Abstract
Melia azedarach extract were applied by feeding the adult female flies on diets mixed with the extracts at different doses. The concentrations of Melia azedarach utilized were 1.8, 2.4 and 3.6%. The gonotrophic cycles of length of 90, 753, 67.6 and 84, 72, 68 hours were obtained after feeding at age 24 hours with diet mixed with doses of 1.8, 2.4 and 3.6% fruit extract; respectively. 98 & 96 hours were the length of gonotrophic cycle in the control groups. The length of 86.7, 72.3, 57.3 and 89.3, 75, 61 hours were obtained after feeding adults at age 48 hours with diets mixed with different doses of fruit extract of the same plant 97.3 and 98.7 hours were the length of the control groups. Proportions of the egg hatching reached 69, 55.3, 49 and 72.9, 64.2, 52 in groups of eggs obtained from 24 hours adults feeding with diets mixed with doses of 1.8, 2.4 and 3.6% fruit extract; respectively. Also 68.7, 53.3,48 5 and 81 2, 70, 56.3 were the proportions of egg hatching obtained from groups at age 48 hours after feeding with diets mixed with the same doses. 85, 77.6, 62.2 and 92.6, 88.9, 84.9 were the proportions of the egg hatching obtained from groups feeding with diets mixed with doses of 1.8, 2.4 and 3.6% fruit extract of Melia azedarach; respectively. The pupae showed larval-pupal intermediates which failed to complete the pupal period and died after emerging from the third larval instar.
Author Contributions
Academic Editor: Narcisa Vrinceanu, Faculty of Engineering, "Lucian Blaga†University of Sibiu / 4 Emil Cioran Street, 550025 Sibiu, Romania.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2019 Radwan E H, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
The house fly Musca vicinais one of the common species found in human habitat in tropical and subtropical regions. It has gained importance as a serious public health hazard. Serious world problems in public health have arisen as insects can develop resistance against insecticides 1. The discovery of Melia azedarach as an insecticide of an entirely new type created quite a stir among entomologists interested in the practical uses of insect researches 2. It was found of great value to study the effect of crude extracts of fruits of Zanzalacht on Musca vicinawith the aim of investigating the effect of the Melia azedarach extract on the development capacities of the insect. The experiments stressed on the potential these plants have as effective and economic insecticides.
Jatwani and Srivastava 3; Schmutterer 4 reported that the common species is Melia azedarach L as it contains six tetera-nortriterpenoids. Chiu 5 mentioned that the evaluation of the petroleum ether extracts of the seed kernels of Melia azedarach in the laboratory showed their potential as antifeedants for the control of the nymphs of Nilaparvatalegens. The ethanol extract of the seed kernels of Melia azedarach inhibited feeding by 99.8%. The effect of azadirachtin and Azadirachtaindica were similar to those of insect growth regulators against the immature stages of the house fly, Musca domestica6.
Hashem and Youssef 7; Radwan 8 observed the developmental changes induced by methanolic extract of leaves and fruits of Melia azedarach L. on the larvae of house fly Musca domesticavicina Macq. They noticed that the pupae and the adults displayed morphological abnormalities as well as pronounced anomalies. Heshem et al.9 studied the effect of Melia azedarach extract on the larvae of Spodopteralittoralis and found that the fruit extract effectiveness depended on the age of the larvae, the concentration of the extract and the period of the treatment on the larval instars. The fruit extract-treatment of the chinaberry tree caused abnormalities in larvae and adults of the insect. Several studies dealt with the effect of azadirachtin on the mortality of different stages of insect species 10. Azadirachtin increased the duration of the immature stages, length of pupal stages 11, 12, 13.
The effects of tri-terpenoid extracted from neem seed were similar to those of insect growth regulators against the immature stages of the house fly, Musca domestica14. Garcia et al.15 claimed that the triterpenoid azadirachtin strongly interfere with the neuroendocrine control of insect hormone titers. Bidman et al.16 studied the juvenilizing effect of azadirachtin by its injection into the first half of the last larval instar of the blowfly Calliphoravicinaand found that it caused inhibited adult emergence. In adult insects, the effect of azadirachtin was a retardation of egg maturation 17. They reported that the inhibition of oogenesis by azadirachtin is discussed on the basis of its interference with the neuroendocrine control of hormone synthesis. Mehrotra and Gujar 18 reported that topical application of 10 Mg azadirachtin reduced adult fecundity in Spodopteralitura. Crude neem oil extract was evaluated for their effect on different stages of three fly species, namely, Musca domestica, Haematobiaexiguaand Chrysomyamegacephala, marked reductions in the hatchability of treated eggs of the three fly species were observed 19.
Material and Methods
Musca vicina in this study were all produced from a colony raised at the laboratory of the Department Zoology, Faculty of Science, and University of Alexandria. This colony was initiated by adult flies borrowed from the Entomology Department, Faculty of Agriculture, and University of Alexandria. The original colony of the Faculty of Agriculture was established since 1995. The colony was raised in a constant room temperature maintained at 27 ± 2°C and 70 ± 2% RH. The adult flies were kept in breeding cages which were made of wooden frames measuring 38 x 30 x 30 cm. The sides and tops of those cages were fitted with mosquito-proof wire mesh. The front side, measuring 25 cm x 25 cm. had been fitted with a cloth sleeve protected with a wooden cover hinged to the cage. A Petri dish, 9 cm in diameter and 2.5 cm in height, containing a piece of cotton wool moderately soaked in diluted milk (3 volumes of milk added to 1 volume of water) was placed inside the cage to be replaced by fresh ones every 24 hours. The Petri dish was placed in the cage to provide diet for the adult flies. The female flies usually lay their eggs on the milk pads. The pads containing the eggs were transferred to two pound jam jars containing fresh milk pads to provide food for the newly hatched larvae. The jars were tightly covered with finely perforated tin lids. The fruits and leaves were washed in running tap water and after drying them up in the air for several days they were put in an oven at 60°C to constant weight and then pulverized by means of a hummer mill. Extraction was conducted in a 250 ml Soxhlet apparatus using methanol as a solvent. The extraction period lasted a total of 20 hours over a course of four days until the chinaberry leaves and fruits became colorless. At the end of the extraction process the resulting solution was put in a porcelain dish and placed in an oven (37°C) for evaporation of the solvent from the obtained solution. After removal of the methanol from the elute it is concentrated to a volume of approximately 25 ml of dark green oil. The crushed chinaberry fruit (160 gm) finally produced 40 ml of thick brown oily extract. The thick crude extracts (from the leaves and fruit) were preserved in tightly capped dark glass, vials and stored in the freezer until used for tests.Statistical analysis Data were subjected to student's T-test and least significant difference (LSD) test 20.
Results
The feeding experiments were conducted with the aim of demonstrating the effect of Zanzalacht (Melia azedarach) extraction at different concentrations on the gonotrophic cycle of the adult female Musca vicina. Two parameters have been taken into account while studying the effect of the different concentrations of Melia azedarach extraction on the female Musca vicina. These two parameters were; the time required for the completion of the first gonotrophic cycle; the number of hatched eggs. The onset of each larval instar and the time interval between the two successive ecdysises had been taken as a third parameter to demonstrate the effect of the Melia azedarach extraction on the instars' growth.
