Abstract
Introduction
Antimicrobial stewardship (ASP) is of the utmost importance as a way to optimize the use of antimicrobials to prevent the development of resistance and improve patient outcomes. So, it is worthwhile to assess the knowledge, attitude and awareness regarding antimicrobial stewardship in hospitals.
Objective
The aim of this study is to assess knowledge, attitudes and practices (KAP) of prescribers towards antimicrobial stewardship at hospitals in Khartoum state and to identify the associations between prescriber’s demographic information and their knowledge. Methodology This descriptive cross-sectional study multi-centered study conducted in 10 hospitals at Khartoum state -Sudan, during period from November to December 2018. Study population included all prescribers who is available at study’s hospitals during study period and willing to participate in the study. A self-administered questionnaire addressing participants’ knowledge, attitudes, and practice (KAP) regarding antibiotic resistance and ASP distributed in the selected hospitals among attending house-officers, registrars and consultants completed then analyzed.
Results
Of the 294 medical staff targeted, 287 responded to the survey (response rate 97.6%). Only (26.4%) were familiar with the term ASP and (31.5%) claimed that it is effective in reducing resistance. (43.0%) of respondents believe that ASP play vital role on antibiotic prescribing. Only (9.5%) had ASP in their hospital and (13.5%) having policy and team. (45.3%) of participants had good level of knowledge about antimicrobial stewardship, but majority show negative attitude (63.1%), and poor practices (92.0%) regarding ASP. There was no observed correlation between knowledge and attitude, knowledge with practice (p-value ≥ 0.05). Only attitude with practice shows significance correlation (P=0.0001), which means that prescribers with positive attitude had the better practices towards antimicrobial stewardship. Age, occupation and experience are the only significant predictors of prescriber's knowledge and attitude towards antibiotic stewardship, while no association between these factors and practice.
Conclusion
The present study concludes that the knowledge of prescribers regarding ASP is moderate and their attitude is negative. Unfortunately, practices regarding ASP were poor, despite, the good knowledge regarding the effects of ASP on antimicrobial resistance.
Author Contributions
Academic Editor: Lucio Mango, University of International Studies (UNINT) – Rome, Italy.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2020 Alneima Salah Ali Alamin, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Antimicrobial resistance (AMR) is increasing; however, antimicrobial drug development is slowing. In the early days of antibiotics, booming drug development meant that even when resistance developed, a new drug was always available to treat the increasingly resistant bacteria. However, the pace of antimicrobial drug development has slowed dramatically, with only a handful of new agents, few of which are novel, being introduced into clinical practice each year1,2 The reasons for this are simple: drug development is risky and expensive, and drugs to treat infections are not as profitable as those that treat chronic disease3. Furthermore; the profit available from such development might be threatened by policies aiming to contain resistance by reducing the consumption of antimicrobials (profit is a function of price and volume)4. The absence of guidelines of antibiotic use, protocols for national therapeutic and infection control committees have led to overuse and misuse of antimicrobials in health care settings, resulted in the emergence of antibiotic-resistant pathogens, fueling an ever-increasing need for new drugs.5 Until this next giant step is achieved by developing new drugs; we have a great job to do: conserve the antibiotics we have and reduce its inappropriate use thought to be the best way to control its negative consequences. Now more than ever before, antimicrobial stewardship is of the utmost importance as a way to optimize the use of antimicrobials to prevent the development of resistance and improve patient outcomes1,6. Antibiotic stewardship (ASP) has been defined in a consensus statement from the Infectious Diseases Society of America (IDSA), the Society for Healthcare Epidemiology of America (SHEA), and the Pediatric Infectious Diseases Society (PIDS) as “coordinated interventions designed to improve and measure the appropriate use of (antibiotic) agents by promoting the selection of the optimal (antibiotic) drug regimen including dosing, duration of therapy, and route of administration” 7. Effective antimicrobial stewardship programs, also known as antimicrobial management programs, can be financially self-supporting and can improve patient care8,9. Antimicrobial stewardship includes not only limiting inappropriate use but also optimizing antimicrobial selection, dosing, route, and duration of therapy to maximize clinical cure or prevention of infection while limiting the unintended consequences, such as the emergence of resistance, adverse drug events, and cost. Given the emergence of multidrug-resistant pathogens and their impact on clinical care, appropriate use of antimicrobial agents has become a focus of patient safety and quality assurance, along with medication errors, allergy identification, and drug-drug interactions. The ultimate goal of antimicrobial stewardship is to improve patient care and health care outcomes.10
It is essential that before implementing the antimicrobial stewardship program to create an effective team with a given budget and personnel constraints. Core members of a multidisciplinary antimicrobial stewardship team include an infectious diseases physician and a clinical pharmacist with infectious diseases training who should be compensated for their time, with the inclusion of a clinical microbiologist, an information system specialist, an infection control professional, and hospital epidemiologist being optimal11 An important first step in building an ASP should be to identify current institutional resistance patterns and antimicrobial use. Not all hospitals need the same level of interventions. ASP should be tailored to institutional problem pathogens and overuse of particular classes of drugs. Every institution should work within its resources does not have to fit a particular mold, but administrative support should be maintained for the necessary infrastructure to measure antimicrobial use and to track use on an ongoing basis.1
There are 2 major approaches to antimicrobial stewardship strategies, with the most successful programs generally implementing a combination of both 1/the front-end or pre-prescription approach which means that uses restrictive prescriptive authority, i.e. certain antimicrobials are considered restricted and require prior authorization for use by all except a select group of clinicians, and 2/the back-end or post prescription approach, uses prospective review and feedback. i.e. the antimicrobial steward reviews current antibiotic orders and provides clinicians with recommendations to continue, adjust, change, or discontinue the therapy based on the available microbiology results and clinical features of the case12,13. The following elements may be considered and prioritized as supplements to the core active antimicrobial stewardship strategies based on local practice patterns and resources; education,14 formulary restriction,15 antimicrobial cycling,16 antimicrobial order forms,17 streamlining or de-escalation of therapy,18, guidelines and clinical pathways19, pharmaco-dynamic dose optimization,20 and parenteral to oral conversion 21.
