Abstract
In autologous hematopoietic stem cell transplantation patients for whom granulocyte-colony stimulating factor fails to mobilize a sufficient number of peripheral blood stem cells, plerixafor proposes an option for successful rescue mobilization. This paper evaluates the efficacy of plerixafor to mobilize peripheral blood stem cells (PBSCs) in patients who failed previous mobilization with G-CSF alone, by retrospectively analysing the PBSC results from lymphoma and myeloma (MM) patients between 2006 and 2011. Patients were classified according to the CD34+ cells/kg yield collected by apheresis: < 2 x 106 CD34+ cells/kg was considered collection failure, whereas ≥ 5 x 106 CD34+ cells/kg was considered good mobilization. 797 patients underwent one or more apheresis. The first mobilization success rate was 82%; 140 patients proved to be poor mobilizers. Suboptimal first mobilization was significantly associated with age >50 years (p=0.005) and the absence of chemotherapy in prior PBSCs stimulation (p=0.04). 149 rescue protocols were used in the 140 poor mobilizers, and 71 patients received plerixafor. In univariate analysis the remobilization rate without plerixafor was 42% and increased to 65% when plerixafor was added. In multivariate analysis, plerixafor administration reduced the PBSC remobilization failure risk by a half (OR=0.47). The median value of CD34+ cells/kg in transplants increased from 1.43 (range, 014.03) without plerixafor to 3.85 (range, 0–18.25; p=1 x 10-4) with plerixafor. There were more good mobilizers after plerixafor use (35% with plerixafor versus 15% without plerixafor; p=0.005). Plerixafor efficacy was similar for lymphoma (60% remobilization) and MM (80%; p=0.12). These data show that plerixafor was effective in poor mobilizers and that it synergized with G-CSF to improve the quantity of collected PBSCs. Plerixafor also increased transplant feasibility by 23%. While the clinical results of this study are promising, economic data were not taken into account and there is a need for real work concerning the cost-effectiveness of this treatment. We propose a subsequent study in which the economic efficacy of plerixafor’s use is evaluated based on the financial aspects of the treatments received by the cohort evaluated in this paper.
Author Contributions
Academic Editor: Umit Tapan, Boston university Medical centre.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2015 E Prunier, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Autologous stem cell transplantation (ASCT) remains an important part of front-line therapy in multiple myeloma (MM) and is mandatory in lymphoma relapse procedures. Mobilized peripheral blood stem cells (PBSCs) are currently used as the sole source of stem cells in these indications. PBSC collection is performed after granulocyte-colony stimulating factor (G-CSF) stimulation alone (steady-state) or following a chemotherapy cycle during the neutrophil recovery phase (chemo-primed). The CD34+ cell count in the autologous graft is one of the strongest contributing factors to successful fast engraftment; nevertheless, 10–15% of patients with haematological malignancies fail to mobilize an adequate quantity of PBSCs1, and poor mobilization may affect patient outcome after transplantation. One way to improve PBSC collection is to repeat the mobilization procedure with an increased dose of G-CSF, but this often fails.2, 3, 4 Use of plerixafor is another way to enhance PBSC collection.5, 6 Plerixafor is a pure antagonist of chemokine receptor-4 (CXCR4) that blocks the interaction of the receptor and its ligand, stromal cell-derived factor 1 (SDF-1).7 After administration of plerixafor, hematopoietic stem cells migrate outside of the bone marrow niche and circulate into the peripheral blood, allowing an efficient and effective apheresis harvest; concomitant G-CSF administration can amplify this process and increase the yield of these circulating cells.
In clinical studies of MM and lymphoma patients, plerixafor combined with G-CSF was well tolerated and significantly increased the number of PBSCs in patients who previously failed to mobilize PBSCs.8, 9, 10 To determine the efficacy of plerixafor, we report here on 3 years of experience using plerixafor as rescue stimulation in a haematological population of patients who failed a previous mobilization procedure plus G-CSF to successfully remobilize PBSCs inlymphoma and MM patients.
Materials and Methods
Patients
We retrospectively analysed the results of consecutive PBSC collections performed in lymphoma and MM patients from 2006 to 2011 at two university hospitals working with the Bourgogne Franche-Comte EFS (French Blood Establishment). Specifically speaking, patients received medical care and ASCT respectively in the Besançon and Dijon university hospital haematology departments. In accordance with local policies, all patients provided informed written consent for the collection and analysis of data in their medical files. When patients were included in a clinical trial, specific written informed consent was obtained. The study was designed and conducted in accordance with the declaration of Helsinki and was approved by the institutional ethics committees.
Mobilization Procedure
For the first collection of cells, PBSC mobilization was performed either using G-CSF alone at a daily dosage of 510 µg/kg (steady state protocol) or after a chemotherapy course of high dose cyclophosphamide in MM patients or after one of two different regimens in lymphoma patients, namely R-CHOP or R-ACVBP (chemo-primed protocol). When ≥ 2 x 106 CD34+ cells/kg were available after the first collection process, the collection was considered a success and ASCT or even 2 ASCTs in some MM patients, could be planned without further PBSC harvest. When < 2 x 106 CD34+ cells/kg were available, the collection was considered a failure. If the first mobilization failed, a new mobilization procedure was performed, most often using the chemo-primed protocol. The decision to add plerixafor was made by the local investigator after the first collection failure in patients considered at high risk of secondary PBSC mobilization failure.