The data obtained from the first set of experiments is represented in table 1, table 2. These experiments were conducted at a temperature of 27 ± 2°C and a relative humidity of 70% ± 2. It is seen from table 1, table 2 that the time required for completing the first gonotrophic cycle of females at age 24 hours, which were fed on Melia azedarach (fruits) extraction at the concentrations of 1.8%, 2.4% and 3.6% had decreased. Table 1 showed that the time required for completing the first gonotrophic cycle in the female groups I, II and III that fed on the previously mentioned concentrations of Melia azedarach fruit extraction was 90, 75.3 and 67.6 hours; respectively as compared with 98 hours for the control group, (Table 1). The time was 84,72 and 68 hours in group Ia, IIa and IIIa; respectively, as compared with 96 hours for the control group (Table 2). table 1, table 2, showed that the number and the percentage of the hatched eggs were decreased in treated groups I, II, III and Ia, IIa and IIIa. Such percentages were found to be 69.0%, 55.3% & 49.1 % for groups I, II and III, respectively, as compared with 98.5% for the control group (Table 1) and 72.9%, 64.5% and 52% for group Ia, IIa and IIIa; respectively, as compared with 98.5% for the control group (Table 2). It is seen form table 1, table 2 that the metamorphosis of the larvae in groups I, II, III and Ia, IIa and IIIa was retarded. It was noted that the mean duration of the first larval instar was prolonged when compared with the control group table 1, table 2. The onset of the first ecdysiast occurred after an average of 88, 97.3 and 102.6 hours for groups I, II and III; respectively as compared with 73.3 hours for the control group (Table 1) and 82.7, 94 and 98.3 hours for groups la, Ila and IIIa; respectively as compared with 73.3 hours for the control group (Table 2).
It is indicated from table 1, table 2 that the mean duration of the second larval instar in groups I, II, III and Ia, IIa and IIIa had been prolonged. The onset of the second larval ecdysis occurred after an average of 137.6, 145 & 155 hours for groups I, II & III; respectively as compared with 122.3 hours for the control group (Table 1) and 130, 136.3 and 147.7 hours for group la, IIa and IIIa; respectively as compared with 123.3 hours for the control group (Table 2). The third larval instar had shown retardation in metamorphosis in groups I, II, III and Ia, IIa and IIIa in table 1, table 2. The onset of the third larval ecdysis occurred after an average of 179.3, 190 & 210 hours for groups I, II & III; respectively as compared with 169.3 hours for the control group (Table 1) and an average of 176.7, 186.7, 205 hours for group Ia, IIa and IIIa; respectively as compared with 168 hours for the control group (Table 2).
Table 1 and Table 2) showed that the onset of the pupation occurred after an average of 110, 145 and 176.6 hours for the larval groups I, II and III, respectively as compared with 98 hours for the control group (Table 1) and 97.7, 125, 148.3 hours in groups la, IIa and IIIa; respectively as compared with 96.3 hours for the control group (Table 2). It is indicated in Table 2 , table 1 that the percentages of larvae reaching the pupal stages in groups I, II, III and la, IIa and IIIa had been decreased, such percentages were found to be 43.8%, 33.2 %, 19% for groups I, II, III; respectively as compared with 99.5% for the control group (Table 1 and a percentage of 46.7%, 38.5% and 19.3% for groups la, IIa and IIIa; respectively as compared with 99.7% for the control group (Table 2).
Table 1a. The larvae of Musca vicina, produced from adult females fed on Melia azedarach fruit extract at age 24 hours, reaching the succeeding instar at the standard time:| Records of larvae reaching the next instar at the standard time. | ||||||
| Feeding medium provided | First instar | Second instar | Third instar | |||
| After 72 hours | After 120 hours | After 168 hours | ||||
| No. | % | No. | % | No. | % | |
| Standard diet | 135 | 99.2 | 133 | 96.3 | 130 | 95.5 |
| Standard diet+1.8%Melia azedarach fruit extract. | 60 | 80.4 | 45 | 60 | 40 | 50 |
| Standard diet+2.4%Melia azedarach fruit extract. | 40 | 76.9 | 30 | 57.6 | 20 | 38.4 |
| Standard diet+3.6%Melia azedarach fruit extract. | 11 | 70.5 | 8 | 51.2 | 4 2 | 5.6 |
| Records of larvae reaching the next instar at the standard time. | ||||||
| Feeding medium provided | First instar | Second instar | Third instar | |||
| After 72 hours | After 120 hours | After 168 hours | ||||
| No. | % | No. | % | No. | % | |
| Standard diet | 135 | 98.5 | 132 | 96.3 | 132 | 96.3 |
| Standard diet+1.8 %Melia azedarach leaves extract. | 70 | 85.3 | 60 | 73.1 | 54 | 65.8 |
| Standard diet+2.4% leaves Melia azedarach extract. | 53 | 80.3 | 45 | 68.1 | 33 | 50 |
| Standard diet+3.6%Melia azedarach leaves extract. | 33 | 76.7 | 23 | 53.4 | 18 | 41.8 |
table 1, table 2demonstrated the retarding effect of the various concentrations of Melia azedarach extraction on the adults Musca vicina. The number and percentages of the larvae reaching the successive instars at the standard time had been recorded in table 1, table 2. The number of molts from the first to the second instar was determined. In the first set of experiments, the percentages of the 1st instar larvae reaching the 2nd instar larvae reaching the standard time were found to be 60%, 57.6% and 51.2 % for groups I, II, III; respectively as compared with 96.3% for the control group (table 1a) and percentages of 73.7%, 68.1% and 53.4% for groups Ia, Ila and IIIa; respectively as compared with 96.5% for the control group (Table 2a). It should be noted that the percentages of the second instar larvae reaching the third instar were 50%, 38.4% & 25.6% for groups I, II, III; respectively as compared with 95.5% for the control group (Table 1a) and a percentage of 65.8%, 50% & 41.8% for groups la, IIa and IIIa, respectively as compared with 96.3% for the control group (Table 2a). It is evident that the time required for the completion of the third larval instars had been prolonged by 11.3, 22 and 42 hours for groups I, II and III, respectively (Table 1a) and 8, 18.6 and 37 hours for groups la, IIa and IIIa; respectively (Table 2a) considering that the standard time required for the completion of the third larval instar is 168 hours (Table 1a and Table 2a).
Three groups of adult Musca vicinaat age 48 hours had been treated with three different concentrations of Melia azedarach fruit extract. The three concentrations were 1.8%, 2.4% and 3.6%. The data obtained have been represented in table 3, table 4 .It is seen from table 3, table 4that the time required for completing the first gonotrophic cycle of females at age 48 hours which have been exposed to fruits of Melia azedarach extraction had decreased. Table 3 showed that the time required for completing the first gonotrophic cycle in the female groups I, II and III that had been exposed to the previously mentioned concentrations of Melia azedarach extraction was 86.7, 72.3, and 57.3 hours; respectively, as compared with 97.3 hours for the control group (Table 3) and after 89.3, 75 and 61 hours in groups Ia, IIa and IIIa; respectively as compared with 98.7 hours for the control group (Table 4).
