Abstract
After processing of cotton seed oil (CSO) the activated bleaching clay (ABC) is converted to low valued waste bleaching clay (WBC). The chemical composition of ABC from Mirpur, Azad Kashmir region of Pakistan is found out as; SiO2 71.34, Al2O3 15.54, CaO 2.72, MgO 1.48, Na2O 0.51, K2O 0.23, Fe2O3 0.02. The clay has bleachability (74 %) and oil retention (32.70 %) by standard method (ASTM). The cotton seed oil recovered (CSOR) with polar/nonpolar solvents (31.1-36.3 %) have different FFA values (0.2-0.85). However, lower percentage of tri-esters (88 %) was found out in dark coloured CSOR as compare to freshly n-hexane extracted CSO (92.5 %) from edible oil refinery. The lower FFA (0.2) valued CSOR with n-hexane is transesterified at optimized conditions to mono alkyl esters (CSOR-FAME). The reaction was optimised by performing series of experiments to observe molar conc., of methanol-oil (3-18:1), catalysts; NaOH, NaOCH3, KOH, KOCH3- oil (0.25-1.5), temperature (20-80°C), reflux time (120 min) and mixing intensity (200-650 rpm). The maximum yield of biodiesel (98.5 %) has been found out by NaOCH3 (1.00 %) as catalyst, methanol-oil (6:1) at temperature (65°C) and stirring intensity (650 rpm). The properties of CSOR-FAME (biodiesel) are also under limits as per standards; ASTM 6751, EN 14214 and WBC/ CSOR-FAME appear to be an acceptable feedstock for fatty acids/biodiesel production as renewable fuel.
Author Contributions
Academic Editor: Atul Sagade, New Satara College of engineering & Management. Maharashtra,India.
Checked for plagiarism: Yes
Review by: double blind
Copyright © 2015 Zeeshan Ali, et al
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
The utilization of waste materials and byproducts is very important to fulfill demands of quality products and to substitute depleting resources of the world. The hydrocarbons of fossils being conventional source of fuels are continuously decreasing; the fluctuating mineral oil prices and environmental impacts have intensified the search for alternate fuels. Biodiesel is defined as the mono-alkyl esters of vegetable oils or animal fats 1, biofuels has attracted a great deal of interest during the past decade as a renewable, biodegradable, non-toxic and eco-friendly clean fuel. Biodiesel is found to be the best substitute of petro-diesel fuel not only for its comparable calorific value but also for its several other advantages such as biodegradability, low toxic emission, higher flash point, excellent lubricity, carbon neutral, & environmentally acceptance fuel 2. The oils/fats are found to be the best candidates as an alternate and ecofriendly energy source. Oils/fats are not only used for edible purposes but varieties of industrial products are also developed from this commodity. However, the use of edible sources comes under heavy criticism since fuel-for food concept, which is regarded as an unethical by many quarters. Alternatively, the use of waste edible oil could be a better solution as it is available in abundant 3 Triglyceride for biodiesel production comes from various sources; edible oil, inedible oil, waste/used oils, animal fats 4 and also from microorganisms 5. The waste bleaching clay (WBC) is used for oil processing is being identified for ecofriendly and renewable fuel; it is an important byproduct of oil processing industries.
The consumption of edible oils/fats only in Pakistan is 2500 mmt, about 565 mmt or 23 % of oils/fats requirements is met through oil seed cultivation and animal resources 6. The cotton is a major crop of the country, cottonseed is the by product and cottonseed oil (CSO) is extracted from the seeds of the cotton plant after the removal of cotton lint. The country contributes about 10 % of the total CSO produced all over the world 7. Cotton (Gossypium hirsutum L.), belonging to the Malvaceae family is an important crop that yields the natural fibre used by the textile industry. It is one of the second best potential sources for plant proteins after soybean and ninth best oil-producing crop 8. The locally available bleaching clay is frequently used to process cotton seed oil to meet the standards for edible usage. The activated bleaching clay (ABC) is used to remove colouring matters, soap, gums, metals (iron, nickel), oxidized compounds and polymers. This substance consists primarily of hydrated aluminium silicate, reports indicated that bleaching clays retains 20-40 % of oil/fat and importantly, the adsorbed oil represents the major part of bleaching cost as reported by Ong 9. After processing of CSO, large quantities of bleaching clays are disposed off in landfills, causes pollution hazard and the retained organics are wasted.
The direct use of oils/fats as fuel is also limited due to two main reasons; high viscosity and low volatility 2. Therefore, oils/fats are chemically reacted with alcohols (transesterification reaction) to produce fatty acid alkyl esters/biodiesel 11. The resulting product not only contains alkyl esters but also unreacted starting material, residual alcohol and residual catalyst 2. The optimization of transesterification reaction depends upon; catalyst type & concentration, methanol-oil molar ratio, reaction temperature, reaction time, stirring intensity, FFA (free fatty acid value) and moisture contents in oils and fats 12. Mostly, transesterification is being carried out by alkaline catalysts in homogeneous phase i.e., NaOH, KOH, NaOCH3 and KOCH313, 14. The transesterification is also reported by enzymatic esterification of CSO 15. However, no work has been carried out to study the base catalyzed transesterification of waste cotton seed oil from processed BC. Keeping in view, the consumption of fixed oils/fatty acids for production of edible oils, oleochemicals and allied products, the thorough investigation regarding base catalysed transesterification of cottonseed oil recovered (CSOR) from WBC has been carried out. The work includes the reaction parametric studies and produced fatty acids methyl esters (FAME) “biodiesel” (CSOR-FAME) properties.
