Abstract
Human obesity is now universal and has drawn serious attention of international academia to unravel its pathophysiology and treatment. In recent years, efforts are being made to understand the complex physiology of both white and brown adipose tissue in detail with relevance to obesity. A large number of secretions from the white fat called the adipokines have been recognized that play a definitive role in obesity and its disorders. Innumerable regulators grouped mainly under the transcriptional, hormonal and signaling factors that govern the thermogenic functions of brown fat have been worked out. Based on these revelations, strong suggestions have been made for treating obesity specially by targeting the brown fat as it plays a key role in energy expenditure as well as through behavioral, pharmacological, physiological and surgical approaches. Such findings have been reported in a nutshell in this communication
Author Contributions
Academic Editor: Laureane Masi, University of Sao Paulo
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2016 Muralidhara DV, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Human obesity has been documented for at least 25,000 years.1 Hippocrates, some 3000 years ago described that proper diet and physical activity can maintain good health thus indirectly referring to obesity and stated that sudden death is more common in those who are naturally fat than in lean.2 However, Venner was the first to introduce the term obesity in 1660s to replace the word corpulency that was in use before.3 Since obesity has become pandemic it is also referred to as ‘globesity’ to stress its global magnitude and ‘diabeisty’ as it is closely associated with diabetes mellitus.4 Obesity has been defined variously as expanded white adipose tissue mass due to hypertrophy and hyperplasia and it is popularly referred to as a condition of excess body fat (not body weight) that is reflected by a body mass index (BMI) of greater than 30 kg/m2.5 This is due to the dysregulation of adipose tissue formation and function. Of late, obesity is considered as a disease of hedonistic life style and also as a mild inflammatory state associated with an increase in certain adipokines such as tumor necrosis factor-α (TNF-α), interleukins 1α and 6 (IL-1α, IL-6). Since the inflammatory condition affects the metabolic status in obesity, it is termed also as a condition of ‘metaflammation’.6
From a physiological view point, obesity is considered to result from a mismatch in energy balance either by an increase in energy intake and/or by a decrease in energy expenditure.7, 8 The energy expenditure components include the following variables variously termed as resting/basal metabolic rate (RMR/BMR), regulatory/adaptive thermogenesis which includes non-shivering thermogenesis (NST), cold induced thermogenesis (CIT), diet induced thermogenesis (DIT), thermic effect of food (TEF) and the energy spent on physical activity known as thermic effect of activity (TEA) which includes spontaneous physical activity/non-exercise activity thermogenesis (SPA/NEAT).9 Obesity is generally attributed to poor life style practices that involve unhealthy dietary intakes, increased dietary caloric consumption and alterations in diet composition, sedentary life, particularly a decrease in occupational physical activity or without much physical activities or both.10 However, metabolic hypothesis that was put forward some time ago describes the development of obesity on the basis of ‘metabolic efficiency’ wherein an individual spends less energy for the daily needs and therefore stores the extra energy as fat, whereas a ‘metabolically inefficient’ person spends more energy on daily requirements and hence stored energy in the form of fat is less.11 This seems to be satisfactory and convincingly explains why some individuals on normal or low energy intakes also become obese at times. Since obesity affects cardiovascular, respiratory, reproductive, digestive, musculoskeletal and other systems of the human body along with metabolic dysfunctions such as lipotoxicity and diabetes mellitus in addition to development of various types of cancers, it is appropriately termed as ‘metabolic syndrome’ in recent years.12 Thus, obesity is a serious health risk factor.
Current Status of Adipose Tissue Physiology:
Though, obesity is a mismatch between energy intake and energy output, basically it results from dysfunction of both white and brown fat tissue. Adipose tissue, particularly white fat was a neglected subject till recently when leptin was identified in 1994.13 This can be substantiated with the following statement made in 1948; “It is to be borne in mind that we know little or nothing concerning the physiology of the tissue most directly concerned with the deposition of fat, namely the fat cells themselves”.14
White Adipose Tissue (WAT):
In recent years adipose tissue physiology has taken the center stage of research to address problems related to obesity, diabetes mellitus, insulin resistance, cancer, metabolic syndrome etc.15 It secretes 100 and over chemicals called adipokines. Multiple functions such as metabolic, immunologic, energy balance, cardiovascular, inflammatory and hormonal signaling have been assigned to these adipokines.16 Implications for a secretory role for WAT was long recognized in 1953 by Kennedy and in 1973 by Coleman when they showed the presence of a circulating factor acting on brain in the control of energy balance.17 However, after the discovery of leptin by Zang et al.13 in 1994, WAT is recognised as more than just a passive fat repository because of its endocrine secretions and is considered the biggest endocrine gland in humans. 17, 18
Listed alphabetically with abbreviations below in the box 1 are a few of the adipose tissue secretions8, 18, 19, 20, 21
Box 1.Some of the adipokines from WAT.