Table 3a. The larvae of Musca vicina, produced from adult females fed on Melia azedarach fruit extract at age 48 hours, reaching the succeeding instar at standard time.| Records of larvae reaching the next instar at the standard time. | ||||||
| Feeding medium provided | First instar | Second instar | Third instar | |||
| After 72 hours | After 120 hours | After 168 hours | ||||
| No. | % | No. | % | No. | % | |
| Standard diet | 138 | 99 | 136 | 97.6 | 135 | 96.9 |
| Standard diet+1.8%Melia azedarach fruit extract. | 56 | 77.7 | 40 | 55.5 | 36 | 50 |
| Standard diet+2.4%Melia azedarach fruit extract. | 33 | 72.8 | 23 | 50.7 | 18 | 39.7 |
| Standard diet+3.6%Melia azedarach fruit extract. | 10 | 66.6 | 6 | 40 | 4 | 26.6 |
| Records of larvae reaching the next instar at the standard time. | ||||||
| Feeding medium provided | First instar | Second instar | Third instar | |||
| After 72 hours | After 120 hours | After 168 hours | ||||
| No. | % | No. | % | No. | % | |
| Standard diet | 136 | 98.1 | 134 | 96.6 | 134 | 96.6 |
| Standard diet+1.8%Melia azedarach leaves extract. | 78 | 79.9 | 56 | 57.3 | 51 | 52.2 |
| Standard diet+2.4%Melia azedarach leaves extract. | 57 | 74 | 42 | 54.5 | 33 | 42.8 |
| Standard diet+3.6%Melia azedarach leaves extract. | 37 | 68.1 | 23 | 42.3 | 16 | 29.4 |
Table 3 and Table 4 showed that the number and the percentages of the hatched eggs were decreased in treated groups I, II, III and Ia, IIa and IIIa. Such percentages were found to be 68.7%, 53.3 % and 48.5% for groups I, II, III; respectively as compared with 99.3 % for the control group (Table 3) and 81.2%, 70% and 56.3% for groups Ia, IIa and IIIa; respectively as compared with 99.1% for the control group (Table 4). It was seen from table 3 and Table 4 that the metamorphosis of the larvae in groups I, II, III and Ia, IIa and IIIa was retarded. It was noted that the mean duration of the first larval instar is slightly prolonged when compared with the control groups (Table 3 and table 4). The onset of the first ecdysis occurred after an average of 96.7, 120 and 141.6 hours for the groups I, II and III; respectively as compared with 73.3 hours for the control group (Table 3) and after 94, 106.7 and 135 hours for the groups Ia, IIa and IIIa; respectively as compared with 78 hours for the control group (Table 4).
It is indicated from table 3, table 4that, the mean duration of the second larval instar in groups I, II, III and Ia, IIa, IIIa had been prolonged. The onset of the second larval ecdysiast occurred after an average of 191, 200 & 225 hours for groups I, II and III; respectively as compared with 126 hours for the control group (Table 3) and an average of 189.3, 191.7 and 208.3 hours for group Ia, IIa and IIIa; respectively, as compared with 130 hours for the control group (Table 4). The third larval instar had shown retardation in metamorphosis in groups I, II, III and Ia, IIa and IIIa, in table 3, Table 4 The onset of the third larval ecdysiast occurred after an average of 186.7, 197.3 and 218.3 hours for groups I, II &III; respectively, as compared with 172 hours for the control group (Table 3) and an average of 183.3, 188 and 205 hours for groups Ia, IIa and IIIa; respectively as compared with 171.3 hours for the control group (Table 4). Table 3 and table 4) showed that the onset of the pupation occurred after an average of 123.3, 156 & 198.3 hours for the larval groups I, II and III; respectively as compared with 95.3 hours for the control group (Table 3) and an average of 108.3, 146.7 and 195 hours for groups Ia, IIa and IIIa; respectively as compared with 98 hours for the control groups (Table 4).
It is indicated in table 3Table 4that the percentages of larvae reaching the pupal stages in group I, II, III and Ia, IIa and IIIa, had been decreased. Such percentages were found to be 35.2%, 29.2% and 13.1% for groups I, II, III; respectively as compared with 99.3 % for the control group (Table 3) and an average of 49.5% 38.1% and 29.9% for groups Ia, IIa and IIIa; respectively as compared with 99.3% for the control group (Table 4). The data obtained from the third set of experiments have been represented in Table 5 and Table 6. It is seen from Table 5 and Table 6 that the time required for completing the first gonotrophic cycle of females at age 72 hours, which had been exposed to Melia azedarach fruits extraction at the concentrations of 1.8%, 2.4% and 3.6% had decreased. Table 5 showed that the time required for completing the first gonotrophic cycle in groups I, II and III that had fed the previously mentioned concentrations of Melia azedarach extractions was 85.3, 80.7 and 75.7 hours; respectively, as compared with 97.7 hours for the control group (Table 5). The time required for completing gonotrophic cyele was 86.7, 83.3 and 78 hours in groups Ia, IIa and IIIa; respectively as compared with 96.7 hours for the control group (Table 6). Table 5 and Table 6 showed that the number and the percentage of the hatched eggs were decreased in the treated groups namely I, II, III and Ia, IIa and IIIa. Such percentages were found to be 85%, 77.6% and 62.2% for groups I, II & III; respectively as compared with 98.8% for the control group (Table 5) and an average of 92.6%, 88.9% and 84.9% for groups Ia, IIa and IIIa; respectively as compared with 99% for the control group (Table 6). It was seen from Table 5 and Table 6 that the metamorphosis of the larvae in groups I, II, III, and Ia, IIa, IIIa was retarded. It was noted that the mean duration of the first larval instar was prolonged when compared with the control group Table 5 and Table 6. The onset of the first ecdysiast occurred after an average of 88.7, 98 and 99.3 hours for groups I, II and III; respectively as compared with 74 hours for the control group (Table 5) and an average of 90, 95.3 and 105 hours for groups Ia, IIa and IIIa; respectively as compared with 75.3 hours for the control group (Table 6) .