In the steady-state protocol, the daily dose of plerixafor was 0.24 mg/kg administered 8-11 hours before the first apheresis session on the evening of the fourth day of G-CSF injection. Alternatively using the chemo-primed protocol, if the CD34+ cell count was < 15/mm3 and the leucocyte count > 1 G/l, plerixafor was administered on the evening 8-11 hours before the first apheresis session. If < 2 x 106 CD34+ cells/kg were collected after the first collection procedure, an additional dose of plerixafor could be delivered on day 2 or 3 following the collection.
Collection Procedure
PBSC harvesting started when the peripheral circulating CD34+ cell count increased to >15/mm3, which was generally on the 5th day of G-CSF administration in the steady-state protocol or when leucocytes increased to > 1 G/l in the chemo-primed protocol. The CD34+ cell count was then checked daily to determine this time point. Blood stem cells were collected with one of two blood cell separators: the Cobe Spectra (Terumo BCT®, Lakewood, CO, USA) or the COM.TEC (Fresenius Kabi®, Bad Homburg, Germany). At least two total blood volumes were processed at each apheresis session. Anticoagulant citrate dextrose solution was used. The quality of the harvested product was evaluated by counting the total nucleated and CD34+ cells, which was expressed as the number of cells/kg body weight of the patient. An apheresis session could be repeated on the following day until at least 2 x 106 CD34+ cells/kg were acquired. Alternatively, sessions were halted if the CD34+ count in the peripheral blood was < 15/mm3. The colony-forming unit-granulocyte macrophage (CFU-GM) content of the PBSCs was quantified systematically only when plerixafor mobilization was used.
Post-Transplantation Engraftment
The post-ASCT haematological engraftment was analysed to determine neutrophil recovery. Neutrophils were counted from the day of ASCT until a neutrophil count ≥ 0.5 G/l was achieved. The duration of the hospital stay was also recorded and was measured from the day of the ASCT infusion until discharge.
Statistical Analyses
The study was divided into two time periods: 2006-2008 (period 1, without plerixafor use) and 2009-2011 (period 2, plerixafor mostly used for remobilization). Patients were classified according to the CD34+ cells/kg yield that was collected by apheresis (before freezing). Patients with a total collected cell count < 2 x 106 CD34+ cells/kg were considered poor mobilizers. Conversely, patients with cumulative yields ≥ 5 x 106 CD34+ cells/kg were considered good mobilizers. Descriptive analyses were used to summarize the patient characteristics, risk factors, CD34+ cell collection parameters and time to neutrophil engraftment. We analysed the features of the first mobilization to identify mobilization failure risk factors. Next the results of rescue protocols to evaluate the efficacy of plerixafor were evaluated. Data are presented as median (range). We performed univariate and multivariate analyses, and the differences between categorical variables were calculated with the χ2 test. Significant factors with p values ≤ 0.05 in univariate analyses were included in a multivariate model that was analysed using logistic regression. All analyses were performed using SAS® software (version 9.2, 2008, Cary, NC, USA).
Results
Patients
During the two periods studied, 797 patients underwent one or more apheresis procedure for PBSC collection. There were no statistically significant differences between the populations recruited in Besançon and Dijon. The detailed baseline and demographic characteristics are summarized in Table 1. Patients were older (p=0.05) and diagnoses of MM were more frequent (p=0.045) during period 2; consequently, the use of bortezomib and lenalidomide was more frequent in the second period. The significant decrease in anthracycline use was mainly due to modifications in therapeutic strategies for MM and in the progressive withdrawal of the referent protocol, the combination of vincristine, adriamycin and dexamethasone (VAD).11
Table 1. Patient demographics and baseline characteristics| Period 1 | Period 2 | Total | |
| Patients, n | |||
| Besançon Hospital | 144 | 155 | 299 |
| Dijon Hospital | 218 | 280 | 398 |
| Total | 362 | 435 | 797 |
|---|---|---|---|
| Median age (range) | 55 (3–76) | 58 (3–77) | 57 (3–77) |
| Sex ratio (male to female) | 1.74 | 1.65 | 1.70 |
| Disease diagnosis | |||
| Multiple myeloma | 145 | 206 | 351 |
| Lymphoma | 169 | 186 | 355 |
| Other | 48 | 43 | 91 |
| Total | 362 | 435 | 797 |
| Previous chemotherapy lines, median (range) | 1 (1–5) | 1 (1–5) | 1 (1–5) |
| Patients (n) previously exposed to: | |||
| Lenalidomide | 23 | 67 | 90 |
| Bortezomib | 105 | 197 | 302 |
| Anthracycline | 261 | 240 | 501 |
| Total | 389 | 504 | 893 |
First mobilization attempt
All 797 patients were included in this analysis. Of these, 140 patients presented mobilization failures. The following parameters were tested in univariate analysis: median age, sex, inclusion period, diagnosis, number of previous cycles of chemotherapy, drugs (anthracyclines, vincristine, lenalidomide, and bortezomib) and type of mobilization procedure (i.e. use of G-CSF alone or in combination with chemotherapy). Patient age (>50 years old), MM diagnosis and the use of a steady state protocol were shown to be major risk factors for mobilization failure. Multivariate analysis of these factors showed that only age and the use of a steady-state protocol remained significant factors that affected mobilization (Table 2).