Several studies have documented the impact of different stewardship strategies in controlling specific resistance problems. Hermsen et al22 used a surgical prophylaxis order form to improve antibiotic choices, and demonstrated a significant increase in appropriate antimicrobial use, appropriate weight-based dosing, and appropriate duration of prophylaxis, as well as a decrease in the mean cost of antimicrobial prophylaxis. Al-Harthi et al.23 studied knowledge and perception regarding antimicrobial stewardship among clinicians in Jeddah, Saudi Arabia. Their findings revealed that although the concept of AMR is clear among general physicians, they lack consistent practice resources, while all participants in the study were also obliged to follow the guidelines for antimicrobials use. This study highlighted the need to adhere to ASP as a basic reference when prescribing antimicrobial agents. In Sudan a two years’ prospective quasi-experimental study shows that there is reduction in the incidence of infection and colonization in addition to amount of antibiotics utilized observed after implementation of antibiotic cycling, but with poor adherence by prescribers to stewardship program enforced in two surgical settings.24,25
Knowledge is the first step in modifying behavior in relation to physicians' adherence to clinical practice guidelines and behavior change based on influencing knowledge and attitude is probably most sustainable than indirect manipulation of behavior alone 27. Pathman et al. 28 described four steps in the process, stating that when physicians comply with practice guidelines they must first become aware of guidelines, then intellectually agree with them; decide to adopt them in the care they provide and then regularly adhere to them at appropriate time. If awareness of guidelines and procedures is deficient, it is unlikely that good practice will follow. The emergence of antibiotic resistance as a global problem underscores the need for physicians to be aware of its existence and factors that drives its development and also it is important to understand what physicians know about antimicrobial resistance and containment and how they acquire and maintain that knowledge28,29. Sudan as many developing countries suffering from misuse of antibiotics and emergence of resistance. Many publications have documented the irrational prescribing pattern of antibiotics in different settings and data confirms that resistance are high and rising.30,32 Relatively few studies of knowledge, attitude and practice are published regarding antimicrobial stewardship.33So, it is worthwhile to assess the knowledge, attitude and awareness regarding antimicrobial stewardship in hospitals. The aim of this study is to assess knowledge, attitudes and practices (KAP) of prescribers towards antimicrobial stewardship at hospitals in Khartoum state (Capital of Sudan) and to identify the associations between prescriber’s demographic information and their knowledge. In hospitals under the Sudanese Ministry of health (MOH), the implementation of ASP is not yet operational. The information obtained from this study can be useful in designing more effective antibiotic stewardship interventions and control programs in Sudanese hospitals.
Method
Study Design, Period, Setting and Participants
This descriptive cross-sectional study multi-centered study conducted in 10 hospitals (6 governmental and 4 private hospitals) at Khartoum state - Sudan, during period from November to December 2018. Study population included all prescribers who attending at study’s hospitals in Khartoum state during study period and willing to participate in the study. A sample size was calculated through OpenEpi software34 in which the population of doctors in public and private hospitals was taken as 8000; design effect was kept as 0.8, response distribution as 0.5, while confidence interval and margin of error was set at 95% and 5% respectively. A total of 294 physicians were needed to be sampled to generalize the findings. Eligible physicians included in the survey were house staff officers, registrars and consultants who were prescribe antibiotics in their clinical practice from internal medicine, surgery, pediatric, obstetrics and gynecology (Obs & Gyn). Medical doctors from psychiatry, radiology, ophthalmology and anesthesiology were not included as they prescribe antimicrobial agents less often than do other physicians.
Survey Instrument
A self-administered questionnaire was distributed in the selected hospitals among attending house-officers, registrars and consultants during morning sessions and grand rounds. The survey questionnaires were distributed and collected back by the main investigator. Each participant was asked to complete all sections of questionnaire. There was no incentive for subjects to participate and no reminders were supplied. The questionnaire content designed after a thorough literature review of the relevant published studies35,36but adopted to Sudanese system and modified for the purposes of our study. No pilot study conducted to validate our modified questionnaire. The questionnaire composed of 3 sections and 25Items addressing participants’ knowledge, attitudes, and practice (KAP) regarding antibiotic resistance and ASP. Section (A) of questionnaire recorded demographic characteristics of the participants, including sex, age, occupancy, specialty and year of experience, in addition to the name, and type of hospital whether govern-mental or private (6 questions). Section (B) consists of 8 questions (4) of them about the participant’s background and knowledge regarding AMR and (4) regarding their attitude in prescribing practice. Section (C) consists of 11 questions measure the level of knowledge (familiarity with the term and knowledge about the effectiveness of ASP) and also attitude, and practice among prescribers towards ASP.