Materials & Methods
Materials
Activated bleaching clay (ABC) of Azad Kashmir (Pakistan) region and waste bleaching clay (WBC) are attained from Hamza vegetable oil refineries, Lahore. Cotton seeds (CS) belongs to Punjab region (Pakistan). Solvents/reagents used are of analytical-grade, mostly purchased from Merck-Darmstadt, Germany and Riedel-de-Haën, Germany. Silicagel HF254, Merck Ref. 7739 was used for TLC and standards are product of BDH, UK. The BF3-methanol complex (Merck-Schuchardt, Germany) was used for fatty acids analysis through GC. The fatty acids’ methyl esters (C12-C24) are attained from Supelco®,USA for GC analysis. The colouring reagent; 2,7-dichlorofluorescein (Merck, Germany) used to identify components under UV light; λ 366 nm.
Evaluation of Freshly Extracted CSO
The analysis of freshly extracted CSO i.e., FFA value, saponification value, iodine value, peroxide value, unsaponifiables was carried out by official methods of American Oil Chemist’s Society (AOCS) 16. The glyceride components & fatty acids analysis were carried out by GC and TLC 17 The gas chromatograph; GC-14A & data processor C-R-4A was used for the identification of methyl esters by using a polar column (2.5 m × 3 mm id), coating material GP-10%-SP-2330 on supporting media 100-120 chromosorb WAW. The FID detector was used with requisite temperature of detector and injector; 250°C and 230°C respectively. It was operated under temperature programming 180-210°C at the rate of 4°C/minute & Nitrogen flow rate of 30 mL/minute. The fatty acid methyl esters were identified by the comparison of their corresponding retention times with standard methyl esters of fatty acids; C12-C24 under the same conditions 18.
Evaluation of ABC
The analysis of ABC ie; compositional analysis, bleachability, moisture contents, oil filtration rate, oil retention and bulk density was carried out by standard methods 19.
Extraction of Oil from WBC & Evaluation of CSOR
The lipids were extracted through cyclic solvent extraction (Soxhlet apparatus) by using solvents; n-hexane, methanol, ethanol, petroleum ether & their combinations with repeated extractions. The solvent was removed under vacuum by rotary film evaporator (Hëidolph, Germany). The analysis of CSOR i.e., free fatty acid value (FFA), saponification value, iodine value, per oxide value, unsaponifiables was carried out 16. TLC used to determine the lipid components & extent of transesterification reactions. The thin layer chromatography (TLC-20 x 20 cm) of 0.5 mm thickness were prepared by coating silicagel for the separation/ identification of lipid components. The solvent system used for the fractionation of lipids components was; hexane-diethylether-acetic acid (80/20/2) & for monoalkyl esters; hexane-diethylether (80/20). The locating agent 2,7-dichlorofluorescein was used, which gave purple yellow colored bands under an ultraviolet light at λ ; 366 nm.
Experimental Procedure
Experiments were carried out to ascertain the methanol-oil molar ratio, catalyst type & concentration, reaction temperature & agitation intensity on transesterification reaction. The reaction time (120 min) was kept constant throughout experimental studies. The molar ratio of methanol-oil was varied as 3:1, 6:1, 9:1, 12:1, 15:1 & 18:1. The catalysts; NaOH, NaOCH3, KOH, KOCH3 were used. NaOCH3 was found the most efficient catalyst so its concentrations were varied; 0.00, 0.25, 0.50, 0.75, 1.00, 1.25 & 1.50 % to found optimum concentration. The employed temperatures were; 20, 35, 50, 65 & 80°C. Agitation rates were; 200, 350, 500 & 650 rpm.
Ttransesterification of CSOR
The chemical reaction was carried out using a 500 mL round-bottomed flask, equipped with thermostat, sampling outlet, mechanical stirrer (Eyela Tokyo, Japan) with tachometer reading 15 × 10 rpm DCM & reflux condensation systems. The CSO (250 g) was preheated to the set temperatures; 20, 35, 50, 65 & 80°C. The fixed amounts of freshly prepared methanolic solutions of catalysts; sodium hydroxide, potassium hydroxide, sodium methaoxide & potassium methaoxide were mixed with oil & considered the time; 0.00 mint. After consistent intervals 2 mL of sample was withdrawn for chromatographic analysis. All the experiments were conducted for maximum of 120 min in order to ensure the complete esterification of CSOR into methyl esters CSOR-FAME.
