microscope (Jeol, Japan) under an accelerating voltage of 80 kV.
Results
Figure 1 and Figure 2 show the sections of cells of the apical meristem of the growing point at different magnifications. In the cytoplasm of meristematic cells, along with typical proplastids, numerous single-membrane vesicles containing fibrils of plastid DNA (arrows 1 in the photomicrograph) are seen. At some places, thin single fibrils form closely contacting conglomerates. In the center of the conglomerate, two ring-shaped structures (arrows 2 in the photomicrograph) are seen (Figure 2a). The vesicles are 1–2 μm in diameter and are filled to a different degree with a dense content (Figure 1, Figure 2, Figure 3). These images can be considered to reflect the process of the step-by-step formation of proplastids from membrane vesicles.
Figure 1.Cells of the apical meristem of the growing point. A general view (а) and a region of the cell at a large magnification (b). Membrane vesicles contain plastid DNA filaments (arrows 1). The developed proplastid is shown by an asterisk; cw, cell wall. Scale bar = 1 μm.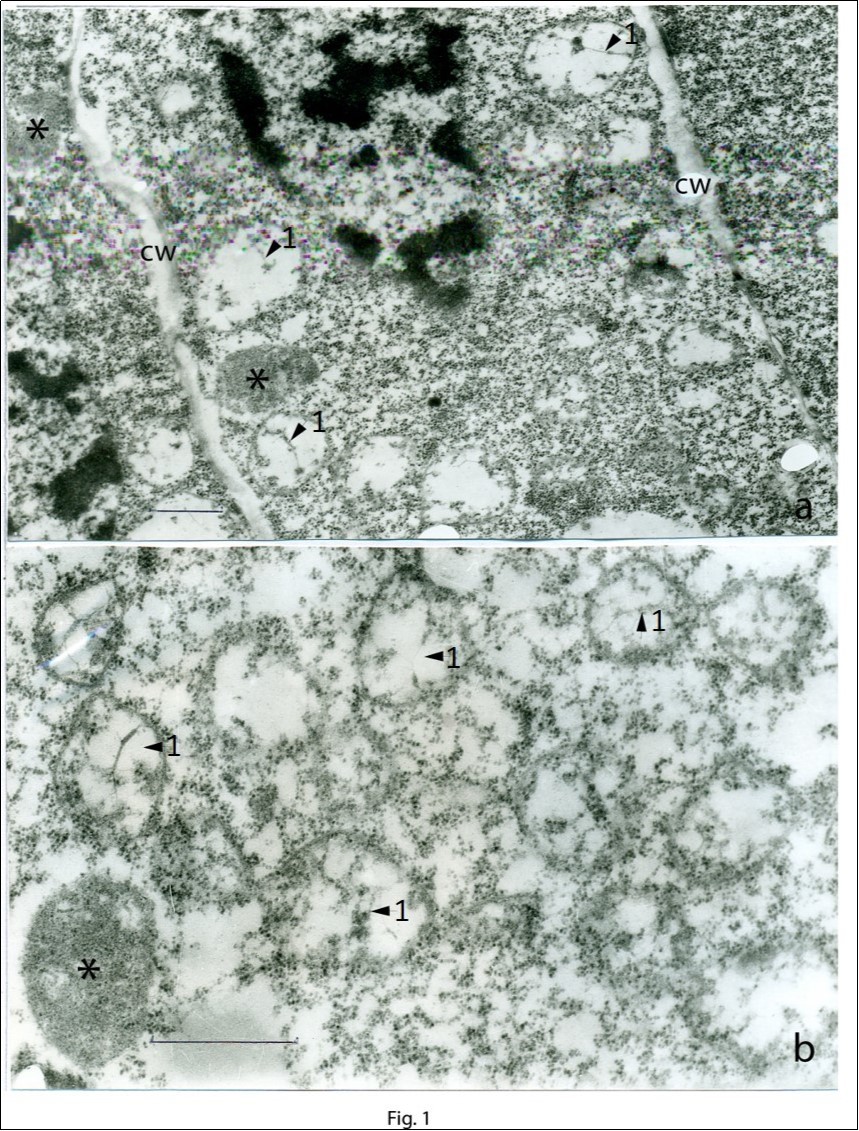
Figure 2.Regions of the cells of the apical meristem at a large magnification.Proplastid contained plastid DNA (a) and proplastids at different degree of maturation (b).The developed proplastids are shown by asterisks; plastid DNA is shown by arrows 2. Scale bar = 0.2 μm.