Table 2. Risk factors that predict failure of the first mobilization| Univariate analysis | Multivariate analysis | ||||||
| Patients, n | PBSCs failure n (%) | P | OR | 95% CI | P | ||
| All patients | 797 | 140 (18) | |||||
| Year of PBSCs | |||||||
| 2006–2008 | 362 | 65 (18) | |||||
| 2009–2011 | 435 | 75 (17) | NS | ||||
| Patient age | |||||||
| ≤50 years old | 235 | 26 (11) | |||||
| >50 years old | 562 | 114 (20) | 0.002 | 1.93 | (1.22–3.06) | 0.005 | |
| Diagnosis of MM | |||||||
| Yes | 351 | 75 (21) | |||||
| No | 446 | 65 (15) | 0.02 | 0.65 | - | NS | |
| Previous exposure to: | |||||||
| Lenalidomide | 90 | 33 (37) | < 0.01 | ||||
| Bortezomib | 302 | 69 (23) | < 0.01 | ||||
| PBSCs stimulation | |||||||
| Chemotherapy + G-CSF | 618 | 97 (16) | |||||
| G-CSF alone | 179 | 43 (24) | 0.01 | 1.54 | (1.03–2.32) | 0.04 | |
Remobilization Procedures and Plerixafor Use
A total of 149 rescue protocols were managed in 117 of the 140 patients who were poor mobilizers; 32 patients were remobilized a third and/or a fourth time. Of these 140 patients, 71 received plerixafor as part of the rescue mobilization protocol (22 after a steady-state protocol and 49 after a chemo-primed protocol). The use of plerixafor in the first rescue protocol was similar between the two sites. In Dijon, 39 (38%) of the 103 patients with a first mobilization failure received plerixafor, while 20 (54%) of the 37 patients with a first mobilization failure in Besançon received plerixafor; this difference was not significant (p=0.09). Univariate analyses were performed using the same criteria previously applied for the whole cohort and the use of plerixafor was added in the model. The results showed that it was easier to succeed in remobilization when a patient suffered from MM (p=3.10-4), when the mobilization took place between 2009 and 2011 (p=4 x 10-5), and when the patient’s cells were mobilized with plerixafor (p=0.003), no matter the age or remobilization protocol, steady-state vs. chemo-primed (p = NS). These three significant factors were then tested in multivariate analysis. Only a diagnosis of MM (p=0.003) and the administration of plerixafor (p=0.004) remained statistically significant factors for successful remobilization (Table 3). Plerixafor was prescribed significantly more often for remobilization in patients with MM (57% of MM rescue protocols) than for patients with lymphoma (33% of lymphoma rescue protocols) (p=0.01) as shown in Figure 1. Regardless of diagnosis, the success rates for plerixafor remobilization were similar: 60% in cases of lymphoma versus 80% in cases of MM (p=0.12).
Table 3. Risk factors that predict failure after remobilization procedures| Univariate analysis | Multivariate analysis | |||||||||
| Rescue protocols, n | PBSC failure, n (% of rescue protocols) | P | OR | 95% CI | P | |||||
| Total | 149 | 70 | ||||||||
| Year of PBSCs mobilization | ||||||||||
| 2006–2008 | 67 | 44 (66) | ||||||||
| 2009–2011 | 82 | 26 (32) | 0.0001 | - | - | NS | ||||
| Patient age | ||||||||||
| ≤50 years old | 29 | 13 (45) | ||||||||
| >50 years old | 120 | 57 (47.5) | 0.8 | |||||||
| ≤55 years old | 50 | 26 (52) | ||||||||
| >55 years old | 99 | 44 (44) | 0.39 | |||||||
| ≤60 years old | 82 | 28 (34) | ||||||||
| >60 years old | 67 | 42 (63) | 0.32 | |||||||
| Hematological diagnosis | ||||||||||
| Myeloma | 79 | 26 (33) | ||||||||
| Other malignancies | 70 | 44 (63) | 0.0003 | 0.35 | (1.75–6.66) | 0.003 | ||||
| Plerixafor administration | ||||||||||
| Yes | 71 | 25 (36) | ||||||||
| No | 78 | 45 (58) | 0.008 | 0.47 | (0.23–0.94) | 0.04 | ||||
Figure 1.Administration of plerixafor according to diagnosis.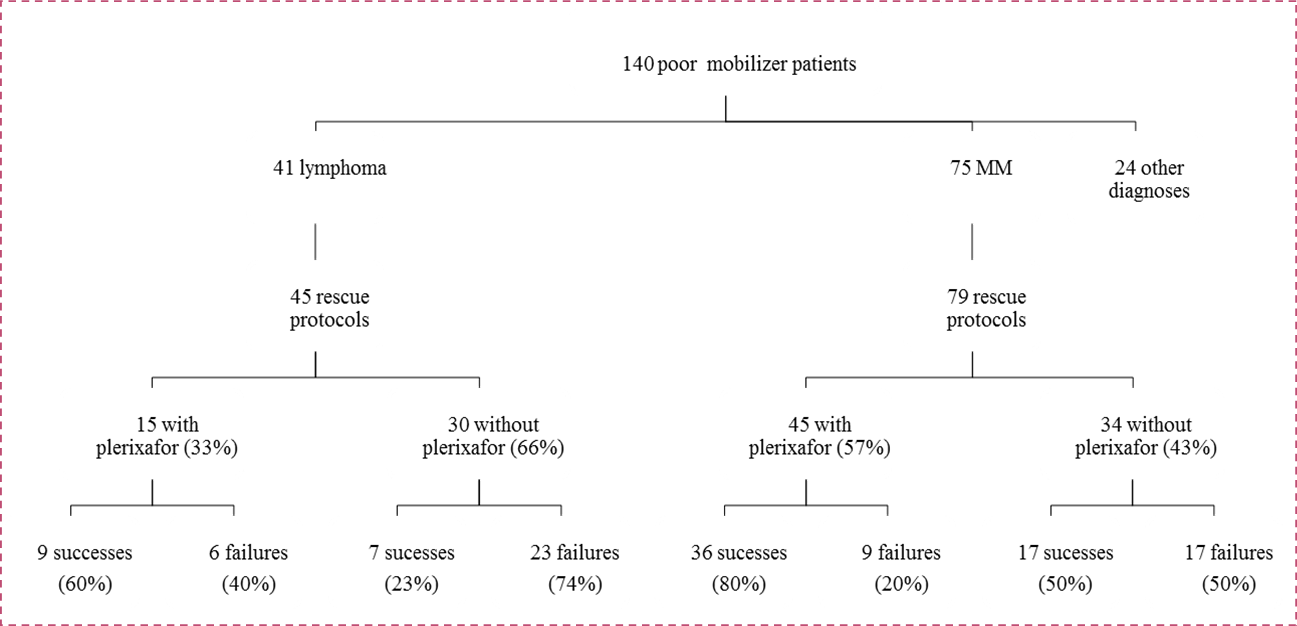
Qualitative Studies of Blood Samples