The location of each adipose tissue (subcutaneous or visceral) itself affects the endocrine function with an unique expression and secretion profiles indicating a high degree of heterogeneity and thereby contribute to disease processes. For example, IL-6 and PAI-1 are secreted more in visceral adipose tissue where as leptin and adinopectin are more from subcutaneous fat tissue. Endocrine secretions from the visceral fat depots are secreted into the portal system and have direct access to liver whereas subcutaneous fat depots secretions are put into the systemic circulation. Local sub-groups of WAT are distributed in visceral, muscle, epicardial, perivascular and kidney areas. Visceral WAT controls local and systemic inflammation while epicardial WAT controls local inflammation and chemotaxis. Muscle WAT is involved in insulin resistance and kidney WAT affects intravascular volume and hypertension. Perivascular WAT is mainly involved with atherosclerosis and hypertension. Such findings may suggest that adipose tissue is perhaps a group of similar but unique endocrine organs.20
Many of the secretions come from non-adipocytic portions of the adipose tissue such as vascular, stromal and connective tissues.22 Some of these secretions act through autocrine, paracrine and endocrine means. In addition, adipose tissue also expresses a number of receptors. Adipokines endocrine functions can be grouped mainly under the following headings. Adipokines associated with 1) Insulin sensitivity and Insulin resistance, 2) Lipid metabolism, 3) Hemostatic functions, 4) Adipose aromatase and intradipose glucocorticoids and steroid metabolism and 5) Other adipocyte proteins such as proteins related to energy balance, immune related proteins, proteins os RAS etc. The different roles of some of the adipokines is summarised in box 28, 18, 19, 20, 21
Box 2.Role of some adipokines.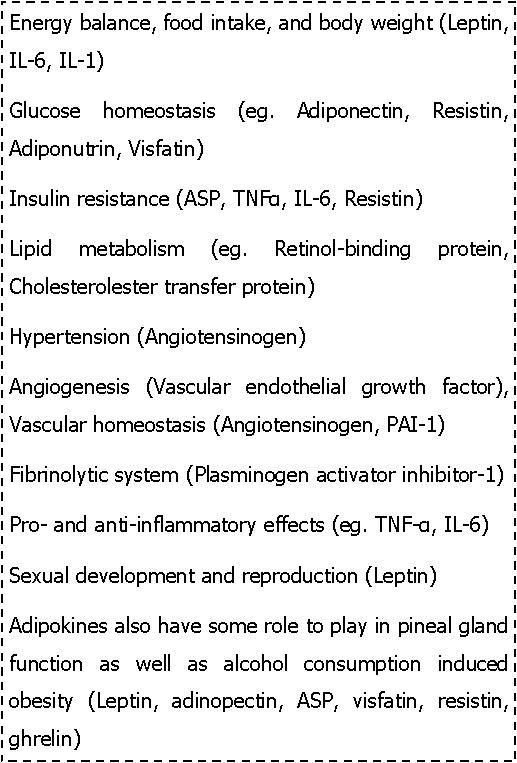
Physiological effects of some important adipokines is briefly considered in the following lines.
Leptin has a profound role in the regulation of whole body metabolism by stimulating energy expenditure through elevated sympathetic activity and by controlling the food intake via anorexigenic and orexigenic peptides at the hypothalamus level. In obesity, insulin resistance has been linked to leptin resistance and decreased plasma adiponectin which is reversed by simultaneous administration of leptin and adinopectin. It also plays a role in immune system modulation, sexual development and reproduction. Leptin is also secreted from placenta, gastric fundus mucosa, skeletal muscle and mammary gland.18, 19, 20
Adinopectin increases insulin sensitivity, fatty acid oxidation, energy expenditure and reduces the production of glucose by the liver. It has an anti-inflammatory, anti-atherogenic effect and is protective against insulin resistance and reduces the risks of coronary artery disease. It suppresses phagocytic activity and macrophage release of TNF-α, and transformation of macrophages to foam cells in vitro. It is also secreted by human cardiocytes and skeletal muscle18, 19, 20
IL-6is secreted by not only adipose tissue but also by brain tissue and skeletal muscle during physical acticity. IL-6 is pro-inflammatory and has a role in tissue injury, lipolysis and it reduces sensitivity to insulin. It decreases adinopectin secretion. But during physical activity, IL-6 secreted from skeletal muscle induces glucose uptake and lipolysis. IL-6 from central nervous system (CNS) decreases body weight and visceral fat by sympathetic activation through increased brown fat activity8, 19, 20, 21
An adipocyte renin-angiotensin system (ARAS) located in the intra adipose tissue regulates fat cell mass and energy stores through paracrine/autocrine effects on adipocyte differentiation and lipid storage.Angiotensinogen (AGT), renin, angiotensin-converting enzyme (ACE), angiotensin II (Ang II) and its receptors (AT1, AT2), and the non-renin-angiotensin enzymes chymase, cathepsins D and G and tonin are all expressed by adipose tissue. Adipose tissue Ang II controls terminal differentiation of preadipocytes to adipocytes through the action of prostacyclin (PGI2) and regulates adipose tissue blood supply. Adipose tissue AGT also influences adipocyte vascular resistance but negatively regulates fat mass by decreasing lipogenesis.It promotesinflammation and insulin resistance and hypertension.However, in humans the role of ARAS in the relation to obesity and hypertension remains to be further explored8, 19, 20, 21.In box 3 , the major biological effects of the other adipokines are listed.16, 18, 19, 20, 21
Box 3.Biological effects of some other adipokines.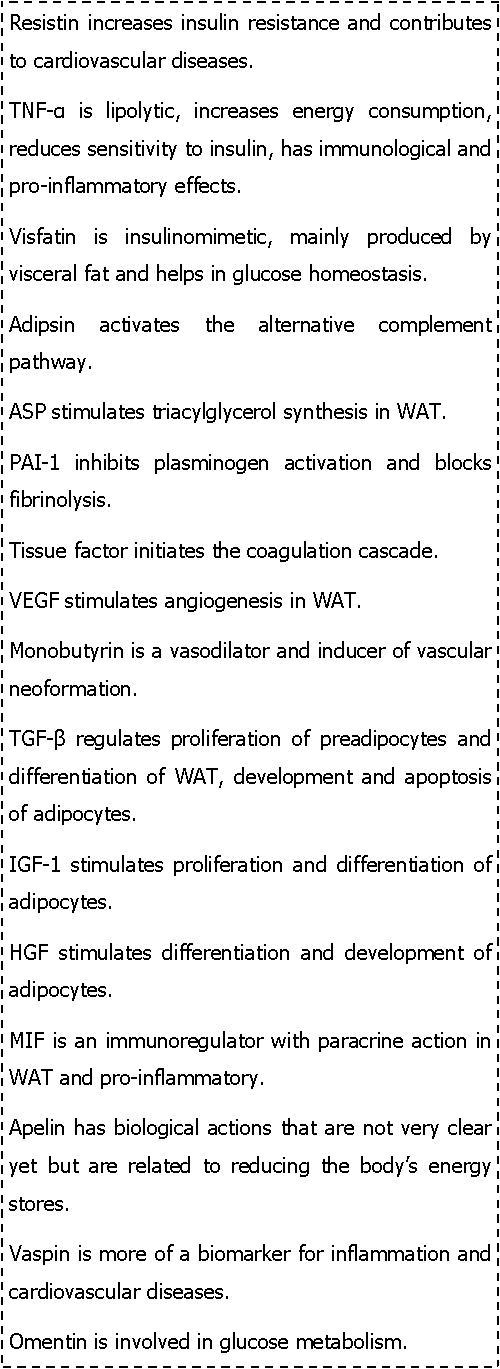
In general, most adipokines affect insulin, glucose metabolism and lipid metabolism negatively leading to development of metabolic syndrome features such as increased adiposity, hypertension, diabetes mellitus, cardiovascular effects and inflammatory process with other manifestations.8, 16Since obesity, hypertension and diabetes mellitus are closely related, the sequence of events linking obesity with insulin resistance and development of hypertension could be explained as follows. Adipocyte hypertrophy in obesity leads to adipocyte stress, possibly involving hypoxia that favours the production of chemo-attractants. This inturn promotes chemo-attraction and infiltration of macrophages into the adipocytes which may lead to the death of adipocytes on reaching the critical size. In such a situation, chronic reabsorption of adipocyte remnants by macrophages is favoured accompanying a massive production of cytokines by macrophages. This would lead to the increased cytokines such as TNFα, resistin, leptin and a decrease in adinopection resulting in reduced insulin sensitivity, endothelial dysregulation leading to atherosclerosis, thrombosis, sodium retention and increased sympathetic activation23, 24. Such changes will contribute to the development of hypertension and insulin resistance in peripheral tissues.25, 26