Figure 3.Stages of formation of proplastids in the apical meristem (a–f): a, b – the onset of the formation of the proplastid body. Membrane vesicles contained plastid DNA filaments are shown by arrows 3. c–e – developed proplastid bodies. Fragments of plastid DNA (arrows 3) and cavities in the matrix of proplastids are seen. f – a mature, completely developed proplastid surrounded by a dense membrane and containing a dense matrix, plastid ribosomes, and plastoglobuli. Scale bar = 0.2 μm.
During the formation of a proplastid body, the single-membrane envelope remains perforated until the complete maturation of the proplastid (Figure 3 a-e). At the first stage of the formation of a proplastid, the membrane of the vesicle, with plastid DNA inside it (arrows 3 in the photomicrograph), is almost closed (Figure 3a and Figure 3b). In the early stages internal cavity of the vesicle and the cytoplasm are in direct contact in a small region where the membrane is disrupted (Figure 3a). At later stages, the area of contact between the inner cavity of the proplastid body and the cytoplasm increases (Figure 3c, Figure 3d, Figure 3e). When the proplastid body is almost completely developed, the membrane of its envelope is perforated to a greater degree, and a direct contact between the proplastid body and the ribosomes of cytoplasm is seen (Figure 3). The density of ribosomes in the cytoplasm of meristematic cells is very high, up to about 3000/μm2. A completely developed proplasid surrounded by a dense closed membrane contains a dense matrix, plastid ribosomes, and plastoglobuli (Figure 3f).
The plastid DNA at a large magnification (arrows 4 in the photomicrograph) is shown in Figure 4. The proplastid, with plastid DNA inside, is surrounded by a perforated membrane. The matrix of the proplastid is reduced, and cytoplasmic ribosomes go into the proplastid body (Figure 4a). The plastid DNA seen among cytoplasmic ribosomes (Figure 4b). The contours of the proplastid itself are poorly distinguished, and the reduced membrane of the proplastid envelope is retained only partially (Figure 4b). Figure 4c shows plastid DNA filaments localized in the matrix of the cytoplasm; to the right, a fragment of the membrane nucleus (n) is seen; the nucleus envelope cannot be distinguished.
The micrographs can also be considered as reflecting the process of proplastid degradation as a result of which plastid DNA becomes submerged immediately into the cytoplasm of the meristematic cell.
Figure 4.A region of the cytoplasm of apical meristematic cells (a–c). а – plastid DNA in an almost completely closed proplastid membrane. The matrix of the proplastid is reduced, and ribosomes of the cytoplasm are seen inside it. b – plastid DNA localized among the cytoplasmic ribosomes; a part of the membrane of the proplastid is seen (arrows 4). c – plastid DNA localized outside the proplastid, among cytoplasmic ribosomes; to the right is seen chromatin of the nucleus (n). Plastid DNA is indicated by arrows 4. Scale bar = 0.2 μm.
Discussion
Plastid DNA visible at the ultrastructural level has a clearly defined morphology. It is represented by a complex of fibrils 2.5–3.0 nm in diameter, which in certain places fuse into dense conglomerates 9. The region occupied by plastid DNA is a nucleoid, which is identified by the level of fluorescence 10
Membrane vesicles similar to those obtained in our study can be seen in micrographs shown in other works. These type of vesicles are found on the sections of ovicells or meristems of various plants; however, either their status is not discussed 5, 11, 12 or they are considered to be vacuoles or degraded proplastids 13.
Japanese researchers suggested to divide proplastids into two groups: plastid initials and mature plastids 14, 15. However, micrographs presented in their papers show that “proplastid initials” containing plastid DNA and a dense matrix in meristematic cells of plant buds, potato stolons, and wheat seedlings are bodies surrounded by two membranes. These bodies can be called true developed proplastids rather than initials. “Mature” proplastids presented in these works contain internal membranes and are actually young chloroplasts. The differences in the enzymatic activity between “initial” and “mature” proplastids revealed by Akita and Sagisaka 14 are in fact the differences between true proplastids and young chloroplasts.
The transition from a proplastid to a young chloroplast is very quick, and this process in meristematic cells is triggered even by weak illumination. Meristematic cells in shoots of various plants contain as a rule plastids with thylakoids 2, 15, 16; in this case, the degree of the development of thylakoids depends on the localization of cells in the apical meristem 17.