Table 5a. The larvae of Musca vicina, produced from adult females fed on Melia azedarach fruit extract at age 72 hours, reaching the succeeding instar at standard time| Records of larvae reaching the next instar at the standard time. | ||||||
| Feeding medium provided | First instar | Second instar | Third instar | |||
| After 72 hours | After 120 hours | After 168 hours | ||||
| No. | % | No. | % | No. | % | |
| Standard diet | 139 | 98.5 | 138 | 97.8 | 137 | 97.1 |
| Standard diet+1.8%Melia azedarach fruit extract. | 73 | 79.9 | 52 | 56.9 | 48 | 52.5 |
| Standard diet+2.4%Melia azedarach fruit extract. | 56 | 72.1 | 35 | 45.1 | 32 | 41.2 |
| Standard diet+3.6%Melia azedarach fruit extract. | 42 | 70.4 | 24 | 40.2 | 21 | 35.2 |
| Records of larvae reaching the next instar at the standard time. | ||||||
| Feeding medium provided | First instar | Second instar | Third instar | |||
| After 72 hours | After 120 hours | After 168 hours | ||||
| No. | % | No. | % | No. | % | |
| Standard diet | 138 | 98.8 | 138 | 98.8 | 136 | 97.4 |
| Standard diet+1.8%Melia azedarach leaves extract. | 106 | 81.5 | 80 | 61.5 | 78 | 60 |
| Standard diet+2.4%Meliaazedarach leaves extract. | 97 | 79.3 | 70 | 57.2 | 61 | 49.8 |
| Standard diet+3.6%Melia azedarach leaves extract. | 85 | 75.6 | 58 | 51.6 | 55 | 44.9 |
It is indicated from Table 5 and Table 6 that the mean duration of the second larval instar in groups I, II, III and Ia, IIa, IIIa had been prolonged. The onset of the second larval ecdysiast occurred after an average of 155, 165 and 195 hours for groups I, II and III; respectively as compared with 122.7 hours for the control group (Table 5) and an average of 144.3, 160 and 185 hours for groups Ia, IIa and IIIa; respectively, as compared with 126 hours for the control group (Table 6 (. The third larval instar had shown retardation in metamorphosis in groups I, II, III and Ia, IIa, IIIa in Table 5 and Table 6. The onset of the third larval ecdysiast occurred after an average of 183.3, 190.7 and 198 hours for groups I, II and III; respectively as compared with 172.7 hours for the control group (Table 5) and an average of 176.3, 184 and 190 hours in groups Ia, IIa and IIIa; respectively as compared with 170 hours for the control group (Table 6). Table 5 and Table 6 showed that the onset of the pupation occurred after an average of 105, 143.3 & 191.3 hours for the larval groups I, II and III; respectively as compared with 97.3 hours for the control group (Table 5); and an average of 101, 136.7 and 185 hours in groups Ia, IIa and IIIa; respectively as compared with 95.3 hours for the control group (Table 6) It is indicated in Table 5 and Table 6 that the percentages of larvae reaching the pupal stage in groups I, II, III and Ia, IIa, IIIa had been similarly decreased. Such percentages were found to be 64.3, 59.2 and 37.3% for groups I, II and III; respectively as compared with 99.3% for the control group (Table 5) and an average of 83.9, 72.5 and 70.3% for groups Ia, IIa and IIIa; respectively as compared with 99.1% for the control group (Table 6). The data obtained from these experiments demonstrated that the adults reared on media containing Melia azedarach extraction seemed to show some morphological variations of larvae, pupae and adults when compared with control groups. These abnormalities could be classified into larval, pupal and adult abnormalities.
Larval abnormalities as small larvae these were larvae which had normal appearance, but they had a comparatively small size. Table 1 showed that this category of the small larvae reached a percentage of 1.7% and 1.2 % in groups I and II; respectively (Table 1) and a percentages of 2.8%, 1.9% in groups Ia, IIa; respectively (Table 2) in the first set of experiments. In the second set of experiments it reached a percentage of 2.8%, 2.2% in groups I, II; respectively (Table 3) reached a percentage of 4.4%, 1.7% in groups Ia, IIa; respectively (Table 4). In the third set of experiments, the percentage of the small larvae reached 2.6%, 2.2% in groups I, II; respectively (Table 5) and a percentage of 0.5% in group Ia (Table 6) the small larvae developed to produce small pupae and small adluts. Only adults belonging to groups I, II, Ia and IIa of the first, second and group I of experiments that were reared on diets containing 1.8% and 2.4% of Melia azedarach extractions had produced small larvae, small pupae and small adults (Figure 5, Figure 11).
2-Pigmented larvae, these were larvae of normal size that had inter-segmental patches of brown pigments (Figure 1). Table 1showed that the larvae attaining this abnormality had reached a percentage of 46.7%, 55%, 70.5% in groups I, II, III; respectively (Table 1) and percentages of 44.3%, 49%, 68.6% in groups Ia, IIa, IIIa; respectively (Table 2) in the first set of experiments. In the second set of experiments the larvae attained this abnormality were 75.9% in groups III and a percentage of 53.4%, 50.5% in groups II, I (Table 3) and reached a percentage of 44.1%, 47.2% and 67.1% in groups Ia, IIa, IIIa; respectively (Table 4). In the third set of experiments, the percentage of larvae that attained this abnormal pigmentation were 20.3%, 23.6%, 50.9% in groups I, II, III; respectively (Table 5) and a percentage of 9.5%, 16.3%, 20.5% in groups Ia, IIa, IIIa; respectively (Table 6).
Pupal abnormalities, larval-pupal intermediates, the puparia of these abnormal pupae were incomplete with parts of the last larval cuticle were still persisting (Figure 2, Figure 3, Figure 4, Figure 5). These larval-pupal intermediates were produced from normal larvae (N.L.), they failed to complete the pupal period. They died after emerging from the normal third larval instar. Table 1 showed that this category of the larval-pupal intermediates had reached a percentage of 5.3%, 9.6% & 10.5% in groups I, II, III; respectively (Table 1) and the percentage of 4%, 8.1%, 9.3% in groups Ia, IIa, IIIa; respectively (Table 2) in the first set of experiment. In the second set of experiments the percentage of this category had reached. In the third set of experiments, the percentage of pupae that attained this larval-pupal intermediate were 9.9%, 12.8% and 3.4% in groups I, II, III; respectively (Table 5) and a percentage of 5.1%, 9.8% &9.2% in groups Ia, IIa, IIIa; respectively (Table 6).
Figure 1.howing larvae with normal size but having brown pigments. X 26.1.
Figure 2.Showing larval–pupal intermediates. X30.5.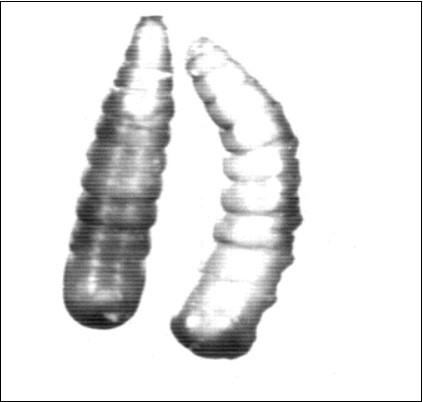
Figure 3.Showing larval – pupal intermediates. X31.2.