The details of lipogenesis, lipolysis and the factors controlling adipogensis is well explained in a review article by Proenca et al.16
Brown Adipose Tissue (BAT):
BAT is a multi-depot organ found generally in inter-scapular, subscapular, axillary, paravertebral, mediastinal, periaortic areas and other regions including neck, intercostal vessels, mammary vessels, suprarenals and greater omentum.27 Its major function is heat production (thermogenesis) that is important for body temperature regulation especially in newborn infants, arousal from hibernation in small mammals, energy balance and body weight regulation in adult humans.28 Such thermogenic functions of BAT is referred to as NST, DIT, CIT or adaptive thermogenesis or metaboloregulatory thermogenesis. It has rich sympathetic innervation and vascularization. Sympathetic innervation helps to a great extent in BAT recruitment and stimulation in CIT.29, 30 BAT also secretes a few hormone like substances (for example IL-6) called batokines just as the WAT secretes adipokines.31 BAT is of two types; a) classical one found in the above mentioned areas while type b) inducible/brite/beige/recruitable type of brown fat cells scattered within the WAT depots which can be found under different conditions such as chronic cold exposure, β-adrenergic receptor agonists treatment or peroxisome proliferator activated receptor gamma (PPAR gamma) treatment or chronic high fat diet feeding.32, 33 However, it is important to note that the adipose tissue has the ability of plasticity and trans-differentiation that contributes to the above phenomenon. Physiologically, sympathetic activation can be considered the ultimate route of BAT stimulation in its thermogenic function.31, 34
As far as the characteristics of WAT and BAT are considered a few major ones can be listed as follows. WAT is unilocular adipocyte (200µm diameter) with lipid storage and mobilization (+++), mitochondria (+), fatty acid oxidation (+), respiratory chain (+), UCP1 (0), PPAR-ˠ coactivator 1α (PGC-1α) (+) while the BAT has the following features; multilocular adipocytes, lipid storage and mobilization (++), mitochondria (+++), fatty acid oxidation (+++), respiratory chain (+++), UCP1 (+++), PGC-1α (+++).35, 36
Thermogenesis in the BAT can be explained in brief as follows. BAT is like a burner for the heater. In other words, the function of BAT is to transfer energy from food to heat. The mitochondria in a eukaryotic cell utilize fuels to produce energy in the form of ATP. In the brown fat mitochondria, inward proton conductance that generates ATP (oxidative phosphorylation) takes place as usual, but in addition there is a second proton conductance (proton run back) that does not generate ATP. This alternative route or ‘short-circuit’ conductance depends on a 32-kDa uncoupling protein (UCP), now called UCP1 (previously known as thermogenin) present in the inner membrane of the mitochondria. This protein causes uncoupling of metabolism and generation of ATP so that more heat is produced.37, 38
The origin of adipode tissue is very interesting to note as both WAT and BAT and other tissues are believed to arise from a common pluripotent stem cell that gives rise to a mesenchymal precursor cell which has the potential to develop into myoblast, chondroblast, osteoblast and adipocyte. As far as origin of brown adipocytes, it is understood that classical BAT are derived from myogenic factor 5 (Myf 5+) positive progenitor cells similar to skeletal myocytes whereas brite adipocytes have been shown to originate from Myf-negative (Myf 5−) progenitor cells much like white adipocytes. Interestingly, the browning of WAT may also involve trans-differentiation of white-to-brown adipose cells.39, 40, 41
Of late, four areas of developments in BAT research have kindled a lot of interest and hopes in the treatment of obesity and related co-morbidities. These include identification of i) active and functional BAT in adult humans, ii) classical and beige /brite types of BAT cells, iii) a number of regulatory factors involved in the control of BAT development and function and iv) a cross-talk between BAT and other peripheral tissues such as skeletal muscle, liver, heart, gut etc.28
Positron-emission tomography and computed tomography (PET-CT) scanning, magnetic resonance imaging (MRI) and magnetic resonance spectroscopy (MRS), infra-red thermography, enzyme/immuno-histochemistry, radio-immunoassay, blood flow measurements, measurement of noradrenaline stimulated oxygen consumption and so on have provided undeniable evidences for the existence of active and functional BAT in humans. The presence of BAT in humans is well confirmed using radioactive glucose analog18F-flurodeoxyglucoe (18-FDG) with PET scanning.42, 43
Thus, both WAT and BAT have become the target to explore the pathophysiology of obesity and its related metabolic disturbances.