Chromatographic Analysis
The chromatographic techniques; gas chromatography (GC) and TLC were frequently used for the analysis of CSOR-FAME. The GC details & conditions have already mentioned in section 2.2. However, for TLC, the chromatograms (20×20 cm) of thickness (0.50 mm) & adsorbent (Silicagel HF254) were prepared by the use of Quickfit TLC applicator. The eluting solvent mix; n-hexane-diethylether (10:90) was used to fractionate & identify methyl esters/ glycerides in order to ensure the complete conversion of the CSOR into methyl esters (CSOR-FAME). The non-destructive locating reagent 2,7-dichlorofluorescein used for coloured spots of esters & glycerides under ultra violet light; λ 366 nm.
Production, Separation & Purification of CSOR-FAME (biodiesel)
After optimization of reaction parameters, the experiment was carried out to produce biodiesel by using NaOCH3 (1 % w/w), methanol-oil (6:1 w/w), temperature (65°C) & stirring intensity (650 rpm) for maximum yield of biodiesel. On achieving the maximum yield of alkyl esters, the reaction stopped & the excess methanol was recovered with rotary film evaporator (Hëidolph, Germany) at 50°C under vacuum. The residue transferred to separating funnel & washed with water (50-55°C) & n-hexane. After, some time the mixture is cooled down & two phases are separated. The upper phase consisted of CSOR-FAME while the lower phase contained the glycerol with other materials (methanol, catalyst, soaps, and some entrained methyl esters and partial glycerides). After separation of the two layers, the upper methyl esters layer was purified by removing residual methanol at 50°C by evaporator under vacuum. The remaining catalyst, methanol and glycerol were removed by successive rinses with distilled water. The residual water was removed by drying with anhydrous Na2SO4 and filtered. The lower glycerol containing phase was acidified with a calculated amount of sulphuric acid, to neutralize any unreacted sodium methoxide and to decompose soaps formed during transesterification reaction. The mixture obtained was subjected to distillation at 65°C under a moderate vacuum to recover the excess methanol. This assisted the separation of glycerol from entrained methyl esters and soaps. The biodiesel yield was determined as: CSOR-FAME (%) = FAME (g)/CSOR (g) × 100
Analysis of Biodiesel
The fuel properties of CSOR-FAME ie; acid value, density, kinematic viscosity at 40°C, oxidative stability, lubricity, cloud point, pour point, cold filter plugging point, flash point, ash content, copper strip corrosion test, ester contents, free glycerine, total glycerine, mono, di and tri glyceride contents were determined according to American Standards for Testing Materials (ASTM) and European Standard (EN). Each experiment was conducted in triplicate and data are reported as mean ± standard deviation (SD).
Results & Discussion
The indigenous BC belonging to Azad Kashmir region of Pakistan is frequently processed to produce ABC. The chemical composition and processing technology relates to bleaching quality of ABC. The ABC is analyzed by standard methods 19 as shown in Table 1,the prominent chemical components of clay are; Silica (71.34), Calcium Oxide (15.54), Magnesium Oxide (2.72), Sodium Oxide (1.48 %) & specific quality parameters of clay ie., bleachability 74 %, oil filtration rate 4.5, oil retention 29.7 %, bulk density 640. The chemical composition and physical properties reveals that clay resembles to bentonite and falls under good category for bleaching of oils/fats. The oil retention is generally depends upon the chemical composition, structure, mesh size, physical parameters, activation process of clay and nature of oil. The bleaching process, impurities and retention time leads to the quantity and quality of recovered cotton seed oil. The cotton seed oil CSO are recovered from WBC with polar and non polar solvents for maximum oil yield as shown in Table 2, the accumulated oil yields (37.2-39.8 %) with combination of solvents are higher as compare to usuall n-hexane extractions at refineries due to different conditions of process in the oil refineries. The colour of polar extractions was also darker (brown) than that of the non-polar extractions. The polar solvents yielded more oil as compare to non-polar due to higher solubility of impurities and unwanted materials. The oil yields extracted with different solvents with respect to solvent cycles and FFA of CSOR are summarized in Figure 1. The ethanol yields more as compare to methanol and non-polar solvents, in agreement to the determinations of Lee CG etal that efficiencies of extractions by the polar alcohols, except for methanol, were higher but with a slower initial rate than the nonpolar hydrocarbons 20.