Figure 4.Showing a constricted pupa. X34.7.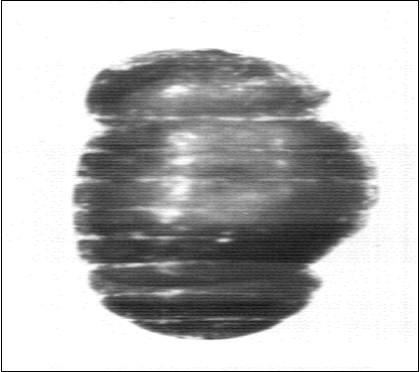
Figure 5.Showing; a-Small pupa, b-Crumpled pupa, c-Normal pupa with normal size. X53.3.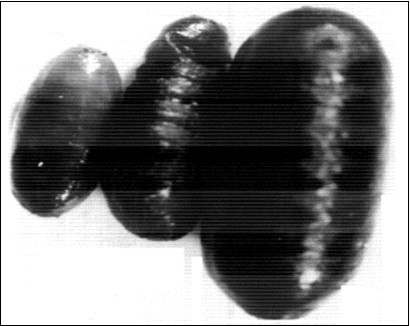
2- Constricted pupa; These were fully formed pupae having conspicuous constrictions in their puparia; they were produced from pigmented larvae (P.L), hence failing to have the characteristic shape of the normal pupae and failing to emerge to the adult stage (Figure 4). (Table 1) showed that this category of abnormal pupae had reached percentages as high as 36.3% in group III, 16% and 11.1% in groups II and I; respectively (Table 1) and a percentage of 10.5%, 13.5% and 20.9% in group I, II and III; respectively (Table 2) in the first set of experiments. In the second set of experiments the percentage of this category of abnormal pupae had reached a percentage of 12.1%, 17.5%, 33.3% in groups I, II, III; respectively (Table 3) and a percentage of 7.8%,10.4%,16.6% in groups Ia, IIa, IIIa; respectively (Table 4).The third set of experiment had shown a marked decrease in the percentage of these constricted abnormal pupae, it reached a percentage of 8%, 9.9%, 8.4% in groups I, II, III; respectively (Table 5) and a percentage of 2.3 %, 3.8%, 4.8% in groups Ia, IIa, IIIa; respectively (Table 6) .
3- Pigmented pupae, these were pupae with an apparently normal appearance but possessing white pigments they were produced from pigmented larvae (P.L.). Table 1 showed that this category of abnormal pupae had reached a percentage of 35.6%, 39.1%, 37.8% in groups I, II, III; respectively and a percentage of 34.1 %, 35.3% and 48.0% in groups Ia, IIa, IIIa; respectively (Table 2) in the first set of experiments. In the second set of experiments the percentage of this category of abnormal pupae had reached a percentage of 38.3%, 36.5%, 42.7% in groups I, II, III; respectively (Table 3) and a percentage of 36.6%, 35.9% and 50.5% in groups Ia, IIa, IIIa; respectively (Table 4(
The third set of experiments had shown a marked decrease in the percentage of these pigmented pupae, it reached a percentage of 12.4 %, 13.8%, 42.5% in groups I, II, III; respectively (Table 5) and a percentage of 7.2%, 7.9%, 8% in groups Ia, IIa, IIIa; respectively (Table 6). It must be noted here that the larval-pupal intermediate and the constricted pupae were produced from the normal larvae. The pigmented pupae were produced from the pigmented larvae .
Small pupae were produced from small larvae (S.L.) they reached as high as 4.4% in group Ia (Table 4).
Adult abnormalities; certain morphological abnormalities were evident in the adults produced after treatment in groups I, II, III and Ia, IIa, IIIa.
These abnormalities seem to fall into two main categories:
1- Half emerged adults were produced from normal pupae (N.P.), they were adults that could not emerge completely and remain trapped in their puparia until they die (Figure 6, Figure 7), they reached in the first set of experiments a percentage of 5.8%, 6.4% and 6.4% in groups I, II and III; respectively (Table 1) and a percentage of 8.5%, 6.5% and 4.6% in groups Ia, IIa, IIIa; respectively (Table 2). In the second set of experiment it reached a percentage of 6.9%, 9.4% & 4.2 % in groups I, II, III; respectively, (Table 3) and a percentage of 10.2% , 9.1% & 6.7% in groups Ia, IIa, IIIa; respectively (Table 4). In the third set of experiments, the percentage of this abnormal adults were 2.9%, 2.1% and 1.1% in groups I, II, III; respectively (Table 5) and percentages of 10.8%, 9% & 9.8% in group Ia, IIa and IIIa; respectively (Table 6)
Figure 6.Showing adult house fly Musca vincina that remains attached to its puparium. X32.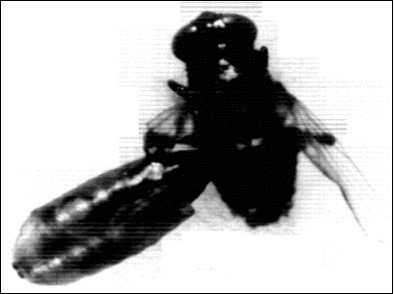
Figure 7.Showing: Head and a leg of the adult fly Musca vicina that remains attached to its light X 63.
2-Adults of relatively small size and possessing different shapes of wings they were produced from small larvae and small pupae. Figure 8, Figure 9, Figure 10, Figure 11, Figure 12 and table 1, table 2, table 3, table 4, table 5, Table 6,demonstrated emerging one winged adults in addition to variety of abnormalities ranging from adults with crumbled incomplete bent to adults with broken wings.
Figure 8.Shows adult house fly Musca vicina with incomplete bent wings and abnormal prolonged legs.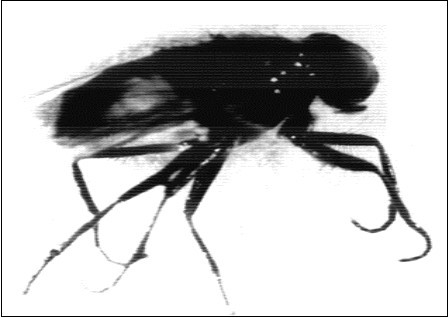
Figure 9.Showing adult with incomplete broken wings. X31.
Figure 10.Showing adult house fly Musca vicina of almost normal size.
Figure 11.Showing small adult house fly Musca vicina with incomplete bent wings. X 30.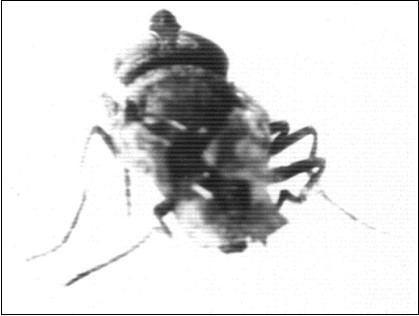
Figure 12.Showing adult Musca vicina of normal size with long crumpled wings and reduced abdomen. X 28.2.
Discussion
The results obtained from the experiments conducted herein indicated the retarding effect of the fruits and leaves extracts of Melia azedarach on the house fly Musca vicina at different ages 24, 48 and 72 hours, when they were treated with various concentrations of Melia azedarach extracts mainly 1.8% 2.4% and 3.6%. The results represented in table 1, table 2, table 3, table 4, table 5, Table 6,indicated the effect of Melia azedarach extracts on the different ages of females Musca vicina which accelerated egg deposition thus the time required for completing the first gonotrophic cycle was decreased and the number of deposited eggs was slightly decreased. It is noticed that Melia azedarach extracts at the different doses used; affect the hatchability of eggs which was decreased. Similar observations were obtained by Riddford and Williams 21 in their work on Silk-worm Hyalophophracercropi by using JHa. Keller 22 reported that the reproductive potential of Diaprepesabbreiatus was reduced by aerial application of JH-6040, plus oil. The JHa reduced the hatchability of eggs and the oil detached them from the leaves of the litters.