Control of BAT Function:
BAT not only plays an important role in energy expenditure through its adaptive thermogenic function in body weight regulation and obesity control but also in insulin mediated glucose disposal.31 BAT metabolism is affected by various physiological factors such as age, gender, BMI, body fat levels and most importantly ambient temperature (cold exposure) and other disease conditions such as diabetes mellitus.34 Malfunctions of the BAT due to hypoxia and hypoglycemia or chronic down regulation of BAT activity can lead to obesity and other metabolic disturbances.44
Extraordinary efforts are made by using cellular, molecular, physiological, pharmacological and clinical approaches to investigate the role of brown fat functions and their regulation.16 Innumerable factors both positive and negative that regulate BAT development and functions can be broadly divided into three groups. 1. Multiple transcriptional factors 2. Signaling pathways and 3. Endocrine factors. Some have been considered briefly here below and further details are available elsewhere.25, 28, 36, 40, 45, 46, 47, 48
Transcriptional Factors.25 28 45 47 48
Adipogenesis consists of growth arrest, clonal expansion and terminal differentiation which are governed by four key transcription factors. These include three CCAAT enhancing binding proteins (C/EPB) ˠ, β and δ and PPAR-ˠ. C/EBPα is required for WAT formation while C/EBPβ plays an important role in regulating thermogenic gene expression in BAT. PPAR-ˠ is a master transcription factor required unconditionally for adipogenesis. Its transcriptional activity is regulated at multiple levels. Another factor, forkhead box C2 (FoxC2) induces the formation of brite cells in WAT with increased mitochondria and thermogenic genes and UCP1.
PGC-1α is a cold inducible transcriptional coactivator of PPARˠ found in brown fat. It regulates mitochondrial biogenesis and oxidative metabolism in BAT. Several transcriptional factors such as receptor-interacting protein-140 (RIP140) and the steroid receptor co-activator SRC2/TIF2/GRIP1 function by modulating the activity of PGC-1α.
PRD1-BF-1-RIZ1 homologous domain-containing protein 16 (PRDM16) is a 140kDa zinc-finger protein that is highly expressed in BAT. It is identified as a molecular switch between myocytes and brown adipocytes. It increases the transcriptional activities of PGC-1α and PPARˠ and the C/EPBs through direct interactions thereby promoting brown fat gene expression, mitochondrial biogenesis and increased cellular respiration. Several regulators such as early B cell factor 2 (EBF2) and microRNAs such as miR-133 and miR-193b are shown to influence PRDM16 activities.
Signaling Pathways:
Norepinephrine influences the proliferation of classical brown adipocyte precursors and mediates thermogenic function via β-1 adrenergic receptor and β-3 adrenergic receptor, respectively. Similarly, nitric oxide signalling also activates cGMP dependent protein kinase in brown adipocytes thereby induces UCP1 expression28.
Transient receptor potential vanilloid (TRPV) signaling involves ion channels that are of four types; TRPV1, TRPV2, TRPV3 and TRPV4. TRPV1 is activated by heat greater than 430C and pungent compounds in chilli peppers. Interestingly, non-pungent capsaicin analogs (capsinoids) activate gastrointestinal TRPV1 and induce thermogenesis in humans and rodents. TRPV4 expressed in adipocytes negatively regulate BAT thermogenic function. Inhibition of TRPV4 activates UCP1 and PGC1α expression in BAT. TRPV2 in BAT when absent or dysfunctional impairs thermogenesis in mouse brown adipose tissue and results in increased body weight and fat upon feeding high fat diet and can not keep constant body temperature when ecposed to cold at 40 C.49, 50
Endogeneous Hormones:
Other than thyroid hormones, catecholamines, glucocorticoids and mineralocorticoids, growth hormones and insulin like growth factor-1 (IGF-1), prolactin, insulin, sex hormones and some of the recently identified endogeneous molecules that control BAT functions.28, 36, 47
Transforming growth factors (TGF) include bone morphogenetic proteins (BMP) that influence BAT functions. BMP7 treatment of fibroblast cultures or adipogenic precursors induce brown fat regulators like PRDM16. BMP4 similarly activates beige cell differentiation in WAT. BMP8 acts on mature BAT and hypothalamus and positively regulate BAT thermogenesis. Other members of TGF-β family such as myostatin negatively regulate BAT differentiation and thermogenesis.40, 51, 52
FGFs are different types and act in an autocrine or paracrine fashion. FGF-21 is secreted from liver and both FGF-19 and FGF-21 promote BAT thermogenic functions. But FGF-19 and FGF-23 are endocrine forms.53, 54They increase the energy expenditure by elevating the metabolic rate and reduce body fat mass partly by activating BAT thermogenesis. FGF-21 which is expressed in BAT and WAT in response to cold exposure also induces brite cells in WAT. It appears that PPAR-gamma transcriptionally controls FGF-21. These are indicators that FGF-21 in adipose tissue may be important and effective in treating obesity, insulin resistance and diabetes.28, 46
Fibroblast Growth Factors (FGF).