Figure 1.CSO yields by different solvents with respect to solvent cycles/extraction time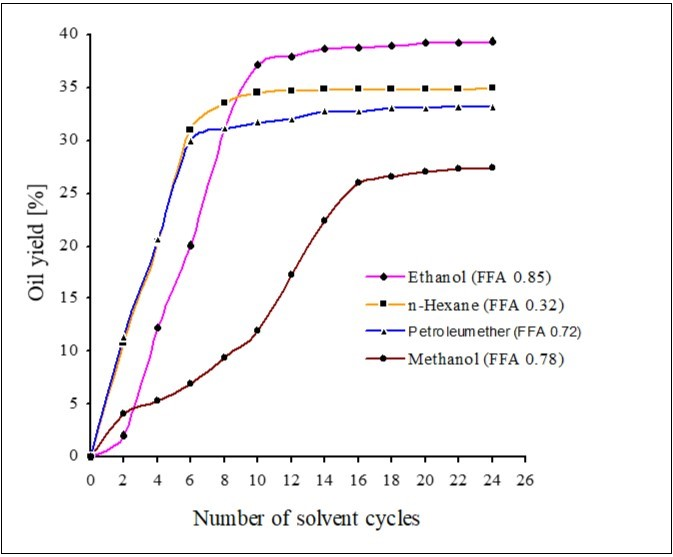
The oil extracted with n-hexane is comparatively cleaner, pure, have less impurities, having less free fatty acids (0.32) and it is also is a generall practice at extraction units/refineries. So, n-hexane extracted oil is being considered for transesterification and for whole proceeding work. The CS yield oil near to WBE (35 %) as shown in Table 1, there is also a little difference in bulk densities of cotton seeds and WBE. So, WBE have almost comparable potential of oil with cotton seeds. The WBE is ignored due to more impurities, dark in colour and limited/nonfesible technology for the recovery of edible oil. But it is equally important as a source of fatty acids required for biodiesel production.The physicochemical values and glycerides components determined by TLC are also comparable as shown in Table 3 and Figure 2, except difference in colour on Lovibond scale. The unsaponifiables (USV) of CSOR are higher as compare to CSO. The Table 3 shows the comparison of CSOR & CSO. The present results support the fact that CSOR have higher values of colour index & unsaponifiables as compare to raw CSO due to higher contents of colouring components, carotenoids, phospholipids, gossypol & sterols.
Table 1. Evaluation of ABC| Chemical Composition | Physical Analysis | Sieve Analysis | |||
| Silica (SiO2) | 71.34 | Bleachability (%) | 74 | -100 | 100 |
| Iron Oxide (Fe2O3) | 0.02 | Moisture (100±2°C ) | 2.85 | -150 | 99.85 |
| Aluminium Oxide (Al2O3) | 15.54 | pH | 3.45 | -200 | 99.78 |
| Calcium Oxide (CaO) | 2.72 | Free acid (as HCl) | 1.26 | -250 | 99.09 |
| Magnesium Oxide (MgO) | 1.48 | Oil filtration rate (100±2°C) | 4.5 | -300 | 98.57 |
| Sodium Oxide (Na2O) | 0.51 | Oil retention | 29.7 | -325 | 98.18 |
| Potassium Oxide (K2O) | 0.23 | Loose bulk density | 480 | -350 | 90.73 |
| Sulphur (as SO3) | Nil | Compact bulk density | 640 | ||
| Loss on ignition (1000±5°C) | 4.82 | ||||
| I st Extract * | II nd Extract * | Total Extract * | |||
| Solvent-I | % | FFA | Solvent-II | % | % |
| n-Hexane | 35.0 ± 0.5 | 0.20 | Methanol (A)Ethanol (B) | 3.7 ± 0.74.6 ± 0.8 | 38.739.6 |
| Methanol | 27.5 ± 0.3 | 0.78 | Petroleum ether (C) | 9.8 ± 0.9 | 37.3 |
| Hexane (D) | 11.3 ± 0.7 | 38.8 | |||
| Ethanol | 39.4 ± 0.4 | 0.85 | Petroleum ether (E) | 0.2 ± 0.05 | 39.6 |
| Hexane (F) | 0.4 ± 0.07 | 39.8 | |||
| Petroleum ether | 33.2 ± 0.6 | 0.72 | Methanol (G) | 4.0 ± 0.5 | 37.2 |
| Ethanol (H) | 6.1 ± 0.7 | 39.3 | |||
| Tests | CSOR | CSO |
| Oil Yield (%) | 35 | 39 |
| Specific gravity (25°C) | 0.9176 | 0.917 |
| Color (Lovibond scale 1″) | 90Y, 18R | 78Y, 7R |
| Refractive index(40°C) | 1.47 | 1.468 |
| FFA (% as oleic acid ) | 0.2 | 0.25 |
| Unsaponifiables (%) | 2.73 | 1.21 |
| Peroxide value (meqKg-1) | 5.5 | 4.56 |
| Iodine value | 110 | 111 |
| Saponification value | 196 | 193 |
| Triglycerides (%) | 88.3 | 92.5 |
| Diglycerides (%) | 5.7 | 3.9 |
| Monoglycerides (%) | 2.3 | 1.8 |
Figure 2.Behaviours of different base catalysts on yield of CSOR-FAME at oil-methanol molar ratio (1:6), rate of stirring (650 rpm), reaction temperature (65°C) & catalyst concentration (equimolecular).