Mehrotra and Gujar 18 found that azadirachtin reduced fecundity and reproduction ofSpodopteralitura. Heyde et al. (1984)23 observed a marked reduction in the fecundity of hemipterous rice pests when adults were treated with 3% neem oil. Chiu et al. 24 noticed that oviposition deterrence by extracts of Melia toosendan for a number of Lepidopteran species. Coudriet et al.25 found that ethanolic extracts of neem seed reduced oviposition of sweetpotato fly, Bemisiatabaci. Wilps 26 studied the effect of azadirachtin on larval development, pupation of Musca domestica. He found that the number of eggs deposited on azadirachtin treated substrate was much less than on the control. Also, he noticed damaging in Musca’s larvae and adults. He indicated that azadirachtin seed kernel extracts (NSKE) could be used as effective inhibitors of growth and development in autogenously insects. Akhter et al.27 observed the egg laying capability after using two preparations of Nicotine dusts against the 3rd instar larvae of the house fly Musca domestica. They found that the egg laying capabilities ranged from 30.4 to 35.8 egg/ fly at the highest doses of Nicotine whereas the same concentrations inhibit the hatching of at least 96.6 and 64.2 egg/fly.
Sterility was indicated by Sukumar 28 when using the extracts of air dried leaves and roots of Catharanthus roseus in both males and females Musca domestica. Rice and Coat 29 treated adults of Musca domestica and their eggs with mono-terpenoids to determine the topical fumigant and ovicidal activity of each compound. Structural activity relationships were evaluated with the toxicity data and comparisons were made between monocyclic, aromatic, a cyclic aliphatic, monocyclic aliphatic to determine the toxicity differences involving the skeletal structure amount of saturation, and associated functional groups of monoterpenoids. They found that ketones were less toxic than an analogous aldehyde, in the topical, fumigant and ovi-cidal bioassays. Saxena et al.30 noticed that topical treatment of Musca domestica L. with the phytochemical plumbagin in doses of 0.005-5 Mg, prevented oocyte development and drastically affected fecundity and fertility in adults. Also, treatment of ‘Wandering” larvae was less effective as the compound only affected fertility, not fecundity. It is clear from the data cited in table 1, table 2, table 3, table 4, table 5, Table 6,that oral administration of Melia azedarach to the adult house fly causes prolongation in the life span of the larval instar of the first generation. Similar observations were obtained by Supavarn et al.31 in their work on Aedes aegypti. Jhansi Rani 32 observed a delay in development of larval-pupal and pupal-adult intermediates of Corcyra cophalonica when insects were treated with lower concentrations of kernel. Koul 33 found that application of Azadirachtin on various stages of Dysdercuskoenigii and Spodopteralitturalarvae caused prolongation in the developmental period wing deformities, development of wingless adults and larval mortality. Koul 34 studied the effect of azadirachtin on blowflyCalliphoravicina by injection. They found that azadirachtin prolonged the 3rd larval instar and the pupae had a lower body weight than in the control also many of the pupae showed malformations. Higher doses caused mortality both in larvae and pupae and only a few adults emerged.
Hashem and Youssef 7 studied the effect of methanolic extracts of leaves and fruits of Melia azedarach L. on the house fly Musca vicina Macq. They found that the reaction of various instars to the concentrations of Melia azedarach extractions is doses dependent. The metamorphosis was retarded and the developmental periods of the larval stages were prolonged. They showed that the younger instars were strongly affected by lower concentrations while the older ones were less affected. They noticed that the fruit extract was more effective on the larvae than that of leaves. They also recorded that the larvae, pupae, and the adults displayed morphological abnormalities as well as pronounced anomalies. It may he reported here that the Melia azedarach extract was considered as the main factor controlling the period of the larval stages. It was evident that the prolongation in the larval life span which occurred as a result of the exposure to Melia azedrarch extraction had made a number of workers make use of Melia azedarach extractions as larvicides. The insect growth regulating properties of petroleum ether extracts of 10 indigenous Indian plants (Acorus calamus, Adhatoda, Vasica, Aristochiaindica, Artemisia vulgaris. Azadirachtaindica, Boerhaviadiffusa, carumcarvi, carumcopticum, Ocimumbasilicum and Ocimum sanctum were tested at 0.01 - 10 ppm against 3rd instar larvae of Culex pipiensfatigans, and Musca domesticaby Deshmukh and Renapurkar 35. They found that Acorus calamus and Ocimum Sanctum inhibited the full development of 20% of the larvae of Musca domestica at 0.1 ppm. Also at 10 ppm extracts of all (10) plants produced 20-80% inhibition in Musca domestica with Acorus calamus and Azadirachtaindica being the most potent.
El Sayed 36; Naqvi et al.37 found that the effects of azadirachtin, a triterpenoid extracted from neem (Azadirachtaindica) seed were similar to those of insect growth regulators against the immature stages of the horn fly, Haematobiairritans the stable fly, Stomoxyscalcitrans and the house fly, Musca domestica. They noticed that, when an ethanolic extract of ground seed was blended into cow manure, the LC50 and LC90 were 10.5 and 20.2 ppm; respectively for house flies larvae. The pesticidal properties of juliflorine and Margosano were determined against 3rd instar larvae of Musca domestica by Jahan et al.38. The teratogenic effects of these pesticides on larvae, pupae and adults were observed. The LC50 was found to be 0.05% and 0.0018% for juliforin and Margosan-o, respectively. The toxicity and abnormalities produced by neem fraction and deltamethrin against second instar larvae of Musca domestica L. were recorded by Naqvi et al.39. They found that LD20 of dektamethrin (25 WP) and a neem extract after 24 hours treatment were 1.56% and 13.5%; respectively. Naqvi et al.40 studied the toxicity of the pyrthroid Coopex 25 EC (permethrin) and a neem extract N-7 against the 3rd instar larvae of the house fly. They found that the LD50 values of both compounds were 0.029% and 3.8%, respectively, which revealed that pyrthroid was more toxic than N-7. Both compounds caused morphogenic effects on various stages of Musca domestica including weight reduction and abnormal development. The first record of abnormality was the appearance of pigmented larvae. The nature of this pigmented area was uncertain. Chiu Shin-Foon 2 noticed appearance of black spots on the body of cabbage larvae soon after treatment with pure compounds isolated from the root bark. Shalaby et al.41; Radwan 8 observed abnormal pigmentation in the second and the third larval instars of the house fly Musca domesticavicina. The second abnormality was the appearance of small larvae.