The mean yield of CD34+ cells harvested after the first mobilization was 6 x 106 CD34+ cells/kg (range, 0–83.50), which was better than the yield after a remobilization procedure, 2.05 x 106 CD34+ cells/kg (range, 0–18.25)(p< 1.10-4). The median level of CD34+ cells in PBSCs collected after remobilization increased from 1.43 x 106 CD34+ cells/kg (range, 0–14.03) without plerixafor to 3.85 x 106 CD34+ cells/kg (range, 0–18.25) with plerixafor (p=1 x 10-4). Thus, there was an increase of good mobilizers after plerixafor employment: 35% with plerixafor versus 15% without plerixafor (p=0.05).
To determine predictive factors for plerixafor efficacy, we retrospectively studied the pre-apheresis blood counts of the 71 patients who received plerixafor. In the patients treated with plerixafor, blood tests performed on the morning of apheresis suggested that the presence of >200 myelocytes/mm3 in the peripheral blood could predict PBSCs collection success (Table 4).
Table 4. Characteristics of PBSCs collected from patients receiving plerixafor.| Successes (%) | Failures (%) | P | ||
| Patients, n | 46 | 25 | 3 x 10 -3 | |
| Median level of CD34+ circulating cells before apheresis | ||||
| Nb cells/mm3 (range) | 25 (3.2–222.2) | 4 (0–50.7) | 1 x 10 -3 | |
| Myelemia count before apheresis | ||||
| Patient with myelemia <200 myelocytes/mm3 | 6 (23) | 20 (77) | < 1 x 10 -5 | |
| Patient with myelemia ≥200 myelocytes/mm3 | 39 (88) | 5 (11) | ||
| Median level of CD34+ cells in the PBSC collection | ||||
| 106/kg (range) | 4.84 (2.06–18.25) | 0.32 (0–1.73) | < 1 x 10 -5 | |
| Median level of CFU-GM in the PBSC collection | ||||
| 104/kg (range) | 84.69 (0–373.6) | 7.7 (0–58.3) | < 1 x 10 -5 | |
Engraftment and Haematological Recovery
Of the 797 patients in the study, 732 (92%) secondarily underwent ASCT. We evaluated haematological recovery by studying the median time to neutrophil recovery (neutrophils >0.5 G/l). For the 657 patients grafted after the first mobilization, the median time to neutrophil recovery was 9 days (range, 4–11). For the 117 patients that required a rescue protocol, the median time to neutrophil recovery increased to 13 days (range, 6–42; p<1 x 10-5). For these 117 patients, the addition of plerixafor did not have a significant impact: the median time to neutrophil recovery was 13.9 days (range, 5–41) in the plerixafor group versus 11.79 days (range, 4–30) in the no plerixafor group (p=0.23). In the 657 patients with a successful first mobilization, the median length of hospital stay after ASCT was 19 days (range, 5–80), which increased to 22 days (range, 7–64 days) when ≥2 mobilization procedures were needed (p<0.001).
Discussion
Mobilization of autologous PBSCs remains of primary interest for performing ASCTs, especially in patients with refractory lymphoma or in young patients with MM. This mobilization process remains one of the factors that limit the feasibility of ASCT. The objective of PBSC mobilization is to obtain as many CD34+ cells as possible, because PBSC counts are correlated with bone marrow aplasia duration.12 A first mobilization with G-CSF alone or in combination with chemotherapy succeeds in 70–95% of patients; this means that 530% of patients will be poor mobilizers.13, 14, 15, 16, 17 Plerixafor represents a new approach to optimizing PBSC mobilization in rescue protocols. It is approved for this use in the European Union in combination with G-CSF to enhance the mobilization of hematopoietic stem cells to the bloodstream for collection and later use in ASCT. It is accepted for use in patients with lymphoma or MM who do not succeed in providing a sufficient PBSC count with G-CSF alone or G-CSF following cytotoxic chemotherapy.
