Abstract
A 57-year-old Hispanic female presented with 3 days of blurry vision in the left eye. Eight years prior, she had a branch retinal artery occlusion in the right eye and a hematologic work-up revealed a 4G/4G polymorphism in plasminogen activator inhibitor-1. With the current episode, she was found to have bilateral branch retinal artery occlusions and mild vitritis in the left eye, simulating a toxoplasma infection. An infectious and inflammatory work up, however, was negative and the vitritis resolved after a short course of steroids. Plasminogen activator inhibitor-1 mutations may be associated with an increased risk of retinal vascular occlusions.
Author Contributions
Academic Editor: Mohamed Zowalaty, Virology, Microbiology and Infectious Diseases (VMID) Research LaboratoryBldg E4 Room 505 School of Health SciencesUniversity of KwaZulu-NatalWestville Campus, Durban 4000, Private Bag X54001South Africa
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2017 Damien C. Rodger, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Retinal vascular occlusions are caused by three primary mechanisms: hematologic abnormalities, vessel wall changes, or perivascular abnormalities, such as sclerosis or a common adventitia. Abnormal hematologic factors can produce increased thrombosis, which is thought to account for 15-28% of unexplained systemic vascular thrombosis in young patients.1 The primary fibrinolytic enzyme in humans is plasmin, which is derived from the inactive precursor plasminogen. The two primary plasminogen activators are urokinase plasminogen activator (uPA) and tissue plasminogen activator (tPA). Plasminogen activator inhibitors neutralize plasminogen activators and have been classified as several distinct inhibitors, the most important of which is plasminogen activator inhibitor-1 (PAI-1), which is the primary inhibitor of tPA.1 In contrast to the systemic circulation where fibrinolytic activity is primarily found in the endothelium of veins and capillaries, high fibrinolytic activity is localized to the endothelium of both arteries and veins within the retinal circulation.2
While mutations in plasminogen activator inhibitor-1 have been associated with an increased risk of systemic thrombosis, the embolic effects of the mutation in the eye have not been well documented and ocular sequelae of PAI-1 inhibitor mutation is not well understood. In this case report, we describe a patient with recurrent branch retinal artery occlusions associated with PAI-1 inhibitor mutation.
Case Report
A 57-year-old Hispanic female was referred to our clinic with decreased vision in the left eye of two months duration. Past medical history included hypothyroidism. Additionally, eight years prior, she experienced a branch retinal artery occlusion (BRAO) in the right eye. At that time an extensive work-up was performed, including a hematologic evaluation, which was found to be normal for lupus anticoagulant, factor VIII, factor II, and anti-thrombin III antigen. However, the patient was found be homozygous (4G/4G) for a mutation in PAI-1. The decision was made to not immediately start a blood thinning agent but to observe the patient. With the current episode of decreased vision, the patient initially reported three days of grey, cloudy vision in the left eye. She presented to an outside clinic with a best-corrected visual acuity (BCVA) was 20/20 and 20/60 in the right and left eyes, respectively. Dilated fundus examination revealed the right eye to have an area of whitening and a sclerotic arteriole superior to the fovea in the right eye while the left eye had 2+ vitritis and two white fluffy focal retinal lesions (Images 1). The clinical features were thought to be consistent with toxoplasmosis so the patient was initially started on oral trimethoprim-sulfamethoxazole 800mg twice a day and oral prednisone 40mg daily. A broad infectious and inflammatory work up, including toxoplasmosis IgG and IgM, syphilis, quanitiferon gold, bartonella, ANA, ANCA, ACE, HLA-B51, and a chest X-ray, were all found to be negative and the patient was referred to our institution. When the patient first presented to our clinic (two months after initial decreased vision), her BCVA was stable and fundus examination of the right eye was relatively normal while the left eye showed mild 1+ vitritis and a small white retinal lesion off the inferior arcade (Image 2). Fluorescein angiography (Image 3) and OCT angiography (OCT-A) (Image 4) revealed multiple areas of arterial occlusion in the left eye while the area where the white lesion had previously been seen showed mild capillary drop out. Oral antibiotics were stopped and an oral prednisone taper was tapered. One month later, the patient’s symptoms and the vitritis had resolved and visual acuity in the left eye improved to 20/30. The patient was referred to a hematologist for management of the thrombotic disease but was then lost to follow-up.