Operational Definitions of KAP 36
A KAP study measures the knowledge, attitude, and practices of prescribers towards a certain issue. The knowledge will provide us with the understanding degree of the prescribers towards AMR and ASP.
Good Knowledge
When the respondents agree on ≥65% of the statement of knowledge.
Poor Knowledge
When the respondents agree on <65% of the statement of knowledge.
Attitude is the perception and internal feeling that prescribers possess towards ASP which may be positive or negative.
Positive Attitude
When the respondents agree on ≥75% of the statements of attitude
Negative Attitude
When the respondents agree on <75% of the statements of attitude.
Practice is the activities of participants towards ASP.
Good Practice
When the respondents agree on ≥ 70% of the statement of practice.
Poor Practice
When the respondents agree on <70% of the respondent of practice.
Data Analysis
Once all necessary data was obtained and checked for completeness, sorted and categorized accordingly. Then the data was entered and analyzed using Statistical Package for Social Sciences software, version 21.0 (IBM SPSS Inc., Chicago, IL) and STATA 11 were usedand Excel 2017. Interviewers obtained written consent before administering the questionnaire. Proportions rate analysis for different response items were calculated for assessing the association between demographic data and level of KAP of prescribers towards antimicrobial stewardship, Chi –square correlation test was made and the (P < .05) was considered statically significance.
Data was Expressed as text, Illustrated Tables and Figures.
Ethical Clearance
The interviewee informed why the information was being collected, and how it would be used, and read them a statement informing them that their participation was voluntary before the start of the interview and confirmed that their answers are anonymous and confidential. Approval for the study obtained from Ethical Committee of Graduate College National University-Sudan. Written approval for conduction of the study was obtained from Ministry of Health- Khartoum State Research Administration.
Results
Demographic Data of the Study Population
Of the 294 medical staff members targeted; 287 completed the survey, giving a response rate of (97.6%). 188 (65.5%) from governmental hospitals and 99 (34.5%) from private hospitals. The proportion of female to male respondents was 58.2% vs. 41.8% respectively. 178 (62.1%) of respondents their age between 20-30 years; and 113 (39.4%) had experience between 3-5 years. 77 (26.8%) of respondents were from medical specialty and 67 (23.3%) were from surgical specialty. About 60.0% of participants were registrars while only 31% were house officers. Details of demographic characteristic of respondents were shown in Table 1.
Table 1. Demographic characteristic of respondents| Characteristic | N( %) |
| Sex | 167 (58.2%) |
| Female | 120 (41.8%) |
| Male | |
| Age (Years) | |
| 20 - 30 | 178 (62.1%) |
| 31- 40 | 87(30.3%) |
| ≥40 | 22 (7.6%) |
| Type of Hospital | |
| Governmental Hospital | 188 (65.5%) |
| Private Hospital | 99 (34.5%) |
| Specialty | |
| Medicine | 77 (26.8%) |
| Surgery | 67 (23.3%) |
| Pediatric | 53 (18.5%) |
| Obs & Gyn | 39 (13.6%) |
| other | 39 (13.6%) |
| Occupation | |
| Registrar | 51 (17.8%) |
| House officers | 171 (59.5%) |
| Consultant | 88 (30.8%) |
| Specialist | 27 (9.3%) |
| Experience (Years) | 1 (0.4%) |
| ≤ 2 | 106 (37.1%) |
| 5-Mar | 113 (39.4%) |
| 10-Jun | 48 (16.8%) |
| ≥ 10 | 20 6.7% |
Prescribers Knowledge about Antimicrobial Resistance and Prescribing Practice
(Table 2) shows that regarding AMR; of total respondents, 27.4% agreed that knowledge and poor skills were the important causes of inappropriate use of antimicrobials, 31.4% thought that providing local antimicrobial guidelines may help in control AMR, 45.3% rated that their colleagues in institution sometimes consider AMR problem before prescribing, 48.8% stated that inappropriate empirical therapy contributing to AMR. Regarding attitude in antibiotics prescribing; 39.4% of prescribers most of the time make sure that antibiotic is cost-effective when prescribing, about 98.0% consider AMR when decided to prescribe antibiotics either most of the time or some of the time, most of them 54.0% prescribe antibiotics for one week and positive microbiological results is the main factor influence decision of 37.2% of the prescribers.