This study retrospectively evaluates the efficacy of plerixafor for remobilizing patients who failed previous mobilizations. We recruited a cohort of consecutive patients that needed ASCT; the study included a large number of patients who were treated at two centres with similar patient management practices and working with the same collection centre for PBSC harvesting. There were no significant differences in plerixafor prescription between the two hospitals (p=0.18), so this cohort offers a reliable and homogeneous representation of the population that needs to undergo ASCT for the treatment of lymphoma or MM. In our study, the first mobilization success rate was 82%, which is similar to rates reported in the literature.13, 14, 15, 16 Plerixafor was administered to half of the 18% who were poor mobilizers.
Various factors have been reported to impact stem cell mobilization and identification of risk factors of PBSC mobilization failure is important for making the decision about whether to prescribe plerixafor or not for the subsequent mobilization attempt. In our multivariate analysis of the first mobilization course, age >50 years old and absence of chemotherapy in prior PBSC stimulation were significantly associated with suboptimal mobilization. These factors have already been identified in previous studies and the nature of pre-mobilization chemotherapy schemes have also been identified as significant risk factors.18, 19, 20, 21, 22 Studying the cohort of poor-mobilizing patients was a useful way to demonstrate plerixafor efficacy. Successful remobilization rates with plerixafor varied from 64% to 88% in different studies.23, 24, 25, 26, 27, 28 In our cohort, the successful remobilization rate without plerixafor was 42%, and increased to 65% when plerixafor was added. In multivariate analysis, plerixafor administration reduced the PBSCs remobilization failure risk by a half (OR=0.47, p=0.003); these results are in accordance with those already published.11, 13, 23, 24, 25, 26, 27, 28, 29 Thus, plerixafor allowed 23% of supplemental poor-mobilizer patients to undergo a successful PBSC collection as compared to remobilization with G-CSF alone. Plerixafor efficacy was similar for both diagnoses, with a 60% remobilization success rate in lymphoma cases and an 80% success rate in MM cases (p=0.12). In 2012, Sancho et al. concluded that plerixafor was effective regardless of the type of haematological malignancy.29 The significant difference between plerixafor prescription rates according to diagnosis (p=0.01) may have been due to differences in treatment strategies for MM versus lymphoma patients. Patients with MM are mobilized rapidly after diagnosis during their front-line treatment, while in the era of rituximab, ASCT is part of the rescue protocol in relapsing or refractory lymphoma patients only and PBSCs were collected mostly after a salvage regimen.
We also found that the use of chemo-primed protocols with plerixafor do not have a beneficial impact on PBSC' remobilization as compared to steady-state protocols. These results are in opposition to those of many studies, which concluded that chemo-primed protocols are superior to steady-state protocols in terms of PBSC collection. The addition of G-CSF can increase by 2.5 times the circulating CD34+ cell level because G-CSF boosts hematopoietic restoration after aplasia induced by chemotherapy.30, 31, 32, 33, 34 However, none of these studies made a difference between the use of plerixafor after a first or subsequent mobilization procedure. Our data showed that during remobilization procedures, the most important risk factor of failure is no longer the use of a steady-state protocol but the amount of previous chemotherapy lines administered.35, 36, 37 For example, a previous treatment with lenalidomide could be an important factor leading to high risk of mobilization failure.36, 37
The third part of the study described the differences in blood test results during the ASCT procedure. Notably, high PBSC content correlates not only with transplant feasibility but also with lower incidences of complications, infections, and transfusion requierements.12 In order to perform apheresis at the optimal time as well as collect as many PBSCs as possible, we looked at preapheresis blood cell counts in plerixafor patients. Currently, the peripheral blood CD34+ cell count is still considered to be the best predictor of apheresis cell yield and is used to determine the adequate time of apheresis.38, 39 In cases of PBSCs success, we effectively observed a significantly higher level of CD34+ cells in the peripheral blood but also the presence of a high myelemia count (> 200 myelocytes/mm3), which was highly significant.
We decided to evaluate only the qualitative blood samples of the 71 patients who received plerixafor. To our knowledge, this outbreak of myelemia has not been studied yet in this setting. Only a limited bone marrow reserve, characterized by a low platelet count, a low peripheral blood CD34+ number and a low bone marrow cellularity is a risk factor for poor PBSC mobilization.40, 41, 42, 43 Other studies have identified impaired glucose tolerance and osteolytic lesions as significant predictors of mobilization failure in MM patients.44, 45, 46 Several studies in the beginning of the G-CSF PBSC mobilization procedures have already shown that the enumeration of immature circulating cells could be of interest to determine the optimal timing of PBSC collection and compare favourably with CD34+ counts.47, 48, 49 This parameter, myelemia, is of potential clinical interest and merits a more extensive study.
Times to haematological recovery were also studied across the entire cohort. Patients who underwent rescue protocols demonstrated no significant difference in outcome between patients who did or did not receive plerixafor (p=0.23). Seeing as that the majority of patients received GCSF from day 5 or 6 post-transplant until neutrophil engraftment, this administration of G-CSF post-transplantation may have influenced these results.