Irisin is a polypeptide released by skeletal muscle through increased PGC-1α expression following exercise in both rodents and humans. Irisin showed a powerful browning effect on WAT in mice both in culture and in vivo. Human irisinin healthy adult subjects showed a 2-fold increase in plasma levels following 10 weeks of supervised endurance exercise training as compared to the non-exercised state. Irisin treatment induces a thermogenic gene program in BAT and protects the animals from diet induced obesity. 55, 56
Cardiac natriuretic peptides (CNP) from atria and ventricle of the heart (ANP, VNP, respectively) and brain natriuretic peptides (BNP) are important endocrine regulators of fluid and hemodynamicfunctions. Cold exposure seem to improve the circulating levels of ANP and BNP and administration of BNP activates the BATthermogenic functions. But, one has to be careful about their effects of on fluid and hemodymanic functions in such situations.57 Prostaglandins (PGs) and vascular endothelial growth factor (VEGF) also play an important role in cold induced brite cell formation, BAT thermogenesis and energy expenditure.58
Interorgan Networking Or Cross-Talk:
BAT mediated interorgan networking in understanding the functional control of BAT is another interesting facet. For example, norepinephrine produced in the CNS and by the macrophages in the adipose tissue is shown to activate the brown fat development and thermogenic function. TGF- β from the skeletal muscle negatively controls BAT function. As stated earlier, irisin, CNP and IL-6 are also important mediators of BAT function.16, 28, 46, 59
Cold exposure not only stimulates tri-iodothyronine (T3) secretion but also releases norepinephrine at sympathetic nerve endings that are plenty in BAT. Such acute cold exposure released norpeinephrine acts via β-3 receptors to increase BAT thermogenic functions. By virtue of a high endogenous expression and activity of intracellular type-2 5’-deiodinase (D2), BAT has the ability to increase substantially the intracellular concentrations of T3 without affecting its circulating concentrations. This will increase the synthesis of UCP1 which turn facilitates the mitochondrial membrane to become leaky to protons rendering the respiration process inefficient.60, 61Bile acid from liver is also reported to activate D2 in BAT thereby promoting thermogenesis thus acting as an endocrine signaler.62
Brown adipocyte stem/progenitor cells CD34+ in skeletal muscle and human multi-potent adipose derived stem cells (hMADs) in subcutaneous tissue can potentially be induced into BAT externally, expanded and implanted back. Available data suggests that subcutaneous transplantation of embryonic BAT corrected type 1 diabetes in immune-competent mice as was evident by reversal of diabetes symptoms, weight regain and normalization of glucose tolerance.63 Other than these, transplantation of BAT into the interabdominal regions has shown improvements in whole body insulin sensitivity in mice. In mice, it is shown that transplantation of fibroblasts expressing PRDM16 and C/EPBs gives rise to fat pads that resemble BAT.64
Along with so many factors as pointed ot above, thecontrolof BAT functions can also be brought about by repressing the translation of PGC1α, inhibition of retinoblastoma (Rb) protein activity, inhibition of RIP140, inhibition of the gender-sensitive α-arrestin domain-containing 3 (ARRDC3) protein, inhibition of retinaldehyde dehydrogenase 1 (ALDH1A1) and so on.25
There are reports on animal experiments that have shown an over-expression of monoacylglycerol lipase (MGL) in the forebrain of transgenic mice that decreases the production of 2-arachidonoyl-sn-glycerol (2-AG) resulting in leanness, increased energy cost of activity, enhanced thermogenesis in BAT suggesting the critical role of endocannabinoid system. In another study, favorable inflammation profile with a lower C-reactive protein (CRP) and alpha-1 antitrypsin level has shownthat CRP could play a protective role against obesity and cardiometabolic risks. Yet, another study claims that obese mice lacked a protein called collagen VI fared much better metabolically.65, 66, 67
Treating Obesity:
Different strategies for treating obesity such as modifying diet, increasing physical activity (behavioral approach), utilizing weight loss medications (pharmacological approach), cold exposure (physiological approach) as well as recommending surgical procedures in appropriate patients could be considered.68, 69, 70, 71, 72The management of obesity through lifestyle is quite challenging. Recently, sibutramine and rimonabant (anti-obesity drugs) are withdrawn for safety concerns.71, 72, 73Despite its effectiveness as a weight loss intervention, bariatric surgery is only applicable to a sub-group of obese patients and as such does not represent a global practical solution.72, 74, 75 Given the limitations of available therapies and ever increasing incidence of obesity-related issues, it is important to identify new and effective therapeutic options for obesity. Increasing energy expenditure through peripheral mechanisms appears very attractive. Data from animal studies have demonstrated that by activating BAT, triglyceride stores within WAT could be utilized for heat production through modulation of adaptive thermogenesis.76
As pointed out earlier, it is suggested that transcriptional factors such as PRDM16 and other associated co-regulators PGC -1α and C-terminal binding protein (CtBP1/2) which controls the switch from WAT to BAT are potential targets for the development of obesity related treatments.77, 78, 79 In addition, brown fat development and functions are regulated by several hormonal factors derived from central and peripheral tissues such as liver, skeletal muscle, heart, and immune cells. Adult human BAT can be recruited by chronic cold exposure and TRPV1 agonists. The observation that cold exposure stimulates BAT thermogenesis has become handy at a time when other forms of obesity treatment (behavioral, pharmacological and surgical) have not found great success.28, 36, 46, 47, 59Cold stimulation is regarded the best potent activator of BAT as of date and safest and most effective method as compared with β-agonist or symapthomimetic stimualtions.31, 80