Table 2. Prescribers’ Knowledge about antimicrobial resistance and attitude of antibiotics prescribing| Knowledge about antimicrobial resistance | % | Attitude of antibiotics prescribing | % |
| Which of these do you think are important causes of inappropriate use of antibiotic? | Do you ever try to make sure that your antibiotic prescribing is cost-effective? | ||
| Knowledge and poor skills | 27.4 | Always | 21.3 |
| Unrestricted availability of antimicrobials | 11.4 | Most of the time | 39.4 |
| Inadequate supervision | 26.0 | Rarely | 30.5 |
| Overwork health care personnel’s | 5.0 | Never | 8.5 |
| Which of the following do you think may help control antimicrobial resistance ? | Do you consider the potential for antibiotic resistant when decided to prescribe antibiotic or not | ||
| Treating infection, not contamination or colonization | 4.6 | Yes, most of the time | 45.7 |
| Physician education | 24.6 | Yes, some of the time | 52.2 |
| Providing local antimicrobial guidelinesKnowledge of pathogens and antimicrobial susceptibility test results | 31.48.2 | No, hardly of the time –(it does not influence my decision) | 2.2 |
| Targeting antimicrobial therapy to likely pathogens | 4.6 | ||
| To what extent do you think other doctors in your institution consider the potential of AMR ? | What your usual duration of empiric antimicrobial therapy | ||
| Always | 13.7 | 3-5 days | 32.4 |
| Sometimes | 45.3 | One week | 54.0 |
| Not considered at all | 19.1 | 2 week | 10.5 |
| I don’t know | 21.2 | ||
| Factors do you think contributing to antibiotic resistance problem ? | Factor may influence your decision to start antimicrobial therapy? | ||
| Wide spread use of antibiotics | 55.7 | Patients clinical condition | 19.5 |
| Inappropriate empiric choices | 48.8 | Positive microbiological results | 37.2 |
| Inappropriate duration of course | 38.7 | Wanting to satisfy the senior treating physician | 2.8 |
| Use of broad spectrum antibiotic | 20.2 | Worry of missing patient with possible infections | 4.3 |
| Patient demands and expectations | 13.2 | ||
Knowledge of Prescribers Towards Stewardship Program
a. Familiarity of Prescriber with Terms
More than quarter of healthcare professionals (26.4%) were familiar with the term antimicrobial stewardship whereas nearly half of healthcare professionals are not familiar with the term antimicrobial stewardship (49.7%). Figure 1:
Figure 1.Distribution of prescribers according to familiarity of prescriber with ASP term.
b. Knowledge about ASP Effectiveness
(Figure 2) shows that; about half of participants they don’t know any information regarding statements of effectiveness of ASP asked. On average about one third of respondents replied that ASA is effective. Where (37.0%) respond that ASP effective in improving patient outcomes, (33.3%) respond its effective in improving patient safety, (34.1%) respond its effective in reduce health costs and about (31.5%) respond ASP is effective in reducing resistance.
Figure 2.Knowledge of prescribers on effectiveness of antibiotics stewardship.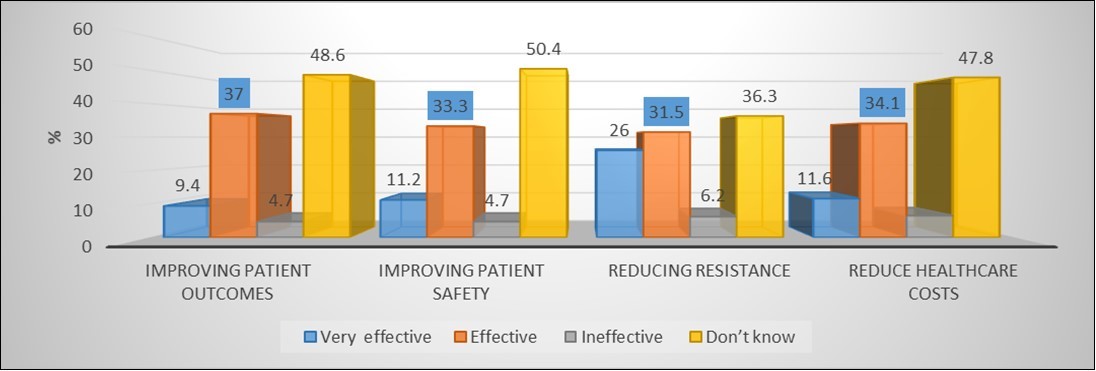
Attitude of Prescribers Towards Stewardship Program
Most of respondents (49.5%) do not know whether ASP play role in antimicrobial prescribing or not, while (43.0%) believe that ASP play vital role and (7.5%) do not believe it is play role on antibiotic prescribing. (23.1%) of respondents strongly agree sufficient education on ASP should be given to hospitals departments, (30%) agree and (2.9%) disagree with this statement. Unfortunately, (44.0%) of them don’t know.
Practices Related to ASP
Only 27 (9.5%) of respondents mentioned that they had ASP in their hospital, 39 (13.5%) having policy and team of ASP in their department and 65 (22.7%) saw evidence of ASP over the past years. An average of (40.0%) they do not have any ASP policy or team or saw ASP evidence and an average of (45.0%) they really don’t know about any of the statements mentioned, Figure 3.