Our study was limited to the investigation of plerixafor efficacy which means it did not take into account economic factors. That said, the cost of plerixafor could limit its use; this drug does not have a current and clear defined place in mobilization protocols for ASCT. Accordingly, it would be worthwhile to study PBSC failure risk factors and plerixafor efficacy in order to optimize the adequate prescription of this drug. Several models of risk adapted algorithms for optimal utilization of the drug have already been made. Some of them recommended a pre-emptive use of plerixafor. In March 2015, a retrospective study compared PBSC mobilization in MM patients using fractioned high-dose cyclophosphamide and G-CSF with G-CSF plus pre-emptive plerixafor. The total average cost of mobilization was slightly higher in the plerixafor group ($7886 vs $7536; p= 0.16), but a chemo–mobilization approach was associated with significantly increased toxicity.50
In other studies plerixafor was administered only to patients who were at high risk for mobilization failure. In general, collected cell counts of < 11.5 x 106 CD34+ cells/kg after at least 4-5 days of mobilization with G-CSF alone has been used as the cut-off marker. Plerixafor management in “just-in-time” approaches enables an optimal increased CD34+ yield in order to decrease mobilization failures as well as the number of apheresis days in addition to limiting costs. In the literature, three studies suggest that plerixafor does not substantially increase overall costs.51, 52, 53 One of them concluded that initiation of plerixafor increases the per-patient cost of PBSC mobilization, but failure rates, number of apheresis days, and number of total mobilization/collection days were lower.54
Conclusion
This study demonstrated that plerixafor was an effective drug for poor mobilizers: it synergized with G-CSF and improved the quantity and possibly the quality of collected PBSC. The successful remobilization rate was significantly increased (23%) when plerixafor was employed. We found that patients >50 years old who were previously mobilized using a steady-state protocol were at very high risk of PBSC mobilization failure and were good candidates for plerixafor use. More studies taking both clinical and economic data into account are needed to analyse the cost effectiveness of plerixafor’s use in rescue mobilization. This is why we will conduct a costeffectiveness analysis on this studies cohort with the objective of determining overall per-patient expenditures with or without plerixafor.
References
- 1.Moskowitz A J, Perales M-A, Kewalramani T, Yahalom J, Castro-Malaspina H et al. (2009) Outcomes for patients who fail high dose chemoradiotherapy and autologous stem cell rescue for relapsed and primary refractory Hodgkin lymphoma. , Br J Haematol 146(2), 158-63.
- 2.Lie A K, Hui C H, Rawling T, Dyson P G, Thorp D et al. (1998) Granulocyte colony-stimulating factor (G-CSF) dose-dependent efficacy in peripheral blood stem cell mobilization in patients who had failed initial mobilization with chemotherapy and G-CSF. , Bone Marrow Transplant 22(9), 853-7.
- 3.Herbert K E, Morgan S, Prince H M, Westerman D A, Wolf M M et al. (2009) Stem cell factor and high-dose twice daily filgrastim is an effective strategy for peripheral blood stem cell mobilization in patients with indolent lymphoproliferative disorders previously treated with fludarabine: results of a Phase II study with an historical comparator. Leukemia. 23(2), 305-12.
- 4.Stiff P, Gingrich R, Luger S, Wyres M R, Brown R A et al. (2000) A randomized phase 2 study of PBPC mobilization by stem cell factor and filgrastim in heavily pretreated patients with Hodgkin's disease or non-Hodgkin's lymphoma. Bone Marrow Transplant. 26(5), 471-81.
- 5.Levesque J P, Hendy J, Takamatsu Y, Williams B, Winkler I G et al. (2002) Mobilization by either cyclophosphamide or granulocyte colony-stimulating factor transforms the bone marrow into a highly proteolytic environment. Exp Hematol. 30(5), 440-449.
- 6.Nervi B, Link D C, DiPersio J F. (2006) Cytokines and hematopoietic stem cell mobilization. , J Cell Biochem 99(3), 690-705.
- 7.Micallef I N, Stiff P J, DiPersio J F, Maziarz R T, McCarty J M et al. (2009) Successful stem cell remobilization using Plerixafor (mozobil) plus granulocyte colony-stimulating factor in patients with non-hodgkin lymphoma: results from the Plerixafor NHL phase 3 study rescue protocol. Biol Blood Marrow Transplant. 15(12), 1578-86.
- 8.Calandra G, McCarty J, McGuirk J, Tricot G, Crocker S A et al. (2008) AMD3100 plus G-CSF can successfully mobilize CD34+ cells from non-Hodgkin's lymphoma, Hodgkin's disease and multiple myeloma patients previously failing mobilization with chemotherapy and/or cytokine treatment: compassionate use data. Bone Marrow Transplant. 41(4), 331-8.
- 9.Yuan S, Nademanee A, Forman S J, Wang S. (2013) Use of plerixafor in patients with Hodgkin lymphoma with poor mobilization of peripheral blood stem cells. Leuk Lymphoma. 54(3), 646-8.
- 10.Keung Y K, Suwanvecho S, Cobos E. (1999) Anaphylactoid reaction to granulocyte colony-stimulating factor used in mobilization of peripheral blood stem cell. , Bone Marrow Transplant 23(2), 200-1.
- 11.Jantunen E, Penttilä K, Pyörälä M. (2011) Addition of Plerixafor to a chemotherapy and G-CSF mobilization in hard-to-mobilize patients. Bone Marrow Transplant. 46, 308-9.
- 12.Limat S, Woronoff-Lemsi M C, Milpied N, Chartrin I, Ifrah N et al. (2000) Effect of cell determinant (CD)34+ cell dose on the cost and consequences of peripheral blood stem cell transplantation for non-Hodgkin’s lymphoma patients in front-line therapy. , Eur J Cancer 36(18), 2360-7.
- 13.Pusic I, Jiang S Y, Landua S, Uy G L, Rettig M P et al. (2008) Impact of mobilization and remobilization strategies on achieving sufficient stem cell yields for autologous transplantation. Biol Blood Marrow Transplant. 14(9), 1045-56.