Studies on cold exposure in BAT research is varied and and less standardized. BAT utilises both carbohydrates and lipids during cold exposure to increase thermogenesis via β-3 adrenergic receptor. The cold exposure methods used include air exposure in environmental chambers, water immersion, liquid-conditioned suit, limb cooling methods, individualized cooling and water perfused mattress or garment. The temperature used also varies from 16-190C. Water cooling has been suggested as the most preferred method. But, the individualized cooling at the lowest temperature for non-shivering conditions may be the most promising approach. Though, this area of cold exposure treatment looks a natural way, it needs proper design and standardised protocols to optimise the benefits before conclusively pronouncing it as an efficient method.34
Similar to cold exposure, exercise and use of nonpungent capsaicin in diet are known to stimulate BAT thermogenesis by enhancing mitochondrial UCP1 production to promote the heat production. Of late, very interestingly, the role played by the gut microbiome in energy balance is reported. It has been shown that there is an intimate relationship between the gut flora and BAT thermogenesis. It is possible that the bacteria in the gut flora is affected by the diet and may alter the metabolic functions of the host. Cold exposure in such situations can influence the microbial flora and help losing the extra fat as well as improve the gastrointestinal absorptive functions.81, 82
Therefore, it appears that several communication pathways linking the brain, skeletal muscle, gut and other organs including the heart and liver involve short-term and long-term signals that may help to balance the energy intake and expenditure. Thus, BAT being a major site for mammalian non-shivering thermogenesis could be a promising target for prevention and treatment of human obesity.36, 45, 47, 60, 69, 70
Conclusion:
To address obesity and related problems, reducing total adipose mass is the important strategy. It may be achievable by considering various ways and means. For example, virtually all known adipose tissue secreted proteins are dysregulated when the WAT mass is markedly altered; either increased in the obese state or decreased in lipoatrophy. Therefore, an in-depth understanding of molecular actions of adipokines and modifying adipokine gene expression, targeting the mechanisms involved in macrophage recruitment, understanding the signaling pathways in target tissues such as the brain, skeletal muscle and elsewhere would certainly provide some ways of dealing with the problem. But, the key challenges will be to identify all of the secreted proteins by WAT to establish the function of each secreted protein and to assess the pathophysiological consequences of changes in adipocyte protein production.
Recent observations of BAT being functional in adult humans provides a rationale for its stimulation to increase energy expenditure through adaptive thermogenesis for an anti-obesity strategy. A large body of data available suggest that targeting endocrine hormones for BAT modulation can yield a positive answer for successful prevention and management of human obesity. Therefore, further understanding of the physiological link between various endocrine/autocrine/paracrine hormones and BAT is necessary for the development of new therapeutic options.
However, there are some suggestions that one should take in treating all obese individuals as there are some divides in opinion. Human studies have shown that some groups of individuals called ‘healthy obese’ who can maintain cardiovascular and metabolic health are genetically programmed to carry extra fat. Some poorly understood or entirely unknown benefits of mild or moderate obesity may be in-built for them and one has to be very cautious in weight loss goals in such individuals. It is better to be overweight or even obese and physically active rather than sedentary and normal weight as the goal of optimum health is not to be ‘thin’ but to be as ‘healthy’ as one can regardless of body weight. Therefore, obesity treatment should be directed at the metabolic syndrome per se and not at the high levels of body fat.83
All in all, obesity is a multidimensional metabolic disorder and is rarely cured once it sets in. Hence, long term treatment should be planned with combined therapy that involves behavioural and physiogical approaches for effectiveness. It may be good to avoid pharmacological and surgical approaches in such situations. Overall, the scientific world eagerly awaits further advanced studies to document possible metabolic interventions using BAT as a primary target to prevent and manage obesity.84
References
- 1.Helmchen L A, Henderson R M. (2004) Changes in the distribution of body mass index of white. , US men,1890-2000.Ann Hum Biol 31, 174-81.
- 2.Hippocrates A F.(1849) The genuine works of Hippocrates. Originally published by the Sydenham society, London, English version published. in 1886. W. Wood and company , New York .
- 6.Gregor M F, Hotamisligil G S. (2011) Inflammatory mechanisms in obesity. , Annu Rev Immunol 29, 415-45.
- 7.Spiegelman B M, Flier J S. (2001) Obesity and the regulation of energy balance. , Cell 104, 531-43.
- 8.Galic S, Oakhill J S, Steinberg G R. (2010) Adipose tissue as an endocrine organ. , Mol Cell Endocrinol 316, 129-39.
- 9.Kevin D H, Heymsfield S B, Kemnitz J W, Klein K, Schoeller D A et al. (2012) Energy balance and its components: implications for body weight regulation. , Am J Clin Nutr 95, 989-94.
- 10.Hill J O, Melanson E L. (1999) Overview of the determinants of overweight and obesity: current evidence and research issues. Med Sci Sports Exerc;31(11Suppl):. 515-21.
- 12.Grundy S M, Brewer HB Jr, Cleeman J I, Smith SC Jr, Lenfant C. (2004) Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. , Circulation 109, 433-38.
- 13.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L et al. (1994) Positional cloning of the mouse obese gene and its human homologue. , Nature 372, 425-32.
- 16.ARG Proença, RAL Sertié, Oliveira A C, Campaãa A B, Caminhotto R O et al. (2014) New concepts in white adipose tissue physiology. , Braz J Med Biol Res 47, 192-205.
- 17.Li Min-Dian. (2011) Leptin and beyond: an odyssey to the central control of body weight. , Yale J Biol Med 84, 1-7.
- 18.Fischer-Posovszky P, Wabitsch M, Hochberg Z. (2007) Endocrinology of adipose tissue - An update. Horm Metab Res. 39, 314-21.