Figure 3.Practices of prescribers Stewardship program in hospitals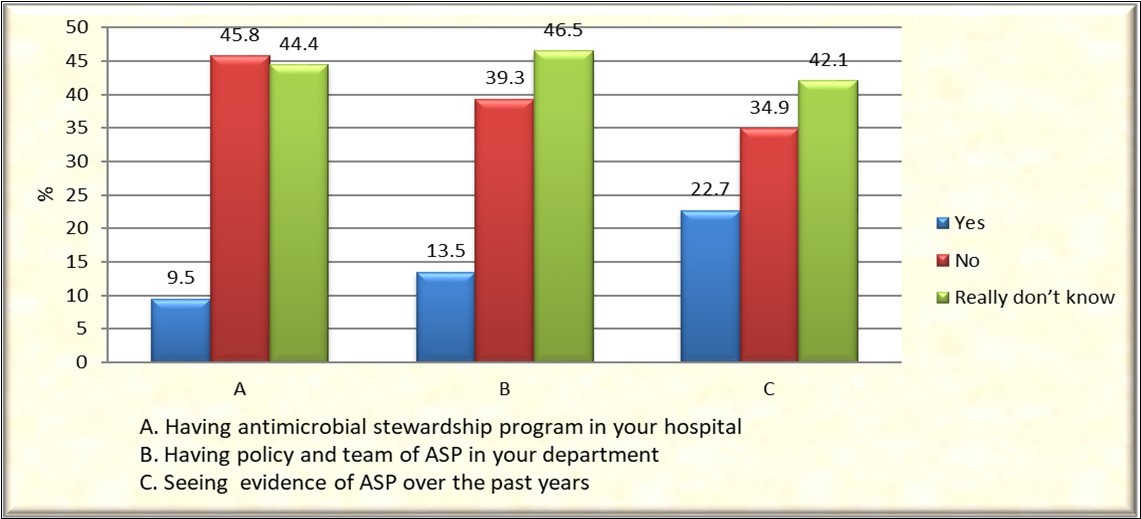
Correlation Between Knowledge, Attitude and Practice and Associations with Demographic Data
The level of knowledge on antimicrobial stewardship was good among 130 (45.3%) of the participants. Positive attitude on antimicrobial stewardship was identified among 106 (36.9%). However; good practice was observed among small fraction 23 (8.0%) of the participants. There were observed correlation only between attitude with practice (P=0.0001), which means that prescribers with positive attitude had the better practices towards antimicrobial Stewardship.
Chi –square correlation test was made between demographic data and level of KAP of prescribers towards antimicrobial stewardship. The results showed that age, occupation and experience are significant predictors of prescriber’s knowledge and attitude towards antibiotic stewardship (P- value < 0.05), but not practice. No significance associations between level of KAP and gender, hospital type and specialty (p-value ≥ 0.05), Table 3.
Table 3. Associations between demographic data and Level of KAP of prescribers towards antimicrobial Stewardship using chi- square test| KAP/ Demographic data | Knowledge | Attitude | Practice | |||
| Level of KAPN (%) | Good | Poor | Positive | Negative | Good | Poor |
| 130 (45.3%) | 157 (54.7%) | 106 (36.9%) | 181 (63.1%) | 23 (8.0%) | 264 (92.0%) | |
| Gender | 0.706 | 0.313 | 0.250 | |||
| Age | 0.024* | 0.032* | 0.184 | |||
| Hospital type | 0.088 | 0.098 | 0.345 | |||
| Experience | 0.019* | 0.015* | 0.568 | |||
| Specialty | 0.100 | 0.312 | 0.322 | |||
| Occupation | 0.000* | 0.001* | 0.302 | |||
Discussion
Antibiotic resistance is an emerging public health problem, and resistance pathogens currently exist for which no first-line treatment is effective and is aggravated by the lack of development of new antimicrobial agents 37. It has been demonstrated that physicians will not alter their management practices unless they are both aware of and in agreement with the changes that are being proposed. Thus, a better understanding the knowledge, attitudes and practices of resident house staffs is crucial to efforts to reduce inpatient antibiotic resistance, as they prescribe a substantial portion of these agents in hospitals where problems of antibiotic resistance.38 However, to our knowledge, the KAP studies regarding ASP are not too many in Sudan, but studies regarding AMR are many. In this concern; we performed this cross-sectional study to assess knowledge, attitude and practices of prescribers toward the antimicrobial stewardship program. This study is focus extensively in registrars and house officers and to lesser extent to consultants and specialist, and includes physicians in medicine, surgery, pediatric and Obs/Gyn in six governmental hospitals and four private hospitals in Khartoum state. Majority of the respondents in our study was females (58.2%) and (62.1%) of the respondents were belongs to age of 20-30 years. (39.4%) of them have an experience of 3-5 years.
Physicians had a reasonable idea of AMR and the major factors contributing to the problem. Wide spread use and inappropriate use were believed to be important general causes of resistance by all of the respondents. A pervious study conducted in Sudan 2013 found that the same reasons were the main cause of AMR for 80.0% of respondents. 39 The prevalence of self-medication with antibiotics in the community in Khartoum State, capital of Sudan is alarmingly high and documented in several occasions. 40,41 However, physician’s decisions in prescribing may be influenced by several factors such as patient clinical condition, cost-effectiveness of antibiotics, duration of empirical therapy. Antimicrobial resistance considered in prescribing decision to approximately all of respondents, only 2.2% of them AMR do not. In terms of practices related to prescribing, 37.2% agreed that microbiology lab results influencing prescribing decision to start antimicrobial therapy. Similar results obtained from a previous study assessing physicians' knowledge and perception of antimicrobial resistance; found updating about local antibiotic resistance pattern and accessing to current antibiogram (37.4 and 34.6% respectively) were the main cofounders.39 Carla et al. 42 reported that "physician’s education about antimicrobial therapy" and "knowing pathogens and antimicrobial susceptibility" were ranked as the 2 most important strategies (44.2% and 30.3%). Current antibiograms for the most hospitals in our country are not readily available and there is need of improving access to results of local antimicrobial susceptibility tests because there is currently no feedback to the staff about the local resistance trends at the surveyed hospitals. In addition to that acquiring antibiotics without a prescription from pharmacies in Khartoum State does seem to be a cultural artifact of the loosely regulated sales of antibiotics in Sudan.