Although, CSOR have impurities but it contains comparable glycerides (96.3 %) to glycerides of CSO (98.2 %) as determined by TLC. The n-hexane extracted CSOR is transesterified to produce biodiesel, the transesterification variables were evaluated. The variables; type of basic catalyst, amount of catalyst, molar ratio of methanol to oil, the reaction temperature & agitation intensity were studied. The reactions were made under the same reaction conditions with different basic catalysts; NaOH, CH3ONa, KOH & CH3OK & their concentrations (0.25-1.50 % w/w). The vegetable oils yield higher percentages of methyl esters for alkaline catalysts at temperature greater then 60°C, molar ratios of methanol to oil is at least 6:1 & minimum reaction time is one hour 12.
Effects of Different Basic Catalyst & Amounts on Transesterification of CSOR
The efficiency of different basic catalysts is illustrated in the Figure 2, KOH showed least amount of CSOR-FAME yield, while highest yield of esters is attained by using CH3ONa. Sodium & Potassium methaoxides exhibited higher yields as compare to hydroxides as shown in Figure 3, the lower yield of esters by using hydroxide catalysts is may be due to formation of water which leads to hydrolysis of esters & formation of soaps 21
Figure 3.Effect of Sodium methaoxide concentration on yield of CSOR-FAME at oil-methanol molar ratio (1:6), reaction temperature (65°C) & rate of stirring (650 rpm)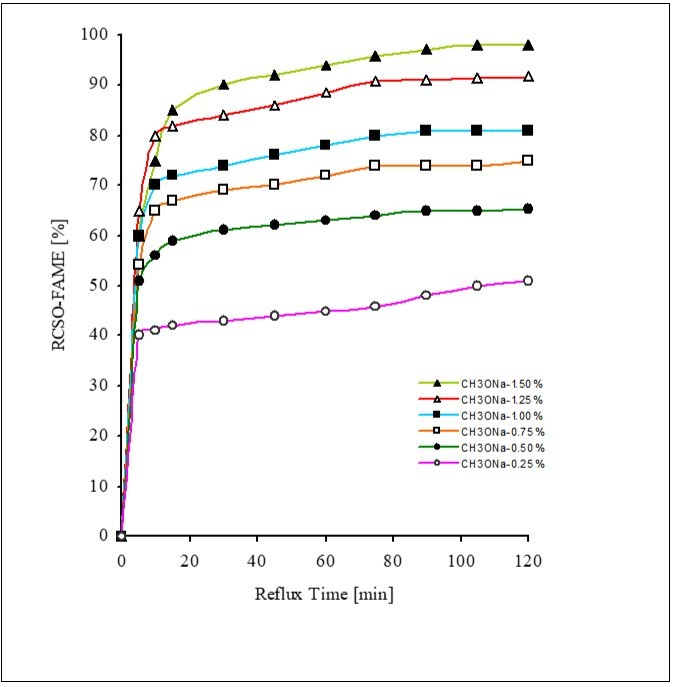
Six experiments were conducted by varying the sodium methaoxide concentrations; 0.25, 0.50, 0.75, 1.00, 1.25 & 1.50 % (oil weight basis). The effect of NaOCH3 concentration on ester formation is shown in Figure 3. The best yields were offered at concentration of 1.00 %. The lowest concentration of sodium methaoxide i.e., 0.25 % was not effective to catalyze the reaction for maximum yield of products. The conversion did not increase by increasing the catalyst amount beyond 1.00 % as well to obtain a fuel meeting biodiesel standards. It was observed that with the increase in catalyst concentration above 1.00 %, the yields were lower. The overload of sodium methaoxide emulsified the product. With the increase in the concentration of catalyst above 1.00 % there was decrease in the yield of methyl esters. This decline in ester yield might be attributed to the increased formation of glycerol & soap. The current work agree with the results that the formation of soap in the presence of higher amount of catalysts increases the viscosity of the reactants thus lowers the ester yield 22, 23. The addition of an excessive amount of alkaline catalyst causes formation of emulsions, increasing viscosity & making recovery of the methyl esters difficult 24, 25. An optimal catalyst concentration is required for successful transesterification, in the present case it is determined to be 1.00 % sodium methaoxide. However, it is found that potassium hydroxide (1.0 %) offered the best yield during the methanolysis of Pongamiapinnata oil 26 & used frying oil 27.
Effect of Methanol/oil Molar Ratio on Trans Esterification of Recovered CSO
The stoichiometrically the transesterification reaction requires 3 mol of alcohol for 1 mol of triglyceride to yield 3 mol of methyl esters & 1 mol of glycerol. The transesterification is a reversible/equilibrium reaction. Therefore an excess methanol is required for successful completion of reaction. The molar ratios of methanol to oil; 3:1, 6:1, 9:1, 12:1, 15:1 & 18:1 have been employed to determine the effects of excess methanol quantities. The yields of methyl esters with respect to time at different molar ratio of methanol-oil from 3:1 up to the level of 18:1 exhibited encouraging effects on the yield of esters (Figure 4). The higher molar ratio then the stoichiometeric value resulted in a greater ester formation 28 & could ensure complete reaction. The limited effect on the ester yield was found with the increase in molar ratio of methanol to oil. On the other h&, the reaction was also incomplete for a molar ratio less then 6:1. It has been shown that beyond the molar ratio of 6;1, further methanol addition had no considerable effect on ester formation. Excess amount of catalyst also complicate ester recovery and raised process cost 29. In case of molar ratios greater then 6:1, a dilution effect is the likely cause while for molar ratios less then 6:1, insufficient mixing of the reactants in the biphasic transesterification reaction system is the likely cause. The current results of optimum yield (98.5 %) of CSOR-FAME with oil/ methanol molar ratio of 1:6 are in agreement with work carried out by Freedman etal and Usta N etal 12, 30which reported optimum conversion of various vegetable oils and tobacoo seed oil in to their corresponding esters with a molar ratio of 1:6. So the unwanted impurities retained by ABC during processing of cotton seed oils have least effects for their conversions to methy esters.