- 14.Gertz M A, Wolf R C, INM Micallef, Gastineau D A. (2010) Clinical impact and resource utilization after stem cell mobilization failure in patients with multiple myeloma and lymphoma. Bone Marrow Transplantation. 45, 1396-1403.
- 15.Cavallo F, Bringhen S, Milone G, Ben-Yehuda D, Nagler A et al. (2011) Stem cell mobilization in patients with newly diagnosed multiple myeloma after lenalidomide induction therapy. Leukemia. 25, 1627-31.
- 16.Bhutani D, Zonder J, Valent J, Tageja N, Ayash L et al. (2013) Evaluating the effects of lenalidomide induction therapy on peripheral stem cells collection in patients undergoing autologous stem cell transplant for multiple myeloma. Support Care Cancer. 21, 2437-42.
- 17.Bensinger W, DiPersio J F, McCarty J M. (2009) Improving stem cell mobilization strategies: future directions. Bone Marrow Transplant. 43, 181-95.
- 18.Micallef I N, Apostolidis J, Rohatiner A Z, Wiggins C, Crawley C R et al. (2000) Factors which predict unsuccessful mobilisation of peripheral blood progenitor cells following G-CSF alone in patients with non-Hodgkin's lymphoma. , Hematol J 1(6), 367-73.
- 19.Fietz T, Rieger K, Dimeo F, Blau I W, Thiel E et al. (2004) Stem cell mobilization in multiple myeloma patients: do we need an age-adjusted regimen for the elderly?. , J Clin Apher 19(4), 202-7.
- 20.Popat U, Saliba R, Thandi R, Hosing C, Qazilbash M et al. (2009) Impairment of filgrastim-induced stem cell mobilization after prior lenalidomide in patients with multiple myeloma. Biol Blood Marrow Transplant. 15(6), 718-23.
- 21.Wuchter P, Ran D, Bruckner T, Schmitt T, Witzens-Harig M et al. (2010) Poor mobilization of hematopoietic stem cell – definitions, incidence, risk factors, and impact on outcome of autologous transplantation. , Biol Blood Marrow Transplant 16, 490-9.
- 22.Kumar S, Dispenzieri A, Lacy M Q, Hayman S R, Buadi F K et al. (2007) Impact of lenalidomide therapy on stem cell mobilization and engraftment post-peripheral blood stem cell transplantation in patients with newly diagnosed myeloma. Leukemia. 21, 2035-42.
- 23.Apperley F, Cook G, Pagliuca A, Stringaris K, Douglas K et al. (2010) Efficacy of plerixafor plus G-CSF for stem cell mobilization in patients with multiple myeloma or lymphoma who have failed prior mobilization: a named patient program (NPP) evaluation. , Haematologica 95(2), 212.
- 24.Calandra G, McCarty J, McGuirk J, tricot G, Crocker S A et al. (2008) AMD3100 plus G-CSF can successfully mobilize CD34+ cells from non-Hodgkin’s lymphoma, Hodgkin’s disease and multiple myeloma patients previously failing mobilization with chemotherapy and/or cytokine treatment: compassionate use data. Bone Marrow Transplant. 41(4), 331-8.
- 25.Duarte R F, Shaw B E, Marin P, Kottaridis P, Ortiz M et al. (2011) Plerixafor plus granulocyte CSF can mobilize hematopoietic stem cells from multiple myeloma and lymphoma patients failing previous mobilization attempts: EU compassionate use data. Bone Marrow Transplant. 46(1), 52-8.
- 26.Hübel K, Fresen M M, Salwender H, Basara N, Beier R et al. (2011) Plerixafor with or without chemotherapy in poor mobilizers: results from the German compassionate use program. Bone Marrow Transplant. 46(8), 1045-52.
- 27.Hübel K, Fresen M M, Apperley J F, Basak G W, Douglas K W et al. (2012) European data on stem cell mobilization with plerixafor in non-Hodgkin's lymphoma, Hodgkin's lymphoma and multiple myeloma patients. A subgroup analysis of the European Consortium of stem cell mobilization. Bone Marrow Transplant. 47(8), 1046-50.
- 28.Worel N, Rosskopf K, Neumeister P, Kasparu H, Nachbaur D et al. (2011) Plerixafor and granulocyte-colony-stimulating factor (G-CSF) in patients with lymphoma and multiple myeloma previously failing mobilization with G-CSF with or without chemotherapy for autologous hematopoietic stem cell mobilization: the Austrian experience on a named patient program. Transfusion. 51(5), 968-75.
- 29.Sancho J M, Morgades M, Grifols J R, Juncà J, Guardia R et al.Predictive factors for poor peripheral blood stem cell mobilization and peak CD34+ cell count to guide pre-emptive or immediate rescue mobilization. , Cytotherapy 14(7), 823-9.
- 30.Bensinger W, Appelbaum F, Rowley S, Storb R, Sanders J et al. (1995) Factors that influence collection and engraftment of autologous peripheral-blood stem cells. J Clin Oncol. 13(10), 2547-55.
- 31.Ford C D, Greenwood J, Anderson J, Snow G, Peterson F B. (2006) CD34+ cell adhesion molecule profiles differ between patients mobilized with granulocyte-colony stimulating factor alone and chemotherapy followed by granulocyte-colony stimulating factor. , Transfusion 46, 193-8.
- 32.Fitoussi O, Perreau V, Boiron J M, Bouzigon E, Cony-Makhoul P et al. (2001) A comparison of toxicity following to different dose of cyclophosphamide for mobilization of peripheral blood progenitor cells in 116 multiple myeloma patients. Bone Marrow Transplant. 27, 837-42.