As far as knowledge of stewardship programs is concerned all health professionals had fair knowledge regarding the familiarity of the term ‘Antimicrobial stewardship program’ only (26.4%) know the term. However, (49.7%) of participants did not know the term and a significant percentage of them had never heard of the term ASP (20.9%), which indicates paucity of implementation strategies and education regarding various stewardship programs across the capital state hospitals. This was high when compared to studies conducted in Ethiopia36 and India43 (10.3% and 15.0% respectively) of health care providers stated they were, not familiar with i.e., never heard the term. This might be due to the absence of education, basic training and promotion of antimicrobial stewardship program across the country. (46.4%) of participants feel ASP is very effective and effective in improving patient outcomes, (44.5%) in improving patient safety, in reducing resistance (57.5%) and reducing healthcare costs (45.7%). There are very few participants who feel antibiotics stewardship is not effective (4.7%, 4.7%, 6.2%, and 6.5% with respect to the statements asked). Surprisingly ranges of (36.3%-50.4%) of respondents answered "I don't know" for the above mentioned points. These findings are in agreement with several studies done about KAP towards ASP.44,45 A major reason for the large scale prescription of antibiotics is inadequate knowledge about the consequences of bacterial resistance. Contradictory reducing antibiotic use through implementation of stewardship programs was believed effective in ameliorating resistance by only (57.5%). This discrepancy may be due to a lack of awareness of the effectiveness of antibiotic restriction or to skepticism about its feasibility in actual practice.
The attitude of the study participants with regards towards stewardship program was found to be casual and lax. About half of respondents (49.5%) don’t know the role of ASP of their practice, (45.3 %) of participants was not believed that other doctors in institution consider the potential of AMR. (43.0%) was believed antimicrobial play a role. (53.1%) of participants feel that they need sufficient education about ASP. Providing education on antimicrobials stewardship for health professional were also suggested as the most important interventions by other studies. 35,46 Regarding the practice of AS polices on their hospitals only (9.5%) apply AS programs. A survey of Australian hospitals 47 found that only 4.8% of private hospitals restricted the use of broad-spectrum antimicrobials versus 93.8% in the public metropolitan sector. For the implementation of an AS program, team efforts and interdisciplinary involvement of different health care professionals are mandatory. Unfortunately, such practices to have team and policy were being performed by only (13.5%) the participants of this study in their hospitals. This is consistent with findings from other surveys, which showed that physicians preferred voluntary changes in prescribing practices, rather than interventions, which imposed limitations. 25,48 There was much less support in interventions that restrict physicians' behavior and practice such as antibiotic restriction and antibiotic polices interventions, perhaps because they restrict physician’s autonomy and complicate antibiotic prescribing practice. The findings of this study draw attention toward interprofessional issues that must be resolved. Otherwise, the positive attributes of AS programs will be difficult to achieve
The level of knowledge of the respondents showed that it was good among moderate numbers of respondents 130 (45.3%). However most of them showed negative attitude towards ASP 106 (63.1%) and majority showed poor practice 264 (92.0%). There was no observed correlation between knowledge and attitude, knowledge with practice (p-value ≥ 0.05). Only attitude with practice shows significance correlation (P=0.0001), which means that prescribers with positive attitude had the better practices towards antimicrobial stewardship. This is consistence with other study.36 Age, occupation and experience are the only significant predictors of prescriber's knowledge and attitude towards antibiotic stewardship, while no association between these factors and practice. Our findings were parallel with other studies results which indicated that demographic characteristics were unrelated to KAP. 49,50 The likelihood of producing behavioural change in professional practice improves if interventions are adapted to address institution specific barriers and limitations. Success of any ASP is ultimately dependent on the education of prescribers to influence change on the prescribing culture of an institution. While many ASP may focus on restricting antibiotics or the development of policies, if prescribers themselves are not actively engaged, effects of ASP interventions will most likely be minimal and short-lived, and efforts may outweigh the benefits of such interventions.
Reviews of ASP from around the world have shown that ASP is still evolving as we gain more experience. Accordingly, the Ministry of health should develop and adopt guidelines and policies to implement and monitor antimicrobial stewardship in all government and private health institutions country wide with continuous audit. Results of audits have to highlighted for us areas that we need to focus on in the future and the need for further audits to review the success of stewardship programs and interventions. This study has some limitations. First, preceptor-ship is a volunteer contribution at surveyed institutions, and there is no official registry of preceptors; therefore, questionnaire was distributed only to those whom the data collector encountered during their routine service hours at the hospitals; as majority interviewed were registrars, with low experience. Secondly, as with most surveys, it is possible that respondents might give socially desirable answers, rather than their true opinions or practices. Finally, one may question whether the attitude of doctors in other parts of Sudan to antimicrobial stewardship is reflected by the results of this survey. As this survey was conducted in the limited numbers of public and private hospitals, the generalizability of the results to other health care settings remains to be demonstrated.
Conclusion
The present study concludes that the knowledge of prescribers regarding ASP is moderate and their attitude is negative. Unfortunately, practices regarding ASP were poor, despite, the good knowledge regarding the effects of ASP on antimicrobial resistance. Considerable unmet training and education needed for physicians in the area of antimicrobial prescribing and stewardship programs to increase their awareness and knowledge. Furthermore; we need to continue in adapting our methods to develop better programs that suit our needs to be encountered in our country’s hospitals. Future large-scale studies that assess the effect of hospitals ASP and clinically relevant outcomes, including antimicrobial resistance, are needed.