- 19.Kershaw E E, Flier J S. (2004) Adipose tissue as an endocrine organ. , J Clin Endocrinol Metab 89, 2548-556.
- 20.Wozniak S E, Gee L L, Wachtel M S, Frezza E E. (2009) Adipose tissue: the new endocrine organ? A review article. , Dig Dis Sci 54, 1847-856.
- 22.Fain J N, Madan A K, Hiler M L, Cheema P, Bahouth S W. (2004) Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. , Endocrinology 145, 2273-282.
- 23.Qatanani M, Lazar M A. (2007) Mechanisms of obesity-associated insulin resistance: many choices on the menu. , Genes & Development 21, 1443-455.
- 24.Carmela R B, Calogero C, Giuseppina C. (2010) The role of adipose tissue and adipokines in obesity-related inflammatory diseases. Mediators of Inflammation.Volume2010ArticleID802078,19pages.
- 27.Sacks H, Symonds M E. (2013) Anatomical locations of human brown adipose tissue: functional relevance and implications in obesity and type 2 diabetes. , Diabetes 62, 1783-790.
- 28.Kajimura S, Saito M. (2014) A new era in brown adipose tissue biology: molecular control of brown fat development and energy homeostasis. , Annu Rev Physiol 76, 225-49.
- 29.Bartness T J, Vaughan C H, Song C K. (2010) Sympathetic and sensory innervation of brown adipose tissue. , Int J Obes 34-36.
- 30.Himms-Hagen J. (1991) Neural control of brown adipose tissue thermogenesis, hypertrophy and atrophy. , Front Neuroendocrinol 12, 38-93.
- 31.Cannon B, Nedergaard J. (2004) Brown adipose tissue: function and physiological significance. , Physiol Rev 84, 277-359.
- 32.Harms M, Seale P. (2013) Brown and beige fat: development, function and therapeutic potential. , Nat Med 19, 1252-263.
- 33.García-Ruiz E, Reynés B, Díaz-Rúa R, Ceresi E, Oliver P et al. (2015) The intake of high-fat diets induces the acquisition of brown adipocyte gene expression features in white adipose tissue. , Int J Obes 39, 1619-1629.
- 34.AA van der Lans, Wierts R, Vosselman M J, Schrauwen P, Brans B et al. (2014) Cold-activated brown adipose tissue in human adults: methodological issues. , Am J Physiol Regul Integr Comp Physiol; 307, 103-13.
- 35.Muralidhara D V, Krithika D M. (2011) Recent advances in human brown fat physiology. , Indian J Physiol Pharmacol 55, 197-206.
- 36.Reddy N L, Tan B K, Barber T M, Randeva H S. (2014) Brown adipose tissue: endocrine determinants of function and therapeutic manipulation as a novel treatment strategy for obesity.doi: 10.1186/s40608-014-0013-5. , Publishedonline2014Aug22.BMCObes; 1, 13.
- 38.Chechi K, Carpentier A C, Richard D. (2013) Understanding the brown adipocyte as a contributor to energy homeostasis. , Trends Endocrinol Metab 24, 408-20.
- 39.Elabd C, Chiellini C, Carmona M, Galitzky J, Cochet O et al. (2009) Human multipotent adipose-derived stem cells differentiate into functional brown adipocytes. , Stem Cells 27, 2753-760.
- 40.Tang Q Q, Lane M D. (2012) Adipogenesis: from stem cell to adipocyte. , Annu Rev Biochem 81, 715-36.
- 41.Timmons J A, Wennmalm K, Larsson O, Walden T B, Lassmann T et al. (2007) Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci. 104, 4401-406.
- 42.Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T et al. (2009) High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. , Diabetes 58, 1526-531.
- 43.Virtanen K A, Lidell M E, Orava J, Heglind M, Westergren R et al. (2009) Functional brown adipose tissue in healthy adults. , N Engl J Med 360, 1518-525.
- 44.Trayhurn P, Alomar S Y. (2015) Oxygen deprivation and the cellular response to hypoxia in adipocytes – perspectives on white and brown adipose tissues in obesity. doi: 10.3389/fendo.2015.00019. Front Endocrinol (Lausanne);. 6, 19.
- 45.Lee Y H, Jung Y S, Choi D. (2014) Recent advance in brown adipose physiology and its therapeutic potential. doi: 10.1038/emm.2013.163. Exp Mol Med. 46, 78.
- 46.Poher A, Altirriba J, Veyrat-Durebex C, Rohner-Jeanrenaud F. (2015) Brown adipose tissue activity as a target for the treatment of obesity/insulin resistance. Publishedonline2015Jan30. doi: 10.3389/fphys.2015.00004. Front Physiol 6, 4.
- 47.Zafrir B. (2013) Brown adipose tissue: research milestones of a potential player in human energy balance and obesity. Horm Metab Res. 45, 774-85.
- 48.Hondares E, Rosell M, Díaz-Delfín J, Olmos Y, Monsalve M et al. (2011) Peroxisome proliferator-activated receptor α (PPARα) induces PPARγ coactivator 1α (PGC-1α) gene expression and contributes to thermogenic activation of brown fat: involvement of PRDM16. , J Biol Chem 286, 43112-122.
- 49.Galgani J E, Ryan D H, Ravussin E. (2010) Effect of capsinoids on energy metabolism in human subjects. , Br J Nutr 103, 38-42.
- 50.Saito M, Yoneshiro T. (2013) Capsinoids and related food ingredients activating brown fat thermogenesis and reducing body fat in humans. , Curr Opin Lipidol 24, 71-77.
- 51.Modica S, Wolfrum C. (2013) Bone morphogenic proteins signaling in adipogenesis and energy homeostasis. Biochim Biophys Acta.1831:. 915-23.