Figure 4.Effect of the oil-methanol molar ratio on CSOR-FAME yield at NaOCH3 (1.0%), rate of stirring (650 rpm) & reaction temperature (65°C)
Optimization of Reaction Temperature.
The transesterification of CSOR were studied at different temperatures i.e., 20, 35, 50, 65 & 80°C. The reaction time of 120 min was constant for each experiment. The Sodium methaoxide (1.0 %) as optimized in the previous section, methanol/oil molar ratio (6:1) & rate of stirring 650 rpm were applied for each experiment at different temperatures. The Figure 5 shows that 85 % ester yield was achieved in just 15 min. After 120 min, the reaction was completed & esters yields; 89.0, 95.5, 98.5 & 98.7 % were found out at 20, 35, 50, 65 and 80°C respectively. The lower ester yields can not be up to the standards due to higher contents of glycerides & fatty acids. The temperature optimization 65°C for conversion of recovered cotton seed oil to esters results are in accordance to the work of Mehr etal 31 regarding optimization of alkali-catalyzed transesterification of Pongamia Pinnata seed oil for production of biodiesel. Encinar etal and Karaosmanoglu etal 32, 33 findings (65°C) are also same for production of biofuels direct from oils of Cynara Cardunculus L and rape seed respectively.
Figure 5.Temperature effects on CSOR-FAME yield at NaOCH3 (1.0%), oil/methanol molar ratio (1:6) & rate of stirring (650 rpm)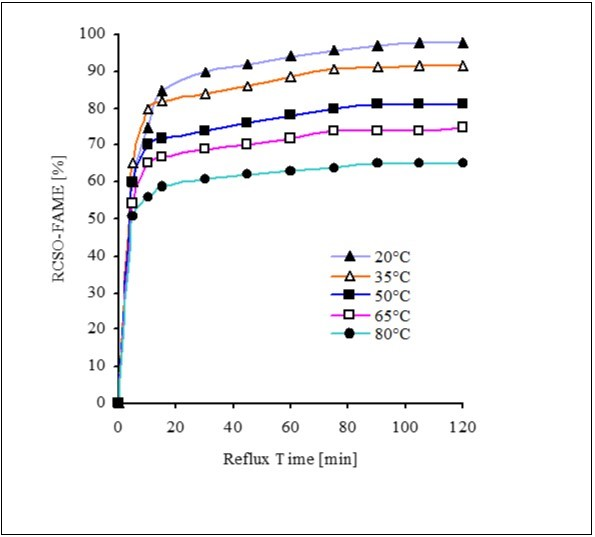
Optimization of Rate of Stirring
The effect of stirring on CSOR-FAME production was investigated by performing four experiments at different stirring rates (200, 350, 500 & 650 rpm). In all experiments, an oil/methanol molar ratio of 1:6, a reaction temperature of 65° C, & a NaOCH3 catalyst (1.00 %) were used. The Figure 6showed direct correlation between the stirring rate & ester yield; i.e., as the rate of agitation was increased, an increase in yield was observed. Accordingly, a mixing rate of 650 rpm afforded the optimum conversion of CSOR to CSOR-FAME (98.5%). The different stirring rates concluded that elevated speeds promoted the homogenization of reactants, leading to higher methyl ester yields. This is in accordance with earlier studies 34.