References
- 1.Doron Shira, Lisa E Davidson. (2011) . Antimicrobial stewardship.Mayo Clin Proc;86(11): 1113-1123.
- 2.Joseph J, Rodvold K A. (2008) The role of carbapenems in the treatment of severe nosocomial respiratory tract infections.Expert Opinion Pharmacotherapy;. 9, 561-575.
- 3.Dellit T H, Owens R C, McGowan J E. (2007) Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship.Clin Infect Dis;44(2):. 159-177.
- 4.Joahnna Coast, Smith Ritchard D. (2003) Solving the problem of antibiotic resistance: Is a global approach necessary?Drug Discovery Today;. 8, 1-2.
- 6.Gerding D N. (2001) The search for good antimicrobial stewardship.JtComm. , J Qual Improv;27 8, 403-404.
- 7.Fishman N. (2012) Policy Statement On Antimicrobial Stewardship by The Society for Healthcare Epidemiology of America (SHEA). , The Infectious Diseases Society of America (IDSA), And The Pediatric Diseases Society (PIDS).Infect Control HospEpidemiol; 33, 322-7.
- 8.Carling P, Fung T, Killion A, Terrin N, Barza M. (2003) Favorable impact of a multidisciplinary antibiotic management program conducted during 7 years.Infect Control HospEpidemiol;. 24, 699-706.
- 9.Lutters M, Harbarth S, Janssens J-P. (2004) Effect of a comprehensive, multidisciplinary, educational program on the use of antibiotics in a geriatric university hospital.J. , AmGeriatrSoc; 52, 112-6.
- 11.Timothy H Dellit, Robert C Owens, John E McGowan, al et. (2007) . Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America Guidelines for Developing an Institutional Program to Enhance Antimicrobial Stewardship. Antimicrobial Stewardship Guidelines: 44 : 159-177.
- 12.Seligman S J. (1981) Reduction in antibiotic costs by restricting use of an oral cephalosporin. , Am J Med 71(6), 941-944.
- 13.Fraser G L, Stogsdill P, Dickens JD Jr, Wennberg D E, al et. (1997) Antibiotic optimization: an evaluation of patient safety and economic outcomes. , Arch Intern Med; 157(15), 1689-1694.
- 14.Belongia E A, Knobloch M J, Kieke B A, al et. (2005) Impact of statewide program to promote appropriate antimicrobial drug use. , Emerg Infect Dis 11, 912-20.
- 15.White A C, Atmar R L, Wilson J, al et. (1997) Effects of requiring prior authorization for selected antimicrobials: expenditures, susceptibilities, and clinical outcomes. Clin Infect Dis;25:. 230-9.
- 16.Gerding D N. (2000) Antimicrobial Cycling: Lessons learned from the aminoglycoside experience. Infect Control Hosp Epidemiol;. 21(1 Suppl): S12–7
- 17.Echols R M, Kowalsky S F. (1984) The use of an antibiotic order form for antibiotic utilization review: influence on physicians’ prescribing patterns. , J Infect Dis; 150, 803-7.
- 18.Paterson D L, Rice L B. (2003) Empirical antibiotic choice for the seriously ill patient: are minimization of selection of resistant organisms and maximization of individual outcome mutually exclusive? Clin Infect Dis;. 36, 1006-12.
- 19.Ibrahim E H, Ward S, Sherman G, Schaiff R, Fraser V J et al. (2001) Experience with a clinical guideline for the treatment of ventilator-associated pneumonia. , Crit Care Med; 29(6), 1109-1115.
- 20.Patel G W, Patel N, Lat A. (2009) Outcomes of extended infusion piperacillin/tazobactam for documented gram-negative infections. , Diagn Microbiol Infect 64(2), 236-240.
- 21.Chan R, Hemeryck L, O’Regan M, Clancy L, Feely J. (1995) Oral versus intravenous antibiotics for community acquired lower respiratory tract infection in a general hospital: open, randomized controlled trial. , BMJ 310, 1360-2.
- 22.Hermsen E D, Smith Shull S, Puumala S E, Rupp M E. (2008) Improvement in prescribing habits and economic outcomes associated with the introduction of a standardized approach for surgical antimicrobial prophylaxis. Infect Control Hosp Epidemiol. 29(5), 457-461.
- 23.S E Al-Harthi, L M Khan, Osman A M M, al et. (2015) Perceptions and knowledge regarding antimicrobial stewardship among clinicians in Jeddah, Saudi Arabia. Saudi medical journal;. 36(7), 813-820.
- 24.Kheder S, Eltayeb I, Shaddad I. (2010) Impact of antibiotic cycling policy in antimicrobial resistance in two Sudanese surgical wards settings: prospective longitudinal interventional study. , JPBMS 5(14).
- 25.Salah I Kheder. (2011) Antibiotic utilization and prescriber’s adherence measurements in a Sudanese hospital settings after introducing antibiotic policy. , Sudan Medical Monitor; 6(1), 39-48.
- 26.Woolf S H. (1993) Practice guidelines: a new reality in medicine: iii. impact on patient care. , Arch Intern Med 153, 2646-55.
- 27.Pathman D E, Konrad T R, Freed G L. (1996) Guidelines compliance: the case of pediatric vaccines recommendations. , Med Care 34, 873-88.