Naqvi 42 found that treated larvae of Aedes aegypti with neutral fraction of winter neem leaves (NFD) produced larval-pupal intermediates. The appearance of small, pigmented and constricted pupae were additional abnormalities observed with various concentrations of Melia azedarach. Wilps 26 found that reduction in pupal weight of Musca domesticaand pupal malformations were found to occur more frequently with increasing azadirachtin concentration in the diet. Koul 34 studied the effect of azadtrachtin on blowfly Calliphoravicinaby injection. They found that azadirachtin prolonged the 3rd larval instar and the pupae had a lower body weight than in the controls, also many of the pupae showed malformations. Shalaby et al.41 noticed a dark pigmentation in pupae of Musca domesticavicinawhen larvae of 2nd and 3rd instars treated with JH-1. The appearance of constricted pupae was another observed abnormality, the pupae were fully formed hut had constrictions in their puparia, so that they failed to emerge to the adult stage.
It became obvious that the exposure of house fly females to Melia azedarach extracts in the treated groups I, II, III and Ia, IIa, IIIa, produced abnormal larvae which gave rise to abnormal pupae (Figure 5) and emerged producing abnormal adults, small adults of normal appearance (Figure 11) adults of normal size with broken wings and abnormal legs which could not deposit eggs and adults that could not emerge completely and remain concealed in the puparia until they died (Figure 8, Figure 9, Figure 10, Figure 11, Figure 12). Koul 33 reported that azadirachtin caused a prolonged development period, wing deformities, non-plasticisation of wing lobes, development of wingless adults, and larval mortality on application to various stages of Dysdercuskoenigii F. and against Spodopteralittura larvae.
Koul 34 found that the injection of azadirachtin to the larvae of the fly (Calliphoravicina) led to inhibition of adult emergence and the adults which succeeded to emerge were smaller and their wings, legs, and proboscis showed typical malformation and their abdomens was often very short. Jahan et al.38 used petroleum ether extracts of (Clerodendruminerme) leaves which afforded a compound that matched the clarodan compound (-)-3-epicaryoptin in physical spectral characteristics. They observed that the tested compound inhibited the development of larvae of Musca domestica and Culex quinquefasciatus. Hashem and Youssef 7 studied the effect of ethanolic extractions of leaves and fruits of Melia azedarach on the house fly Musca vicina. They found that the reaction of various instars to the concentrations of Melia azedarach extractions is dose dependent.
The insecticidal performance of neem products against most insects is not as dramatic as that of the synthetic insecticides and for equivalent effectiveness, considerably higher doses are required. Evidently the (JH-like substance) or the fruit and leaves extracts of Melia azedarach seems to be responsible for the normal development of the larvae, the appearance or disappearance of the larval characters and their normal or abnormal pupation. It became obvious from the results presented herein and which had been confirmed by the work of other authors, that the presence of Melia azedarach fruit and leaves extracts during the period of pupation interferes with the normal process of pupation and induces abnormal pupae and the emergence of anomalous pupal forms. These forms were found to attain some of the larval characters, in addition to the inhibition of the melanization process. The employment of the fruit extract of Melia azedarach is disrupting the course of morphogenesis and preventing the normal development of insects of medical and economic importance would point their importance as unharmful insecticides to human being. House flies Musca vicina are still the world’s number - one vectors of human and domestic animals diseases. Today, we depend almost entirely on synthetic chemical pesticides. The appearance of pesticide resistance had diminished our confidence in convential chemical methods. It is clear that the fruit and leaves extracts of Melia azedarach are effective against house flies Musca vicina.
References
- 2.Chiu Shin Foon. (1986) Experiments on the practical application of chinaberryMelia azedarachand other naturally occurring insecticides in China. Proc. 3rd Int. Neem. Conf , Nairobi 661-668.
- 3.Jatwani M G, Srivastava K P. (1983) A review of neem research. in India in relation to insects.Proc.2nd Int. Neem Conf. Rauischholzhausen: 43-56.
- 4.Schmutterer H, CPW Zebitz. (1984) Effect of methanolic extracts from seed of single neem trees of African and Asian Origin, onEpilachnavaivestisandAedesaegypti. Proc. 2nd Int Neem. Conf.(Rauischholzhausen,1983) 83-90.
- 5.Chiu S F, Huang Z X, Huang D P, Bing-Qui H, Muchen X et al. (1984) Investigation into the toxic principles from seed kernels of Meliaceae and their effects on agricultural insects. China Res. , Bull 3, 1-32.
- 6.Garcia E, Rembold H. (1984) Effect of azadirachtin on ecdysis ofRhodinusprolixusJ. , Insect. Physiol 30, 939-941.
- 7.Hashem H O, Youssef N S. (1991) Developmental changes induced by methanolic extracts of leaves and fruits ofMelia azedarachL. on the house fly,MuscadomesticavicinaMacq. , J. Egypt. Ger. Soc. Zool 3, 335-352.
- 8.Radwan E H. (1999) Effect of crude methanolic extracts of leaves and fruits of chinaberry treeMelia azedarach L. on the adult house flyMuscadomesticavicinamacq.MSc Thesis Faculty of Sci.Univ.ofAlex.
- 9.Hashem H O, Swidan M H, Fetyani A A. (1991) Disrupting biological effects of methanolic leaves and fruit extracts of chinaberry tree,Melia azedarachL. on the development of the cotton leaf worm,Spodopteralittoralis(Boids). , J. Egypt. Ger. Soc. Zool 4, 47-65.
- 10.Rovesti L, ICY Dese. (1991) Effectiveness of neem seed kernel extract againstLeucopteramalifoliellaCosta (Lepi. , Lyonetiidae). J. appl., Ent 111, 231-236.
- 11.Ladd T L, Wathen J D, Klein M G. (1984) Japanese bettle (Coleoptera: Scarabeidae):The effects azadirachtin on growth and development of the immature forms. , J. Econ. Entomol 75, 903-905.
- 12.Schwinger M. Univ. of Hohenheim, FRG (1985) Uber die frassab-schreckende wirkung von Meliaceen inhaltsstoffen (Muls.) und andere insekten : Methoden versuchstechniken-Engebnisse. Ph. D. Thesis
- 13.Klingel M. (1985) Untersuchungen zur frass-und entwicklungsstoren-den Aktivitat von Meliaceen- inhaltsstaffen: Entwicklung and Anwendung eines Testverfahrens für den agyptischen BaumwollwurmSpodopteralilloralis. Wissenschafihiche Arbeit. , Univ. of Hohenheim, R F G
- 14.Miller J A, Chamberlain W F. (1989) Azadirachtin as a larvicidae against the horn fly, stable fly and house fly (Diptera:. , Muscidae). J. Econ. Entomol. V 82(5), 1375-1378.
- 15.Garcia E S, UhI M, Rembold H. (1986) Azadirachtin, a chemical probe for the study of the moulting process inRhodinusprolixus.
- 16.Bidmen H J, Kraser G, Mbus P, Koolman J. (1986) Effect of azadirachtin on blow fly larvae and pupae. Proc. 3rd Int. Neem. Conf , Nairobi 253-271.