- 33.Gertz M A, Kumar S K, Lacy M Q, Dispenzieri A, Hayman S R et al. (2009) Comparison of high-dose CY and growth factor with growth factor alone for mobilisation of stem cells for transplantation in patients with multiple myeloma. Bone Marrow Transplant. 43, 619-25.
- 34.Winkler I G, Levesque J P. (2006) Mechanisms of hematopoietic stem cell mobilisation: when innate immunity assails the cells that make blood and bone. Exp Hematol. 34, 996-1009.
- 35.Wuchter P, Ran D, Bruckner T, Schmitt T, Witzens-Harig M et al. (2010) Poor mobilization of hematopoietic stem cell – definitions, incidence, risk factors, and impact on outcome of autologous transplantation. Biol Blood Marrow Transplant. 16, 490-9.
- 36.Kumar S, Dispenzieri A, Lacy M Q, Hayman S R, Buadi F K et al. (2007) Impact of lenalidomide therapy on stem cell mobilization and engraftment postperipheral blood stem cell transplantation in patients with newly diagnosed myeloma. Leukemia. 21, 2035-42.
- 37.Popat U, Salibat R, Thandi R, Hosing C, Qazilbash M et al. (2009) Impairment of filgrastim-induced stem cell mobilization after prior lenalidomide in patients with multiple myeloma. Biol Blood Marrow Transplant. 15, 718-23.
- 38.Armitage S, Hargreaves R, Samson D, Brennan M, Kanfer E et al. (1997) CD34 counts to predict the adequate collection of peripheral blood progenitor cells. Bone Marrow Transplant. 20(7), 587-591.
- 39.Gutensohn K, Magens M M, Kuehnl P, Zeller W. (2010) Increasing the economic efficacy of peripheral blood progenitor cell collections by monitoring peripheral blood CD34+ concentrations. Transfusion. 50(3), 656-662.
- 40.Hosing C, Saliba R M, Ahlawat S, Korbling M, Kebriaei P et al. (2009) Poor hematopoietic stem cell mobilizers: a single institution study of incidence and risk factors in patients with recurrent or relapsed lymphoma. , Am J Hematol 84, 335-7.
- 41.Kuittinen T, Nousiainen T, Halonen P, Mahlamaki E, Jantunen E. (2004) Prediction of mobilization failure in patients with non-Hodgkin’s lymphoma. Bone Marrow Transplant. 33, 907-12.
- 42.Putkonen M, Rauhala A, Pelliniemi T T, Remes K. (2007) Sepsis, low platelet nadir at mobilization and previous IFN use predict stem cell mobilization failure in patients with multiple myeloma. Cytotherapy. 9, 548-54.
- 43.Ozkurt Z N, Yegin Z A, Suyani E, Aki S Z, Acar K et al. (2010) Factors affecting stem cell mobilization for autologous hematopoietic stem cell transplantation. J Clin Apher. 25, 280-6.
- 44.Advanced lytic lesion is a poor mobilization factor in peripheral blood stem cell collection in patients with multiple myeloma. , J Clin Apher 29(6), 305-10.
- 45.Fadini G P, Pucci L, Vanacore R, Baesso I, Penno G et al. (2007) Glucose tolerance is negatively associated with circulating progenitor cell levels. Diabetologia. 50, 2156-63.
- 46.Fadini G P, Boscaro E, S De Kreutzenberg, Agostini C, Seeger F et al. (2010) Time course and mechanisms of circulating progenitor cell reduction in the natural history of type 2 diabetes. Diabetes Care. 33, 1097-102.
- 47.Delamain M T, Metze K, Marques JF Jr, Reis A R, De Souza CA et al. (2006) Optimization of CD34+ collection for autologous transplantation using the evolution of peripheral blood cell counts after mobilization with chemotherapy and G-CSF. Transfus Apher Sci. 34, 33-40.
- 48.Kozuka T, Ikeda K, Teshima T, Yoshida C, Shinagawa K et al. (2004) Peripheral blood circulating immature cell counts predict CD34+ cell yields in G-CSF-induced PBPC mobilization in healthy donors. Transfusion. 44, 526-32.
- 49.Kozuka T, Ikeda K, Teshima T, Kojima K, Matsuo K et al. (2002) Predictive value of circulating immature cell counts in peripheral blood for timing of peripheral blood progenitor cell collection after G-CSF plus chemotherapy-induced mobilization. Transfusion. 42, 1514-22.
- 50.Antar A, Otrock Z K, Kharfan-Dabaja M A, Ghaddara H A, Kreidieh N et al. (2015) G-CSF plus preemptive Plerixafor vs hyperfractionned CY plus G-CSF for autologous stem cell mobilization in multiple myeloma: effectiveness, safety and cost analysis. Bone marrow Transplant advance online publication.2015Mar9.
- 51.Costa L J, Alexander E T, Hogan K R, Schaub C, Fouts T V et al. (2011) Development and validation of a decision-making algorithm to guide the use of plérixafor for autologous hematopoietic stem cell mobilization. Bone Marrow Transplantation. 46, 64-69.
- 52.Li J, Hamilton E, Vaughn L, Graiser M, Renfroe H et al. (2011) Effectiveness and cost analysis of "just-in-time" salvage plerixafor administration in autologous transplant patients with poor stem cell mobilization kinetics. Transfusion. 51(10), 2175-82.