Electron transparent single-membrane vesicles containing plastid DNA, observed in the present work, can be called plastid initials. They are more similar to “particle initials” in the filicine egg cells, which were given this name by Bell et al. 18, than to those described by Sagisaka and Kuroda 15.
Bell et al. 18 presented micrographs from which it can be assumed that plastid initials are budded off immediately from the nucleus envelope. Based on the images presented by Sagisaka and Kuroda 15, it can be proposed that plastid initials are formed by protrusion and separation from mature proplastids.
In the present study, the division of neither developed nor developing proplastids was observed in the cells of the apical meristem. The localization of plastid DNA immediately in the matrix of the cytoplasm in close contact with cytoplasmic ribosomes shown in micrographs suggests that in this state the replication of plastid DNA in the cytoplasmic matrix is possible. A great number of plastid DNA copies are covered by a membrane and generate those plastid initials that subsequently develop into mature proplastids.
Boffey and Leech 19 showed that the number of plastid DNA copies at the early stages of the development of wheat leaves is 800–1000 per plastid, and at later stages this number decreases to 300. This observation can be explained assuming that, at early stages of plant development, plastid DNA can exist outside plastids.
Author Contribution Statement
GS designed and performed the experiment, analyzed the data and wrote the manuscript.
References
- 1.K A Pyke. (2007) Plastid biogenesis and differentiation. In R. Bock (Ed.),Cell and molecular biology of plastid(1–28),Topics current genetics, Vol. 19.Berlin Heidelberg:Springer-Verlag .
- 2.Chaly N, J V Possingham. (1981) Structure of constricted proplastids in meristematic plant tissues.Biol. , Cell 41, 203-210.
- 4.Gifford EM Jr, K D Stewart. (1967) Ultrastructure of the shoot apex ofChenopodium albumand certain other seed plants.J. , Cell Biol 33, 131-142.
- 5.R F Lyndon, E S Robertson. (1976) The quantitative ultrastructure of the pea shoot apex in relation to leaf initiation.Protoplasma. 87, 387-402.
- 6.Robertson D, W M Laetsch. (1974) Structure and function of developing barley plastids.PlantPhysiol. 54, 148-159.
- 7.A R Wellburn, D C Robinson, F A Wellburn. (1982) . Chloroplast development in low light-grown barley seedlings.Planta,154: 259-265.
- 8.H K Lichtenthaler. (2013) Plastoglobuli, thylakoids, chloroplast structure and development of plastids. In Biswal B, Krupinska K, & Biswal UC (Eds.),Plastid development in leaves during growth and senescence(337–361), Advances in photosynthesis and respiration,Vol. 36 , Dordrecht, The Netherlands:SpringerScience+Business Media .
- 9.Kowallik K V, R G Herrmann. (1972) . , Electron-microscopic investigation ofNarcissuschromoplasts.Protoplasma 74, 1-6.
- 10.Sakamoto W, Uno Y, Zhang Q, Miura E, Kato Y et al. (2009) Arrested differentiation of proplastids into chloroplasts in variegated leaves characterized by plastid ultrastructure and nucleoid morphology.Plant. , CellPhysiol 50, 2069-2083.
- 11.W A Jensen. (1965) The ultrastructure and composition of the egg and central cell of cotton.Am. , J Bot 52, 781-797.
- 12.E J Robertson, K A Pyke, R M Leech. (1995) arc6, an extreme chloroplast division mutant ofArabidopsisalso alters proplastid proliferation and morphology in shoot and root apices.J. , Cell Sci 108, 2937-2944.
- 13.P R Bell, Mühlethaler K. (1962) The fine structure of the cells taking part in oogenesis inPteridium aquilinum(L.). , Kuhn.JUltrastructRes 7, 452-466.
- 14.Akita T, Sagisaka S. (1995) Functional and structural differences among proplastids as seen by immunogold staining and electron microscopy.Biosci. , Biotech Biochem 59, 1477-1484.
- 15.Sagisaka S, Kuroda H. (1991) Changes in the ultrastructure of plastids after breaking of dormancy in perennials.Agric. , Biol Chem 55, 1671-1673.
- 16.V K Sawhney, P J Rennie, T A Steeves. (1981) The ultrastructure of the central zone cells of the shoot apex ofHelianthus annuus.CanadJ. , Bot 59, 2009-2015.
- 17.Charuvi D, Kiss V, Nevo R, Shimoni E, Adam Z et al. (2012) Gain and loss of photosynthetic membranes during plastid differentiation in the shoot apex ofArabidopsis.Plant. , Cell 24, 1143-1157.