Figure 6.Stirring effects on CSOR-FAME at NaOCH3 (1.0%), oil-methanol molar ratio (1:6) & reaction temperature (65°C)
Quality of CSOR-FAME (Biodiesel)
The nature of fatty acids plays an important role on the qualities of biodiesel, the fatty acids were analyzed by GC. The major fatty acid is linoleic acid (53.8 %), followed by oleic acid (20.6 %) while palmitic acid (19.5 %) is the predominant saturated acid as shown under Table 4. The recovered oil contains saturated fatty acids (23.8 %), monounsaturated fatty acids (21.2 %) and polyunsaturated fatty acids (54.9 %). The CSOR-FAME were purified with solvent extraction. The impurities had deleterious effects on the fuel properties, especially with regard to diminished temperature performance, manifested by increased cloud, pour, & cold filter plugging points. The important fuel properties; acid value, density, kinematic viscosity (40°C), oxidative stability, lubricity, cloud point, pour point, cold filter plugging point, flash point, ash content, copper strip corrosion of CSOR-FAME as measured according to accepted ASTM methods are depicted in Table 5. In addition to mentioned properties the ester contents, free glycerine, total glycerine, mono, di & tri glyceride contents were also determined. Although CSO contains higher contents of triglycerides (92.5%) as compare to triglycerides of CSOR (88.3%) but lower contents of mono & diglycerides are present in CSO as shown in table 3. Therefore, higher yield of esters have been found out by the optimised transesterification of CSOR as shown in table 5. The properties of biodiesel depend heavily on its raw materials 35, however, properties of produced CSOR-FAME investigated in this study also satisfied nearly all prescribed ASTM D 6751 & EN 14214 specifications. Therefore the indigenous ABC, abundantly used for processing of CSO have appreciable contents of fatty acids, after certain optimized processing the product is economical & ecofriendly source of energy.
Table 4. Fatty acids of CSOR-ME (Biodiesel)| Composition | Symbol | (%0 |
| Myristic acid | C14: 0 | 0.8 |
| Palmitic acid | C16: 0 | 19.5 |
| Palmitoleic acid | C16: 1 | 0.6 |
| Stearic acid | C18: 0 | 2.6 |
| Oleic acid | C18: 1 | 20.6 |
| Linoleic acid | C18: 2 | 53.8 |
| Linolenic acid | C18: 3 | 1.1 |
| Arachidic acid | C20:0 | 0.4 |
| Behenic acid | C22:0 | 0.3 |
| Lignoceric acid | C24:0 | 0.2 |
| Property | CSOR-FAME | ASTM D6751 | EN 14214 |
| Acid value (mg KOH/g) | 0.10±0.03 | 0.50 max | 0.50 max |
| Density (25°C, kg m−3) | 873±12.7 | – | 860–900 |
| Kinematic viscosity (mm2/s; 40 °C) | 4.03±0.08 | 1.9–6.0 | 3.5–5.0 |
| Oxidative stability (h) | 1.82±0.13 | 3 min | 6 min |
| Lubricity (HFRR; μm) | 143.5±2.6 | – | – |
| Cloud point (°C) | 1.10±0.11 | Report | – |
| Pour point (°C) | 1.98±0.09 | – | – |
| Cold filter plugging point (°C) | 1.00±0.12 | – | – |
| Flash point (°C) | 154±3.0 | 93 min | 120 min |
| Ash content (%) | 0.013±0.001 | 0.02 max | 0.02 max |
| Copper strip corrosion (50°C, 3 h) | 1a | No. 3 max | No. 1 min |
| Methanol content (%) | 0.187±0.002 | – | 0.20 max |
| Free glycerin (%) | 0.015±0.001 | 0.240 max | 0.250 max |
| Total glycerin % | 0.225±0.015 | 0.020 max | 0.020 max |
| Ester contents % | 98.5±1.26 | – | 96.5 min |
| Monoglyceride % | 0.35± 0.04 | – | 0.80 max |
| Diglyceride % | 0.13 ±0.02 | – | 0.20 max |
| Triglyceride % | 0.07 ± 0.01 | – | 0.20 max |
Abberivations
ABC:
Activated bleaching clay
AOCS:
American Oil Chemists’ Society, Official methods
ASTM:
American standard for testing materials
CSO:
Cottonseed oil
CSOR:
Cotton seed oil recovered
CSOR-FAME:
Cotton seed recovered oil-fatty acid methyl esters (biodiesel)
FFA:
Free fatty acid value
GC:
Gas chromatography
SD:
Standard deviation
TLC:
Thin layer chromatography
WBC:
Waste bleaching clay
References
- 1.Knothe G. (2013) Production and properties of biodiesel from algal oils, Algae for Biofuels and Energy. Developments in Applied Phycology Springer 5, 207.
- 2.Knothe G, Gerpen J V, Krahl J. (2005) Introduction in the biodiesel handbook Urbana:AOCS Press Il.
- 3.Gui M M, Lee K T, Bhatia S. (2008) Feasibility of edible oil vs non-edible oil vs waste edible oil as biodiesel feedstock Energy. 33, 1646-53.
- 4.Haas M J, Foglia T A. (2005) Alternate feedstocks and technologies for biodiesel production, Biodiesel Handbook Urbana:AOCS Press Il.
- 5.Meng X, Yang J, Xu Z, Zhang L, Nie Q. (2009) Biodiesel production from oleaginous microorganisms Renew Energy. 34, 1-5.
- 6.Ministry of (2009) food & agriculture, Government of Pakistan. Agricultural Statistics of Pakistan table-169 249.
- 9.Ong JT L. (1983) Oil recovery from spent bleaching clay. , disposal of the extracted material J Am Oil Chem Soc 60, 314-5.
- 10.Encinar J M, Gonzalez J F, Rodriguez-Reinares A. (2005) Biodiesel from used frying oil. Variables affecting the yields & characteristics of the biodiesel Ind Eng Chem Res. 44, 5491-99.