Images 1.Fundus photos on first presentation showing a white lesion in the right eye while the left eye showed moderate vitritis with white lesions off the superior and inferior arcades.
Image 2.Fundus photos two months after first presentation showing resolution of the white lesion in the right eye and improvement of the vitritis in the left eye.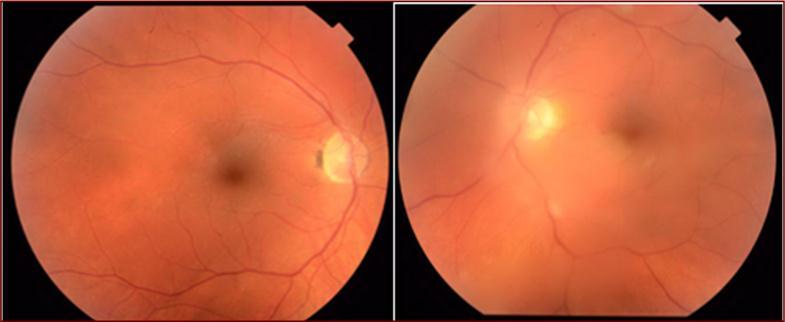
Image 3.Fluorescein angiography two months after first presentation showing mild capillary drop out in the right eye (arrow) and several arterial occlusions in the left eye (arrows).
Image 4.OCT angiography non-segmented images two months after first presentation showing mild loss of inner retinal perfusion of the right and severe loss of perfusion in the left eye (arrows).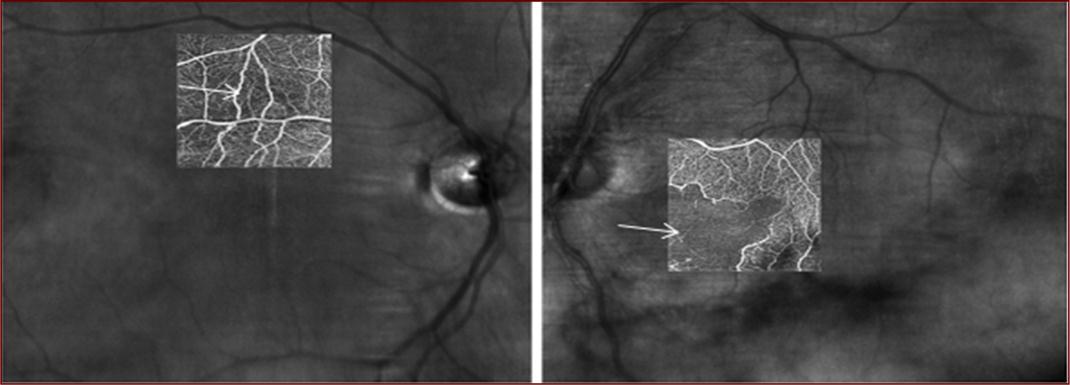
Discussion
Plasminogen activator inhibitor-1 is a protein whose primary function is to inhibit tissue plasminogen activator and urokinase (uPa). As tPa and uPa are responsible for fibrinolysis of clots, inhibition of these functions leads to a hypercoagulable state. Numerous studies have found mutations of PAI-1 to be associated with systemic thrombosis. However, the size of the effect remains unknown and some studies have failed to find an association. One meta-analysis of the 4G allele in PAI-1 mutation included 2,644 cases and found that in patients with a history of venous thrombosis, the PAI-1 mutation was associated with a statistically significant increased odds ratio of 1.153.3 A meta-analysis of PAI-1 4G polymorphism and the risk of ischemic stroke found that when all prospective studies were pooled, the mutation was associated with a significantly increased risk of stroke with a relative risk of 1.91.4 However, when both cohort and case-control studies were included the relative risk fell to 1.18, which was not statistically significant. Additional studies have reported PAI-1 mutation to be associated with increased risk of severe preeclampsia,5 serious pregnancy complications,6 myocardial infarction in young patients, 7 and internal organ thrombosis.8
The thrombotic implications of PAI-1 polymorphism in the eye is poorly understood. Most studies evaluating the effects of PAI-1 mutations have evaluated occlusions of the retinal venous system. In a study of 17 patients with retinal vein occlusions (RVO) and 234 controls, 88% of RVO patients were found to have a 4G heterozygous or homozygous polymorphism in PAI-1 while 63.7% of controls had the polymorphism (p=0.03).9 Additionally, patients with the 4G/4G genotype and RVO had significantly higher levels of hypofibrinolytic activity compared to controls (p=0.05). In a study of 112 patients with a history of retinal vein occlusion (RVO), elevated PAI-1 activity was highly significantly associated with RVO after controlling for other risk factors in multivariate analysis (OR=4.93; p=0.003).10 In the most comprehensive study to date, authors performed a meta-analysis of the associations between RVO and thrombophilia polymorphisms and found that the PAI-1 mutation was significantly associated with an increased risk of RVO (OR=1.27; p=0.036).11 While high fibrinolytic activity has been found in both the arterial and veins systems within the retinal circulation,2 few studies have evaluated retinal arterial occlusions and PAI-1 mutations. One study evaluating risk factors for retinal artery occlusion did not find the PAI-1 mutation to be associated increased risk of occlusion.12 However, this study was insufficiently powered as it only included 4 patients with the mutation.