- 28.Burke A, Cunha M D. (1998) Antibiotic resistance control strategies. , Critical Care Clinics; 14, 309-327.
- 29.Arjun S, Xiaoyan S, Ann R. (2004) A Survey of knowledge, attitudes, and beliefs of house staff physicians from various specialties concerning antimicrobial use and resistance. Arch. International Med;164: 1451-56.
- 30.Ib Abdalla. (2005) El-Obeid Ea, Ei-Nima Ei. , Omdurman Journal of Pharmaceutical Sciences; 1, 85-92.
- 31.I B Abdalla, Khalid S A Babiker S A, al et. (2005) Resistance patterns in aerobic bacterial pathogens and incidence of noscomial infections in khartoum teaching hospital. IN 1st (Ed.) Khartoum. The annual report of tropical medicine reseach institute. 173-80.
- 32.Abdullahi Nur Hassan, Dya Eldin M. (2007) Elsayed, Mohamed Mahjoub. Uropathogens and thier antibiotic resistance patterns. , Sudan Medical Monitor; 2, 51-54.
- 33.Salah I Kheder. (2011) Implementation of strategies to control antimicrobial resistance in hospitals.Sudan. , Medical Monitor; 6(2), 135-38.
- 35.Vandana B, Vidisha V P, Priti G. (2018) Study of knowledge, attitude and practice amongst medical professionals about antimicrobial stewardship in tertiary care teaching hospital in India: a questionnaire based study.International. , Journal of Basic & Clinical Pharmacology; 7, 511-517.
- 36.Tegagn G T, Yadesa T M, Ahmed Y. (2017) Knowledge, attitudes and practices of healthcare professionals towards antimicrobial stewardship and their predictors in Fitche Hospital.JBioanalBiomed9:. 091-097.
- 37.Spellerg B, Powers J H, Brass E P. (2004) Trends in antimicrobial drug development: implications for the future.Clin Infect. , Dis; 38, 1279-86.
- 38.Cabana M D, Ebel B E, Copper-Patric. (2000) Barriers pediatricians face when using practice guidelines.ArchPediatricAdolescent. Med;154: 685-93.
- 39.Salah I. (2013) Kheder.Physicians'' Knowledge and perception of antimicrobial resistance: a survey in Khartoum State Hospital Settings.British. , Journal of Pharmaceutical Research; 3(3), 347-362.
- 40.Abdelmoneim A, Idris E, Lloyd M. (2005) Self-medication with antibiotics and antimalarial in the community of Khartoum State. Sudan.J Pharm Pharmaceutical Sciences;8: 326-31.
- 41.Cherghali A M, Amjad M I. (2009) Availability, affordability, and prescribing pattern of medicines in Sudan.Pharm World Sci;. 31, 209-15.
- 42.Carla M, Carlos A, Armando R. (2007) Physicians' perceptions, beliefs, attitudes and knowledge concerning antimicrobial resistance in Brazilian teaching hospital.Infect. Control and Epidemiology. 28(12), 1411-14.
- 43.Bucke W, Hersh A, Pavia A, Jones P, Caraccio J. (2016) Antimicrobial stewardship knowledge, attitudes and practices among healthcare professionals at small community hospitals.Journal of Hospital Pharmacy;. 51(2), 149-157.
- 44.Erku D A. (2016) Antimicrobial stewardship: a cross-sectional survey assessing the perceptions and practices of community pharmacists in Ethiopia.InterdiscipPerspectInfect Dis.;. 5686752, 1-7.
- 45.M R Khdour, H O, Al‐Deyab M, al et. (2018) Impact of antimicrobial stewardship program on hospitalized patients at the intensive care unit: a prospective audit and feedback study.British journal of clinical pharmacology;. 84, 708-715.
- 46.Abera B, Kibret M, Mulu W. (2014) Knowledge and beliefs on antimicrobial resistance among physicians and nurses in hospitals in Amhara region. , Ethiopia.BMCPharmacolToxicol; 15(26), 1-7.
- 47.Loh1 Jeannine A M, Jonathan D Darby1, al John R Daffyet.Implementation of an antimicrobial stewardship program in an Australian metropolitan private hospital: lessons learned.Healthcare. , Infection; 20, 134-140.
- 48.Murray M D, Kohler R B, McCarthy M C. (1988) Attitudes of house physicians concerning various antibiotic-use control program. , Am J Hosp Pharm;45: 584-88.
Cited by (2)
- 1.RANI AITHA SWETHA, MADHAVI PUDUTHA, T. CHAKRADHAR, 2022, A RETROSPECTIVE STUDY TO EVALUATE THE EFFICACY OF INJECTION AUGMENTIN IN COVID-19 PATIENTS WITH PNEUMONIA AT A TERTIARY CARE TEACHING HOSPITAL, TELANGANA, International Journal of Pharmacy and Pharmaceutical Sciences, (), 28, 10.22159/ijpps.2022v14i10.45730
- 2.Altayb Hisham N., Elbadawi Hana S., Baothman Othman, Kazmi Imran, Alzahrani Faisal A., et al, 2022, Genomic Analysis of Multidrug-Resistant Hypervirulent (Hypermucoviscous) Klebsiella pneumoniae Strain Lacking the Hypermucoviscous Regulators (rmpA/rmpA2), Antibiotics, 11(5), 596, 10.3390/antibiotics11050596