- 12.Freedman B, Pryde E H, Mounts T L. (1984) Variables affecting the yields of fatty esters from transesterified vegetable oils. , J Am Oil Chem Soc 61, 1638-43.
- 13.Karamoanoglu F Centinkaya. (2004) Optimisation of base catalyzed transesterification reaction of used cooking oil Energy & Fuels. 18, 1888-95.
- 14.Latero E, Liu y, Lopez D E, Suwannakarn K, Bruce D A. (2005) Synthesis of biodiesel via acid catalysis Ind Engg Chem Res. 44, 5353-63.
- 15.Royon D, Daz M, Ellllenrieder G, Locateelli S. (2007) Enzymatic production of biodiesel from cotton seed oil using t-butanol as a solvent Bioresour Technol. 98, 648-53.
- 17.Ali Z, Siddiqui H L, Mahmud S. (2009) Lipids classification of Mangifera indica kernel fat. , J Chem Soc Pak 31(1), 131-37.
- 18.Ali Z, Siddiqui H L, Mahmood S. (2007) Cis/trans monoenoic fatty acids of hydrogenated mango kernel fats. , Pak J Sci Ind Res 50(6), 377-79.
- 20.Lee C G, Seng C E, Liew K Y. (2000) Solvent efficiency for oil extraction from spent bleaching clay. , J Am Oil Chem Soc 77(11), 1219-22.
- 21.Alcantara R, Amores J, Conoira I, Fidalgo E, France M J. (2000) Catalytic production of biodiesel from soyabean oil, used frying oil & tallow Biomass Bioenergy. 18, 515-27.
- 22.Encinar J M, Gonzalez J F, Rodriguez J J, Tejedor A. (2002) Biodiesel fuels from vegetable oils, transesterification of cynara cardunculus L. oils with ethanol Energy Fuels. 16, 443-50.
- 23.Dorado M P, Ballesteros E, Lopez F J, Mittelbach M. (2004) Optimization of alkali-catalyzed transesterification of Brassica carinata oil for biodiesel production Energy &. , Fuel 18, 77-83.
- 24.Zhang Y, Dube M A, Mclean D D, Kates M. (2003) Biodiesel production from waste cooking oil. Economic assessment & sensitivity analysis Bioresource Technology 90, 229-40.
- 25.Ma F, Clements L D, Hanna M A. (1998) The effects of catalyst, free fatty acids, & water on transesterification of beef tallow Trans Am Soc Agric Eng. 41, 1261-64.
- 26.Meher L C, Vidya S S, SNN Dharmagadda. (2006) Optimization of alkali-catalyzed transesterification of Pongamia pinnata oil production of biodiesel Bioresour Technol. 97(131), 92-7.
- 27.Encinar J M, Gonzalez J F, Rodriguz-Reinares A. (2005) Biodiesel form used frying oil. Variables affecting the yields & characteristics of the biodiesel Ind Engg Chem Res. 44, 5491-99.
- 28.Fukuda H, Kondo A, Noda H. (2001) Biodiesel fuel production by trans-esterification of oils. , J Bioscience & Bioeng 92, 405-16.
- 29.Goff M J, Bauer N S, Lopes S, Sutterlin W R, Suppes G J. (2004) Acid-catalyzed alcoholysis of soybean oil. , J Am Oil Chem Soc 81, 415-20.
- 30.Usta N. (2005) Use of tobacco seed oil methyl ester in a turbocharged indirect injection diesel engine Biomass Bioenergy. 28, 7786.
- 31.Meher L C, Vidya S S, SNN Dharmagadda. (2006) Optimization of alkali-catalyzed transesterification of Pongamia pinnata oil production of biodiesel. , Bioresour Technol 97, 1392-97.
- 32.Encinar J M, Gonzalez J F, Sabio E, Ramiro M J. (1999) Preparation & properties of biodiesel from Cynara cardunculus L Ind Eng Chem Res. 38, 2927-31.
- 33.Karaosmanoglu F, Akdag A, Cigizoglu K B. (1996) Biodiesel from rapeseed oil of turkish origin as an alternative fuel. , Appl Biochem Biotechnol 61, 251-265.
Cited by (2)
- 1.Agu Chinedu Matthew, Orakwue Charles Chukwudozie, Ani Onuabuchi Nnenna, Chinedu Mmesoma Promise, Kadurumba Chukwuma Henry, et al, 2024, Methyl ester production from cotton seed oil via catalytic transesterification process; characterization, fatty acids composition, kinetics, and thermodynamics study, Sustainable Chemistry for the Environment, 5(), 100064, 10.1016/j.scenv.2024.100064
- 2.Costa Jonei Marques da, Lima Luiz Rogério Pinho de Andrade, 2023, Bentonite functioned by potassium compounds as a solid catalyst for biodiesel production, REM - International Engineering Journal, 76(3), 265, 10.1590/0370-44672022760011