- 17.Rembold H, Forster H, Ch Czoppelt, Rao P J, Sieber K Y. (1984) . Natural Pesticides from the neem tree and other Tropical plants pp., 153 (Schmuterer H, Ascher KPS eds.) Eschborn, GTZ .
- 18.Mehrotra K N, Gujar G T. (1984) Neem an insect growth inhibitor. Natn. Seminar on “Neemin Agriculture”, IARI,April12-13,1983. Neem News1 1, 6.
- 19.Abraham C C, Ambica B. (1979) Effect of leaf and kernel extract of neem on moulting and vitillogenesis inDysdercuscingulatusFabr. , (Heteroptera: Pyrrhocoridae). Curr. Sci 48, 554-556.
- 20.Snedecor G W, Cochran W G. (1967) . Statistical methods.6thEdition , Ames, Lowa, the Lowastateuniversity .
- 21.Riddford L M, Williams C M. (1967) The effect of juvenile hormone analogues on the embryonic development of silk worm. , Proc. Nat. Acad. Sci 57, 595-601.
- 22.Keller U. (1985) Untersuchungen zur frass-und entwicklung schemenden Aktivitat von Meliaceenin-haltsstoffen:Epilachnavarivestisim dual choice-Inochoice Test und. in Langzeitversuchen Wissenschafiliche Arbeit, Univ. of Hohenheim, FRG .
- 23.JVD Heyde, Saxena R C, Schmutterer H. (1984) Neem oil and neem extracts as potential insecticides for control of Hemipterous rice pests. In Proceeding, 2nd International Neem Conference, Rauisch-Holzhausen Castle,1983.German Agency for Technical Cooperation,Eschborn 377-390.
- 24.Chiu S F, Huang Z X, Huang D P, Bing-Qui H, Muchen X et al. (1984) Investigation into the toxic principles from seed kernels of Meliaceae and their effects on agricultural insects. China Res. , Bull 3, 1-32.
- 25.Coudriet D L, Prabgaker N, Heyerdirk D E. (1985) Sweetpotato whitefly (Homoptera: Aleyrodidae): Effects of neem seed extract on oviposition and immature stages. , Envir. Ent 14, 776-779.
- 26.Wilps H. (1986) Growth and adult moulting of larvae and pupae of the blow fly Phormia terrae-Novae in relationship to azadirachtin concentrations. Proc. 3rdInt. Neem. Conf , Nairobi 299-314.
- 27.Akhtar K, Rizivi S A, SNH Naqvi. (1986) Effect on fecundity produced by nicotine dusts against 3rd instar larvae ofMuscadomesticaL. , (PCSIR strain). Pak. J. Entomol. Karachi 1(2), 31-38.
- 28.Sukumar K. (1987) Effect of catharanthus alkaloids on the reproductive performance of the house fly,Muscadomestica. Pesticide Div., Regional Res. , Lab., Hyderabad, India, Entomol 12(1), 1315-8.
- 29.Rice P J, Coats J R. (1994) Insecticidal properties of several monoterpenoids to the house fly corn root worm (Coleoptera: Tenebrionidae and southern corn root worm (coleopterr: chrysomelidae). , J. of Econ. Entomol 87, 1172-1179.
- 30.Saxena R C, Liquido N J, Justo H D. (1981) Neem seed oil, a potential antifeedant for the control of the rice brown plant hopper,NilaparvatalugensProc. 1stInt. Neem. Conf (Rottach-Egern 17, 1-188.
- 31.Supavarn P, Knapp F W, Sigafus R. (1974) Biologically active plant extracts for control of mosquito larvae. , Mosq. News 34(4), 398-402.
- 32.Jhansi Rani B. (1984) Studies on the biological effecincy of deoiled neem (AzadirachtaindicaA.Juss) kernel against a few insects. M.Sc. Thesis Post Graduate School, IARI. , New Delhi
- 33.Koul O. (1985) Part 3.Azadirachtin interaction with development ofSpodopteralitturaFab. , Indian J. Exp. Biol 23, 160-163.
- 34.Koul O. (1984) Azadirachtin I. Interaction with the development of red cotton bugs. , Entomol. Exp. Apple 36, 85-88.
- 35.Deskmukh P B, Renapurkar D M. (1987) Insect growth regulatory activity of some indigeneous plant extracts. Jai Res. Foundation. PO valvada, Umergoan District, Bulsar-396-108, Gujarat, India. Insect. Science and its application 8(1), 81-8309.
- 36.El-Sayed E I. (1982) Neem (AzadirachiaindicaA. Juss) seeds as antifeedant and ovipositional repellent for the Egyptian cotton leaf - worm,Spodopteralittoralis(Boids.). , Bull. Ent. Soc. Egypt, Econ. Ser 49, 1982-1983.
- 37.SNH Naqvi, Ahmed A, Ahmed I, Khan M F, Azmi M A et al. (1990) Toxicity and alkaline phosphatase inhibition effects of Soya” extract against 3rd instarMuscadomesticaL. larvae in comparison with Coopex (pyrethroid: permethrin + bioallathrin). , Pakistan J. Entomol. Karachi,5(1-2): 71-80.
- 38.Jahan M, Ahmed I, SNH Naqvi. Department of Zoology- Entomology, University of Karachi, Pakistan (1990) Toxic and teratogenic effects of juliflorine and Margosan-O on theMuscadomesticaL. larvae. Proceedings of Pakistan Congress of Zoology 10, 293-320.
- 39.SNH Naqvi, Tabassum R, Jahan M, Yasmen N, Azmi M A et al. (1994) Toxicity and abnormalities produced by neem fraction (NM) and deltamelthrin against second instar larvae ofMuscadomesticaL. , (PCSIR Strain). Proceeding of Pakistan-Congress of Zoology 14, 283-290.
- 40.SNH Naqvi, Jahan M, Tabassum R, Qamar S J, Ahmed I. (1995) Toxicity and teratogeny caused by Coopex 25 EC and a neem extract (n-7) against 3rd instar larvae ofMuscadomesticaL. , PAK. J. Zool 27(1), 27-31.
Cited by (3)
- 1.Eissa Ebrahim E., Radwan EH, Hakeem N Abdel, KK Abdel Aziz, Hashem HO, et al, 2020, Impact if Chlorpyrifos on the Second Instar Mosquito Larvae as Bioindicator in El-Beheira Governorate, Egypt, International Journal of Limnology, 1(2), 1, 10.14302/issn.2691-3208.ijli-20-3268
- 2.Shishehbor Parviz, Hemmati Seyed Ali, 2022, Investigation of secondary metabolites in bean cultivars and their impact on the nutritional performance ofSpodoptera littoralis(Lep.: Noctuidae), Bulletin of Entomological Research, 112(3), 378, 10.1017/S0007485321000948
- 3.Jafari Hasan, Hemmati Seyed Ali, Habibpour Behzad, 2023, Evaluation of artificial diets based on different legume seeds on the nutritional physiology and digestive function ofHelicoverpa armigera(Hübner), Bulletin of Entomological Research, 113(1), 133, 10.1017/S0007485322000402