- 52.Koncarevic A, Kajimura S, Cornwall-Brady M, Andreucci A, Pullen A et al. (2012) A novel therapeutic approach to treating obesity through modulation of TGFβ signaling. , Endocrinology 153, 3133-46.
- 53.Fisher F, Kleiner S, Douris N, Fox E, Mepani R et al. (2012) FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. , Genes Dev 26, 271-81.
- 54.Beenken A, Mohammadi M. (2009) The FGF family: biology, pathophysiology and therapy. , Nat Rev Drug Discov 8, 235-53.
- 55.Pedersen B K, Febbraio M A. (2012) Muscles, exercise and obesity: skeletal muscle as a secretory organ. , Nat Rev Endocrinol 8, 457-465.
- 56.R De Matteis, Lucertini F, Guescini M, Polidori E, Zeppa S et al. (2013) Exercise as a new physiological stimulus for brown adipose tissue activity. Nutr Metab Cardiovasc Dis. 23, 582-90.
- 57.Ramos H R, Birkenfeld A L, A J de Bold. (2015) Interacting disciplines: cardiac natriuretic peptides and obesity: perspectives from an endocrinologist and a cardiologist. Endocr Connect. 4, 25-36.
- 58.Elias I, Franckhauser S, Ferré T, Vilà L, Tafuro S et al. (2012) Adipose tissue overexpression of vascular endothelial growth factor protects against diet-induced obesity and insulin resistance. , Diabetes 61, 1801-813.
- 59.Trayhurn P, Drevon C A, Eckel J. (2011) Secreted proteins from adipose tissue and skeletal muscle - adipokines, myokines and adipose/muscle cross-talk. , Arch Physiol Biochem 117, 47-56.
- 60.Butler P W, Mentuccia D, Celi F S. (2010) Stimulating brown fat: a potential future therapeutic approach for obesity and insulin resistance?. , Heart Metab; 48, 19-22.
- 61.Ouellet V, Labbe S M, Blondin D P, Phoenix S, Guerin B et al. (2012) Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. , J Clin Invest 122, 545-52.
- 62.Watanabe M, Houten S M, Mataki C, Christoffolete M A, Kim B W et al. (2006) Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. , Nature 439, 484-489.
- 63.Gunawardana S C, Piston D W. (2012) Reversal of type 1 diabetes in mice by brown adipose tissue transplant. , Diabetes 61, 674-82.
- 64.Stanford K I, RJW Middelbeek, Townsend K L, An D, Nygaard E B et al. (2013) Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. Published online 2012 Dec 10. doi: 10.1172/JCI62308.J Clin Invest; 123, 215-223.
- 65.Jung K M, R C Jason, Fu Jin, D’Agostino Giuseppe, Ana G et al. (2012) 2-arachidonoylglycerol signaling in forebrain regulates systemic energy metabolism. , Cellmetabolism; 15, 299-310.
- 66.Karelis A D, Faraj M, Bastard J P, St-Pierre D H, Brochu M et al. (2005) The metabolically healthy but obese individual presents a favorable inflammation profile. , J Clin Endocrinol Metab 90, 4145-50.
- 67.Khan T, Muise E S, Iyengar P, Wang Z V, Chandalia M et al. (2009) Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. , Mol Cell Biol 29, 1575-91.
- 68.Wadden T A, Webb V L, Moran C H, Bailer B A. (2012) Lifestyle modification for obesity: new developments in diet, physical activity, and behavior therapy. , Circulation.125: 1157, 1170.
- 69.Yoneshiro T, Aita S, Matsushita M, Kayahara T, Kameya T et al. (2013) Recruited brown adipose tissue as an antiobesity agent in humans. , J Clin Invest 123, 3404-408.
- 70.Cypess A M, Kahn C R. (2010) Brown fat as a therapy for obesity and diabetes. Curr Opin Endocrinol Diabetes Obes. 17, 143-149.
- 71.Kaplan L M. (2005) Pharmacological therapies for obesity. , Gastroenterol Clin North Am 34, 91-104.
- 72.Holly R W. (2013) Update on treatment strategies for obesity. Published online2013Feb26.doi: 10.1210/jc.2012-3115.J Clin Endocrinol Metab. 98, 1299-1306.
- 74.Schauer P R, Kashyap S R, Wolski K, Brethauer S A, Kirwan J P et al. (2012) Bariatric surgery versus intensive medical therapy in obese patients with diabetes. , N Engl J Med 366, 1567-576.
- 75.Sjöström L, Peltonen M, Jacobson P, Sjöström C D, Karason K et al. (2012) Bariatric surgery and long-term cardiovascular events. , JAMA 307, 56-65.
- 76.WD van Marken Lichtenbelt, Schrauwen P. (2011) Implications of nonshivering thermogenesis for energy balance regulation in humans. , Am J Physiol Regul Integr Comp Physiol; 301, 285-296.
- 77.Seale P, Kajimura S, Spiegelman B M. (2009) Transcriptional control of brown adipocyte development and physiological function of mice and men. , Genes Dev 23, 788-97.
- 78.Kajimura S, Seale P, Spiegelman B M. (2010) Transcriptional control of brown fat development. , Cell Metab 11, 257-62.
- 79.Sanchez-Gurmaches J, Guertin D A. (2014) Adipocyte lineages: tracing back the origins of fat. , Biochim Biophys Acta 1842, 340-51.
- 80.Blondin D P, Labbé S M, Turcotte E E, Haman F, Richard D et al. (2015) A critical appraisal of brown adipose tissue metabolism in humans. DOI 10.2217/clp.15.14 (doi: 10.2217/clp.15.14) , Clin Lipidol 3, 259-80.
- 82.Yi C X, Tschöp M H. (2012) Brain-gut-adipose-tissue communication pathways at a glance. , Dis Model Mech 5, 583-87.