An interesting aspect of this case is that while both eyes have had branch retinal arterial occlusions, the patient presented with mild unilateral vitritis of unknown etiology. An infectious and inflammatory work-up revealed no underlying etiology and the patient had no systemic symptoms. The ocular inflammation resolved after a short course of steroids. While it is possible a separate etiology underlies the patient’s disease, the PAI-1 polymorphism may be culpable given the recurrent, bilateral nature of the occlusions in a patient with no systemic symptoms and a negative systemic work up.
In conclusion, we present a patient with recurrent branch retinal artery occlusions and a 4G/4G polymorphism in plasminogen activator inhibitor-1, a genotype that has been associated with retinal vascular occlusion. Ophthalmologists should be aware of the increasing understanding of the fibrinolytic system and should institute appropriate hematological work-up in select patients presenting with retinal vascular occlusion.
References
- 1.Vine A K, Samama M M. (1993) The role of abnormalities in the anticoagulant and fibrinolytic systems in retinal vascular occlusions. , Surv Ophthalmol 37(4), 283-92.
- 2.Pandolfi M. (1967) Fibrinolytic activity of retinal vessels in man and monkey. , Am J Ophthalmol 63(3), 428-34.
- 3.A E Tsantes. (2007) Association between the plasminogen activator inhibitor-1 4G/5G polymorphism and venous thrombosis. A meta-analysis. Thromb Haemost. 97(6), 907-13.
- 4.A E Tsantes. (2007) Plasminogen activator inhibitor-1 4G/5G polymorphism and risk of ischemic stroke: a meta-analysis. Blood Coagul Fibrinolysis. 18(5), 497-504.
- 5.Yamada N. (2000) The 4G/5G polymorphism of the plasminogen activator inhibitor-1 gene is associated with severe preeclampsia. , J Hum Genet 45(3), 138-41.
- 6.C J Glueck. (2000) The 4G/4G polymorphism of the hypofibrinolytic plasminogen activator inhibitor type 1 gene: an independent risk factor for serious pregnancy complications. Metabolism. 49(7), 845-52.
- 7.Isordia-Salas I. (2009) Association of the plasminogen activator inhibitor-1 gene 4G/5G polymorphism with ST elevation acute myocardial infarction in young patients. Rev Esp Cardiol. 62(4), 365-72.
- 8.Balta G, Altay C, Gurgey A. (2002) PAI-1 gene 4G/5G genotype: A risk factor for thrombosis in vessels of internal organs. Am JHematol. 71(2), 89-93.
- 9.C J Glueck. (1999) Heritable thrombophilia and hypofibrinolysis. Possible causes of retinal vein occlusion. Arch Ophthalmol. 117(1), 43-9.
- 10.A M Gori. (2004) Impaired fibrinolysis in retinal vein occlusion: a role for genetic determinants of PAI-1 levels. Thromb Haemost. 92(1), 54-60.
